

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50295955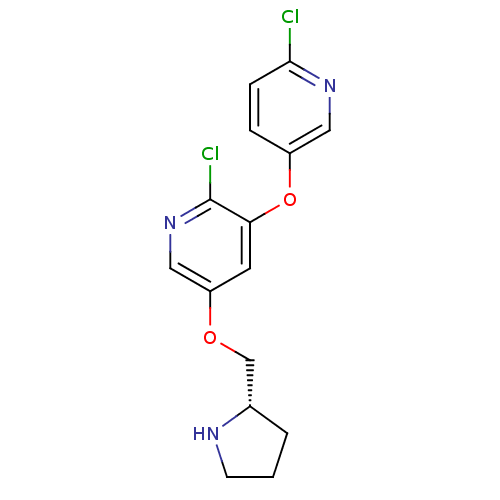 (5-(((S)-Pyrrolidin-2-yl)methoxy)-3-((6-chloropyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50295954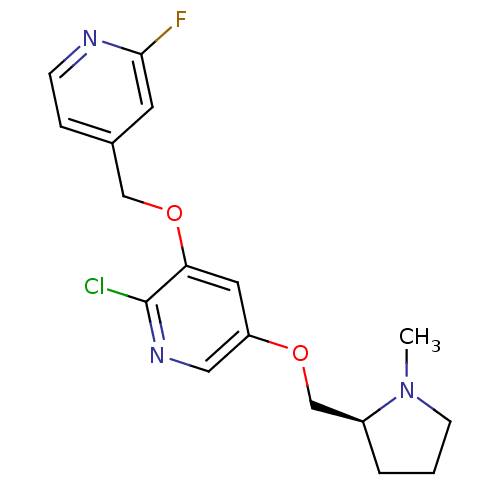 (2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-((1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295955 (5-(((S)-Pyrrolidin-2-yl)methoxy)-3-((6-chloropyrid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295959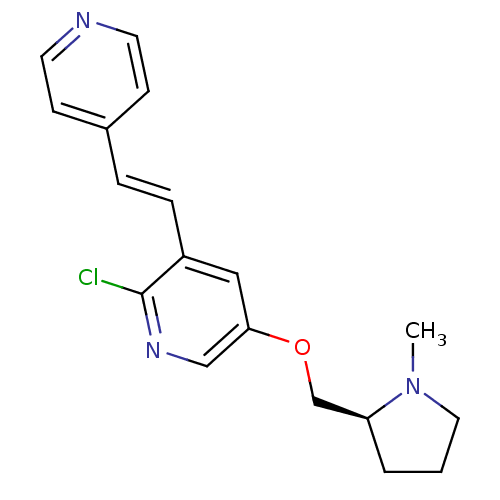 ((S)-2-chloro-5-((1-methylpyrrolidin-2-yl)methoxy)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [125I]5-A-85380 from alpha4beta2 nicotinic receptor in rat brain | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50295956 (2-Chloro-3-(2-chloro-5-(pyridinyl)methoxy)-5-((1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50295953 (2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-(2-(S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50295955 (5-(((S)-Pyrrolidin-2-yl)methoxy)-3-((6-chloropyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50295958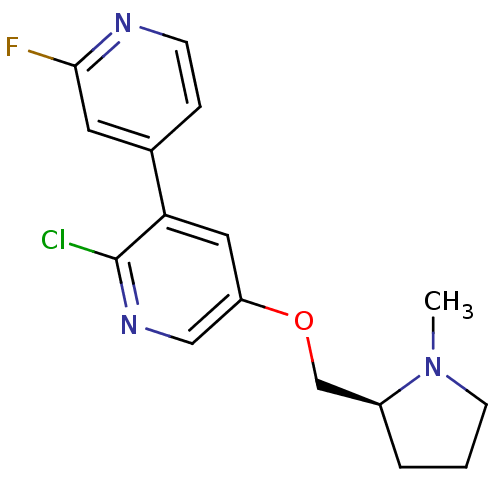 ((S)-2-chloro-2'-fluoro-5-((1-methylpyrrolidin-2-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295956 (2-Chloro-3-(2-chloro-5-(pyridinyl)methoxy)-5-((1-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295954 (2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-((1-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295958 ((S)-2-chloro-2'-fluoro-5-((1-methylpyrrolidin-2-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50039257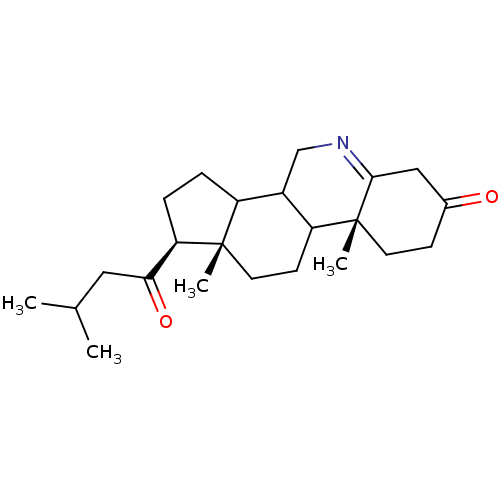 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta4 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295953 (2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-(2-(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta4 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50043604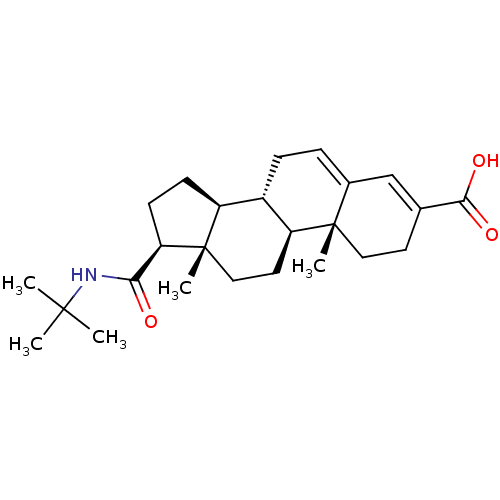 ((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50043604 ((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 | Bioorg Med Chem Lett 4: 2327-2330 (1994) Article DOI: 10.1016/0960-894X(94)85034-8 BindingDB Entry DOI: 10.7270/Q25Q4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50295953 (2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-(2-(S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50295956 (2-Chloro-3-(2-chloro-5-(pyridinyl)methoxy)-5-((1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50295954 (2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-((1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50403606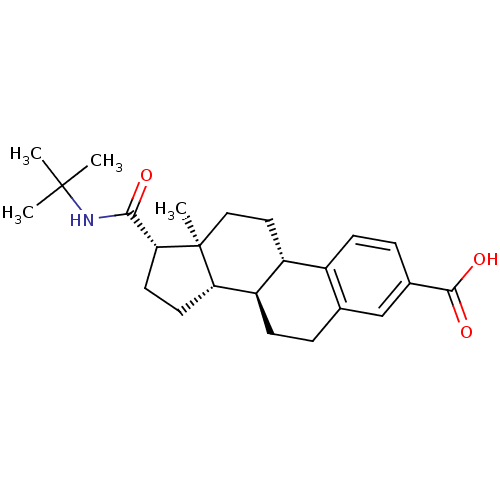 (CHEMBL1627951) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295955 (5-(((S)-Pyrrolidin-2-yl)methoxy)-3-((6-chloropyrid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta4 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TNNI3K (Homo sapiens (Human)) | BDBM50578225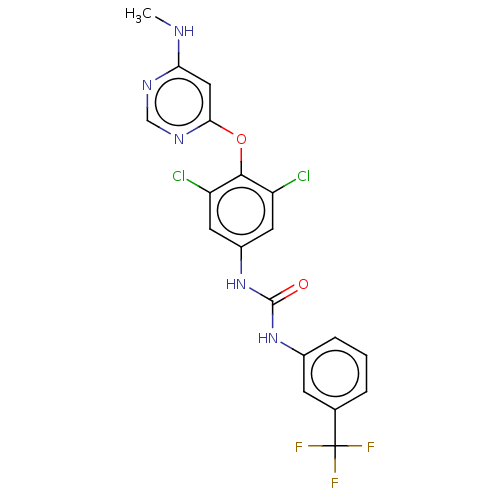 (CHEMBL4869303) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to full length human His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00700 BindingDB Entry DOI: 10.7270/Q23X8BG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50295958 ((S)-2-chloro-2'-fluoro-5-((1-methylpyrrolidin-2-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50403324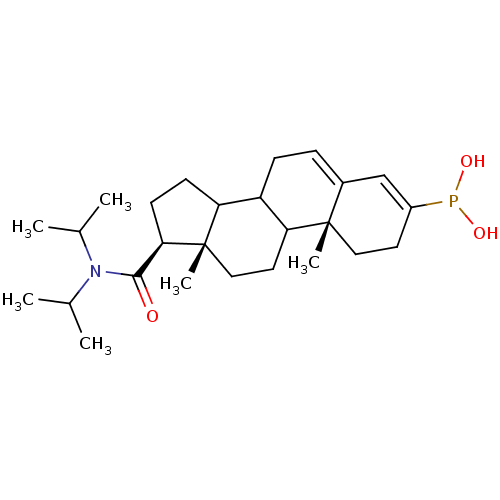 (CHEMBL78060) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 | Bioorg Med Chem Lett 4: 2327-2330 (1994) Article DOI: 10.1016/0960-894X(94)85034-8 BindingDB Entry DOI: 10.7270/Q25Q4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50295957 (2-Chloro-3-(2-chloro-5-(pyridinyl)methoxy)-5-((1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50039285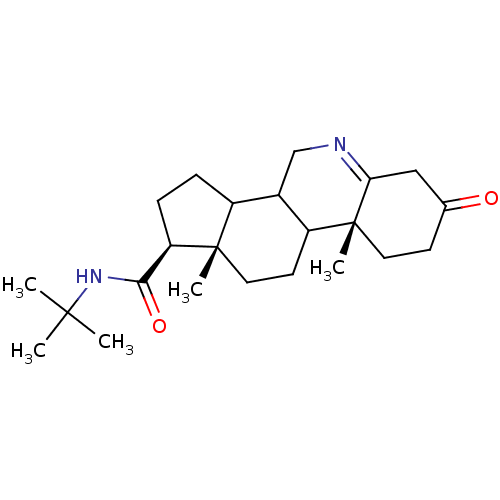 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50100130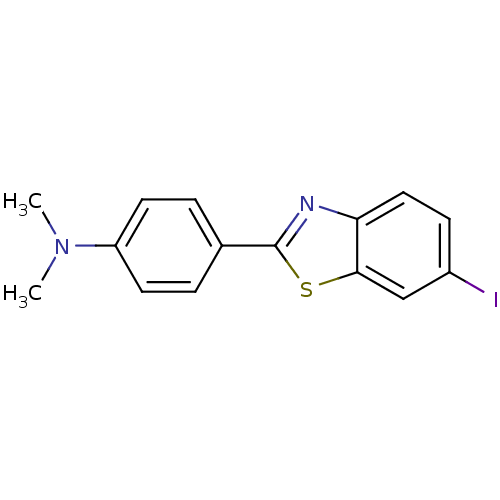 (4-(6-Iodobenzo[d]thiazol-2-yl)-N,N-dimethylaniline...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition constant against [125I]-7 (TZDM) binding to Amyloid beta 1-40 aggregates | J Med Chem 46: 237-43 (2003) Article DOI: 10.1021/jm020351j BindingDB Entry DOI: 10.7270/Q2WH2PBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295953 (2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-(2-(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta4 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295957 (2-Chloro-3-(2-chloro-5-(pyridinyl)methoxy)-5-((1-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50403610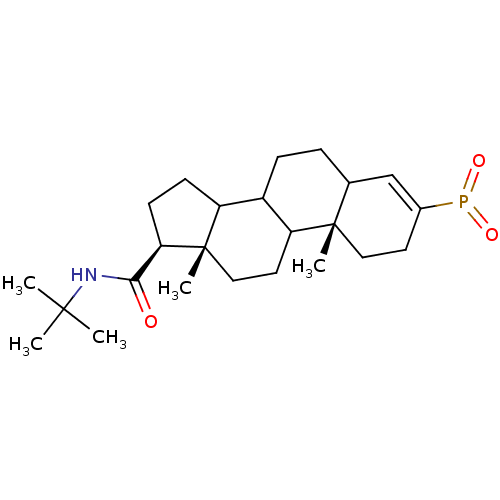 (CHEMBL143220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50066788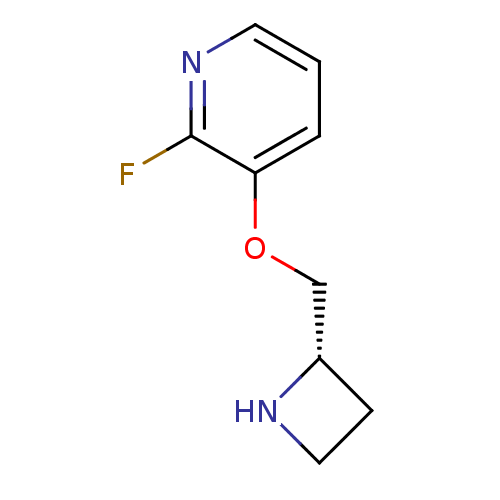 ((S)-3-(azetidin-2-ylmethoxy)-2-fluoropyridine | 3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50066788 ((S)-3-(azetidin-2-ylmethoxy)-2-fluoropyridine | 3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2 (Rattus norvegicus (Rat)) | BDBM50295957 (2-Chloro-3-(2-chloro-5-(pyridinyl)methoxy)-5-((1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50295954 (2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-((1-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta4 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 4367-77 (2009) Article DOI: 10.1016/j.bmc.2009.05.021 BindingDB Entry DOI: 10.7270/Q2DN453T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129794 (CHEMBL328660 | [4-(6-Bromo-benzothiazol-2-yl)-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. | J Med Chem 46: 2740-54 (2003) Article DOI: 10.1021/jm030026b BindingDB Entry DOI: 10.7270/Q23R0S87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129784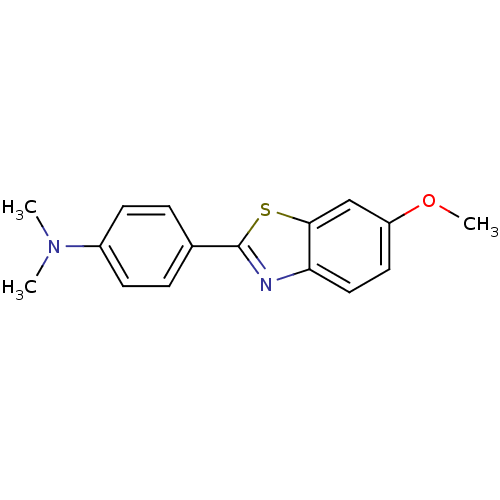 (4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylanil...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. | J Med Chem 46: 2740-54 (2003) Article DOI: 10.1021/jm030026b BindingDB Entry DOI: 10.7270/Q23R0S87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 | Bioorg Med Chem Lett 4: 2327-2330 (1994) Article DOI: 10.1016/0960-894X(94)85034-8 BindingDB Entry DOI: 10.7270/Q25Q4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50213061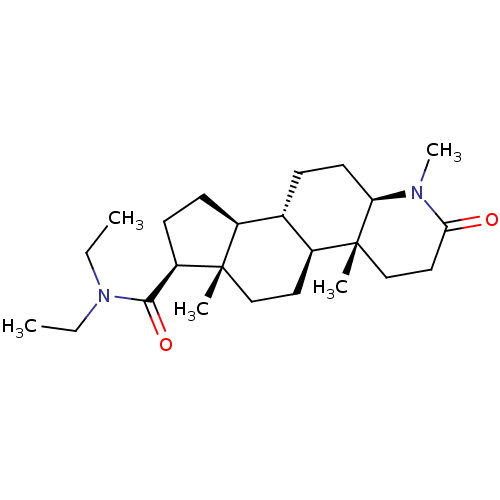 (CHEMBL2298601) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1565 total ) | Next | Last >> |