

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123720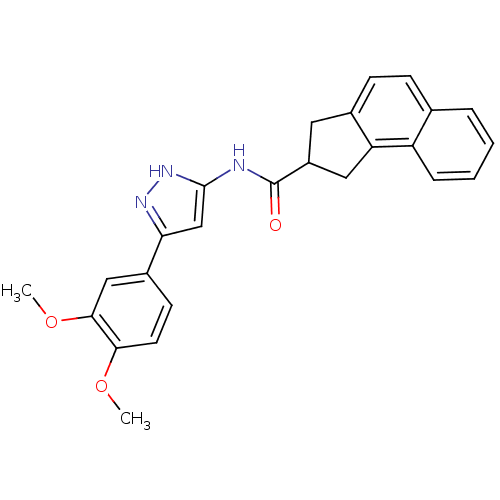 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123724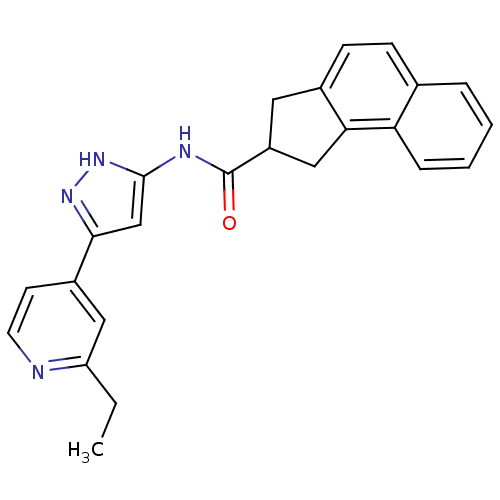 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123724 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123724 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50073047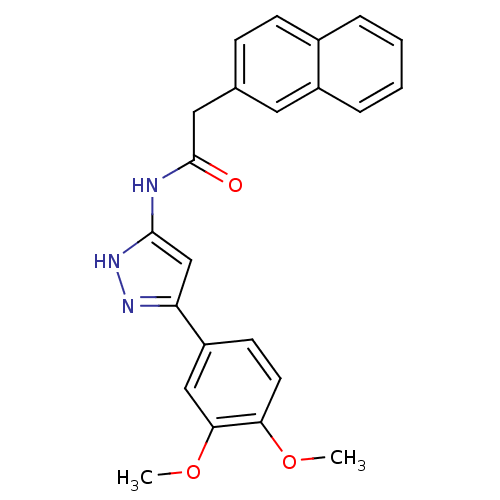 (CHEMBL291666 | N-[5-(3,4-Dimethoxy-phenyl)-1H-pyra...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123729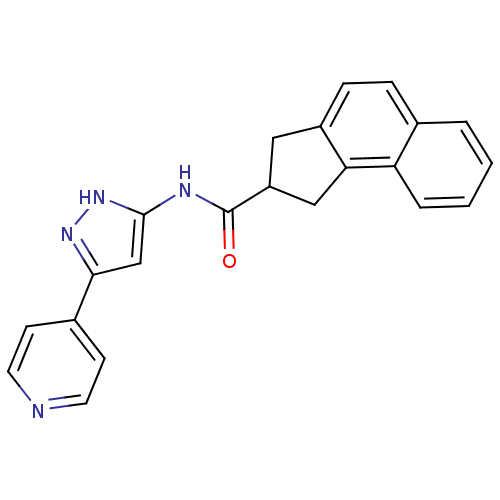 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50409778 (CHEMBL2110162) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123735 (CHEMBL160443 | Indan-2-carboxylic acid [5-(3,4-dim...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123717 (CHEMBL435036 | Indan-2-carboxylic acid [5-(4-chlor...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123726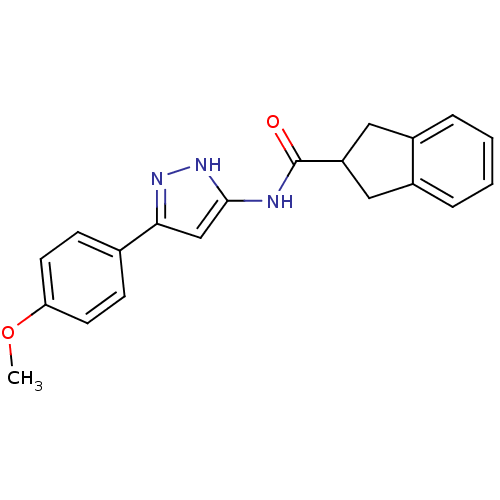 (CHEMBL159070 | Indan-2-carboxylic acid [5-(4-metho...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123727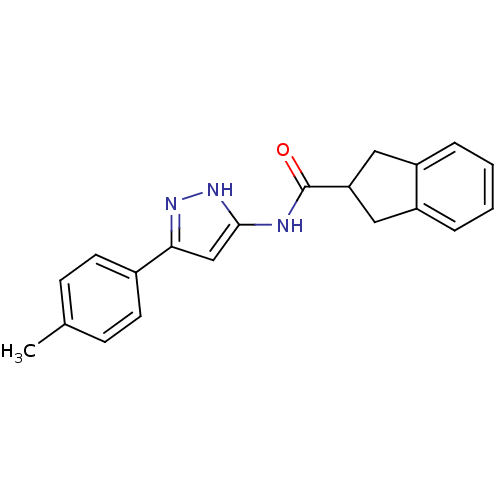 (CHEMBL158759 | Indan-2-carboxylic acid (5-p-tolyl-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123728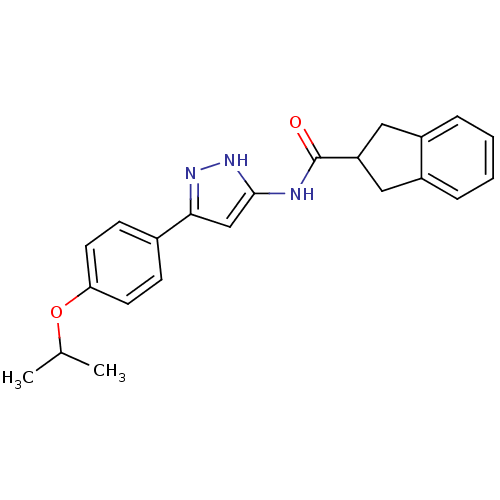 (CHEMBL160722 | Indan-2-carboxylic acid [5-(4-isopr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123734 (CHEMBL158940 | Indan-2-carboxylic acid [5-(3-chlor...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123732 (CHEMBL161398 | Indan-2-carboxylic acid (5-phenyl-1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123730 (CHEMBL157588 | Indan-2-carboxylic acid [5-(2-chlor...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50401285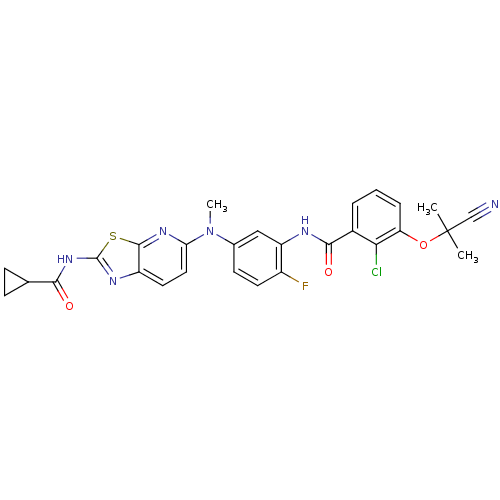 (CHEMBL2204532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 in HUVEC assessed as reduction of VGF-stimulated cell proliferation after 5 days | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50401285 (CHEMBL2204532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM99471 (US8497274, 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged wild type BRAF (unknown origin) expressed in baculovirus system using GST-MEK1(K96R) as substrate after 20 mins | J Med Chem 56: 6478-94 (2013) Article DOI: 10.1021/jm400778d BindingDB Entry DOI: 10.7270/Q2W95BPS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50401288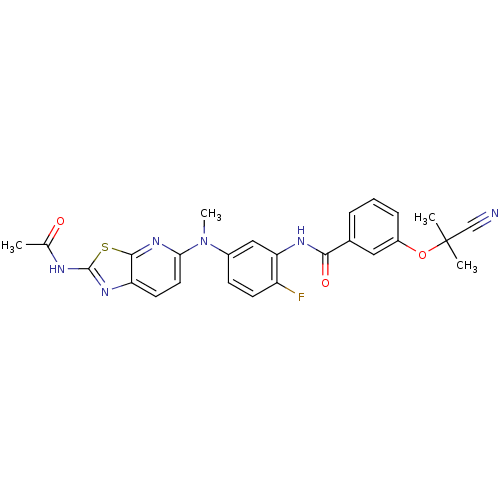 (CHEMBL2204530) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 in HUVEC assessed as reduction of VGF-stimulated cell proliferation after 5 days | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123724 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of compound was determined by its ability to inhibit NPY induced [Ca2+]i increases in CHO cells which expressed the recombinant... | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123724 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of compound was determined by its ability to inhibit NPY induced [Ca2+]i increases in CHO cells which expressed the recombinant... | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50401287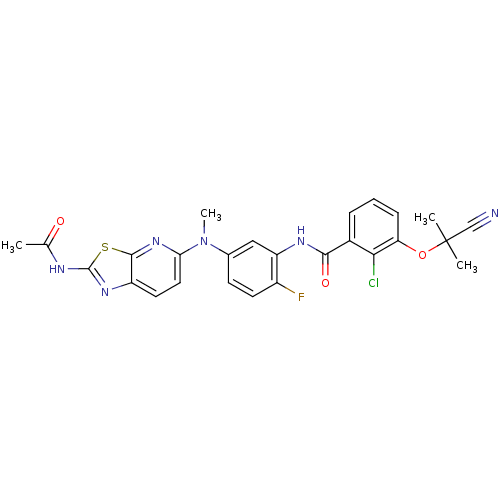 (CHEMBL2204531) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 in HUVEC assessed as reduction of VGF-stimulated cell proliferation after 5 days | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50409778 (CHEMBL2110162) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50401285 (CHEMBL2204532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-RAF | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136699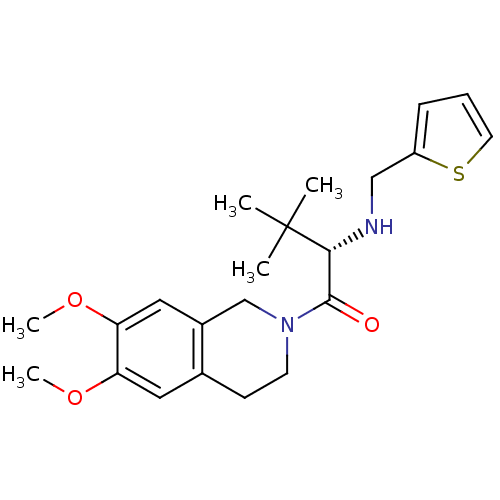 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136711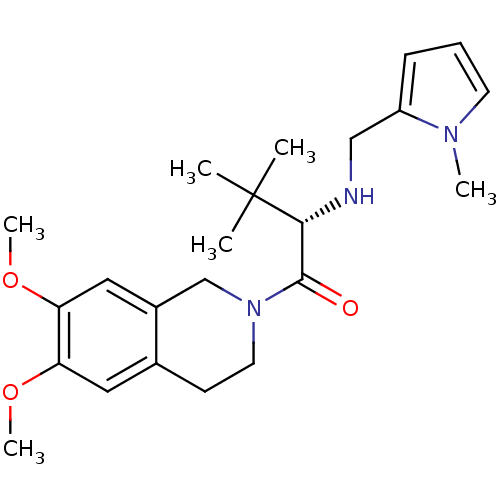 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136714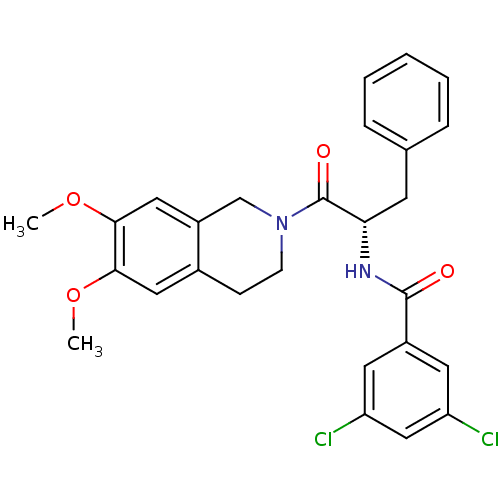 ((S)-3,5-dichloro-N-(1-(6,7-dimethoxy-3,4-dihydrois...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136718 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136720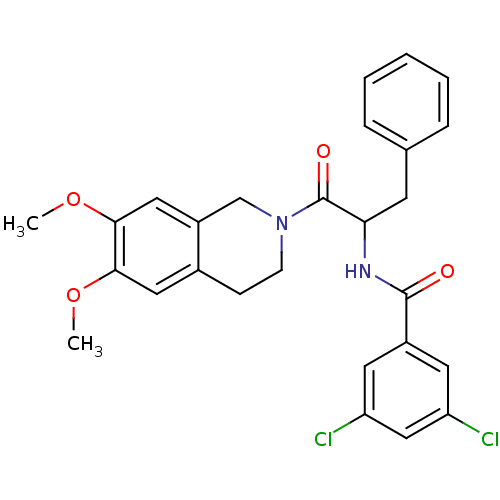 (CHEMBL343551 | N-[1-Benzyl-2-(6,7-dimethoxy-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136694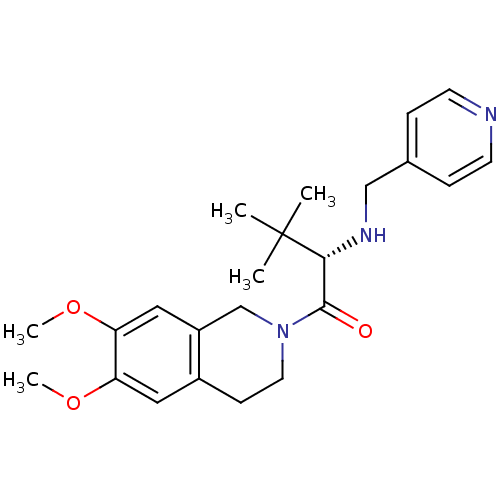 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50401285 (CHEMBL2204532) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50401285 (CHEMBL2204532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123721 (CHEMBL158732 | N-[5-(4-Chloro-phenyl)-1H-pyrazol-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136693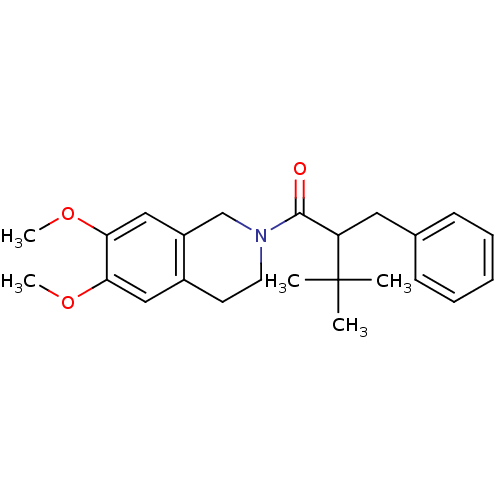 ((+/-)-2-benzyl-1-(6,7-dimethoxy-3,4-dihydroisoquin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136701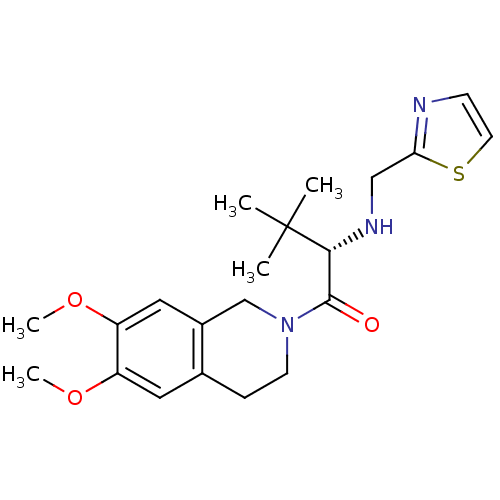 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50401286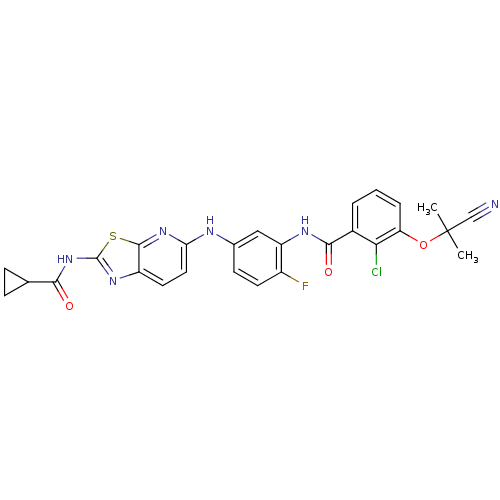 (CHEMBL2204533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 in HUVEC assessed as reduction of VGF-stimulated cell proliferation after 5 days | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM99471 (US8497274, 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal His-6-tagged Aurora kinase B (unknown origin) expressed in baculovirus system after 60 mins | J Med Chem 56: 6478-94 (2013) Article DOI: 10.1021/jm400778d BindingDB Entry DOI: 10.7270/Q2W95BPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50401285 (CHEMBL2204532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of wild type human BRAF (445 to 726) expressed in Sf9 insect cells | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136703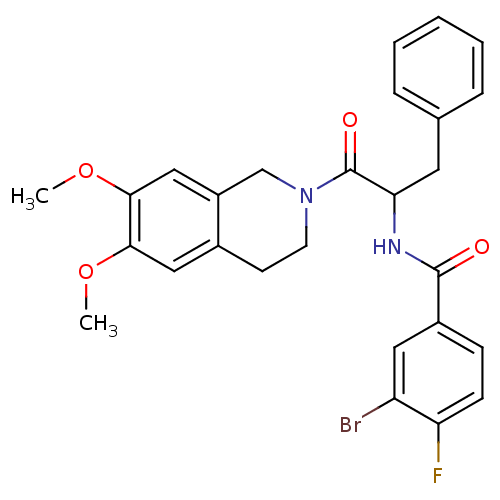 ((+/-)-3-bromo-N-(1-(6,7-dimethoxy-3,4-dihydroisoqu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50401285 (CHEMBL2204532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of FGFR3 | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50401285 (CHEMBL2204532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta | Bioorg Med Chem 20: 5600-15 (2012) Article DOI: 10.1016/j.bmc.2012.07.032 BindingDB Entry DOI: 10.7270/Q2765GH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306307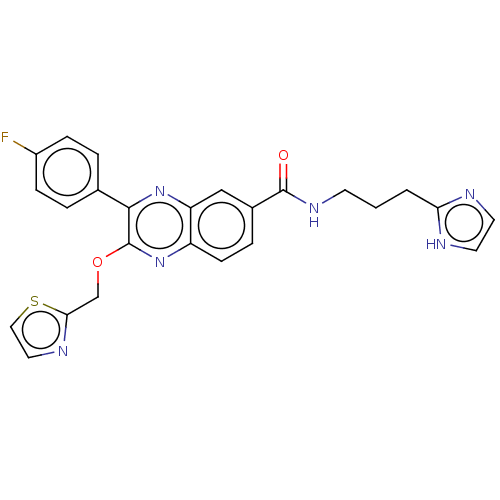 (US10144742, Compound I-469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306309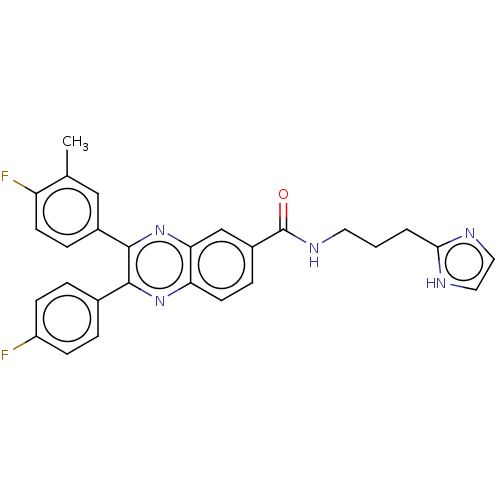 (US10144742, Compound I-471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306310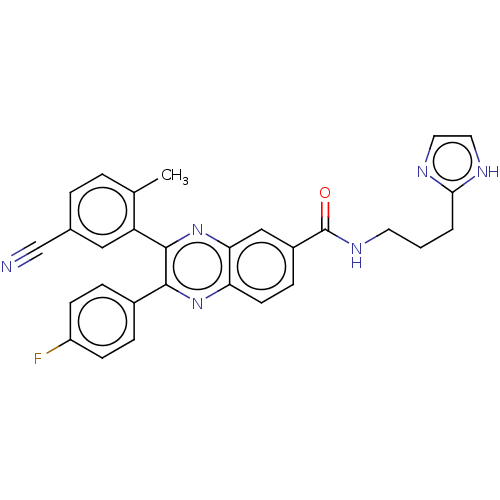 (US10144742, Compound I-472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306315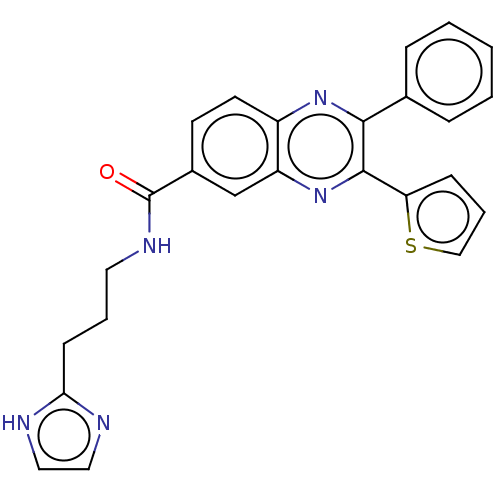 (US10144742, Compound I-477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306316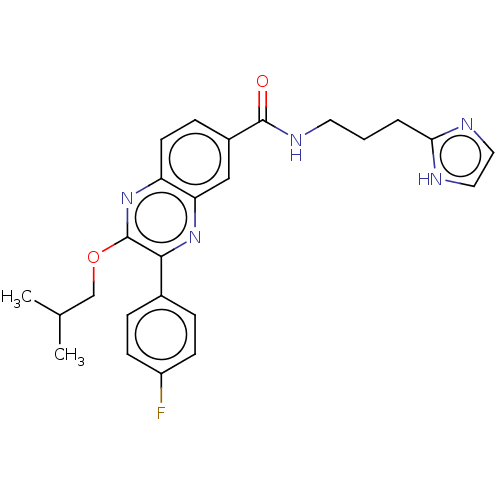 (US10144742, Compound I-478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306318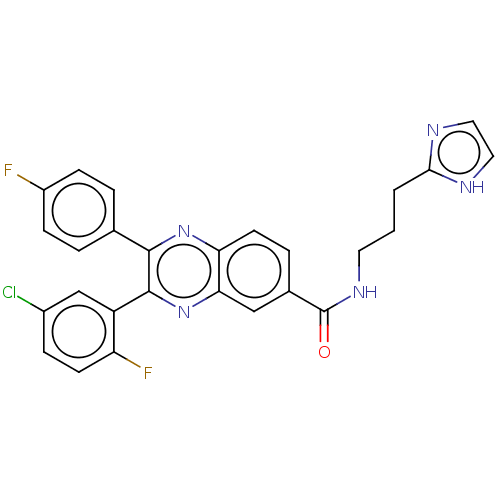 (US10144742, Compound I-480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306319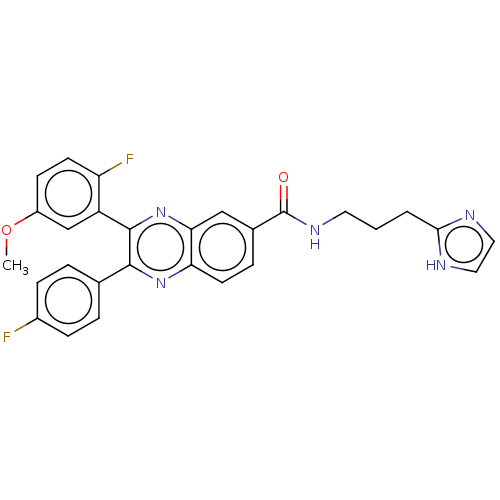 (US10144742, Compound I-481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306322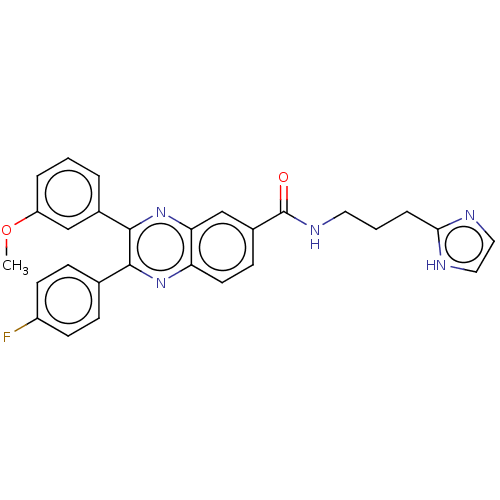 (US10144742, Compound I-484) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM306323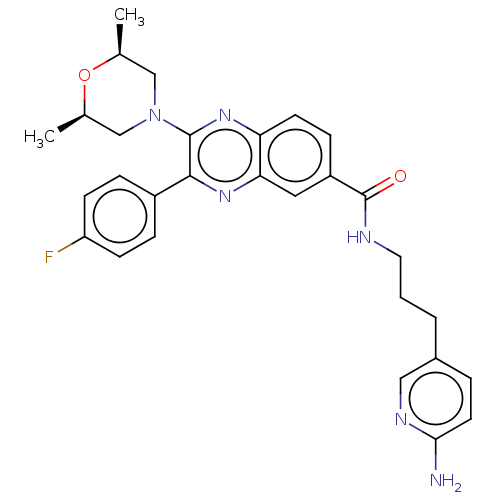 (US10144742, Compound I-485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To measure the inhibition of NAMPT activity hNAMPT protein stock and anti 6His-Tb (Cisbio; Cat. No. 61HISTLB) is diluted to 3× final concentration wi... | US Patent US10144742 (2018) BindingDB Entry DOI: 10.7270/Q2NV9M91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 670 total ) | Next | Last >> |