 Found 49 hits with Last Name = 'horn' and Initial = 'jr'
Found 49 hits with Last Name = 'horn' and Initial = 'jr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 receptor from guinea pig brain membrane |
J Med Chem 54: 5858-67 (2011)
Article DOI: 10.1021/jm200591t
BindingDB Entry DOI: 10.7270/Q2KK9C5Z |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50035131
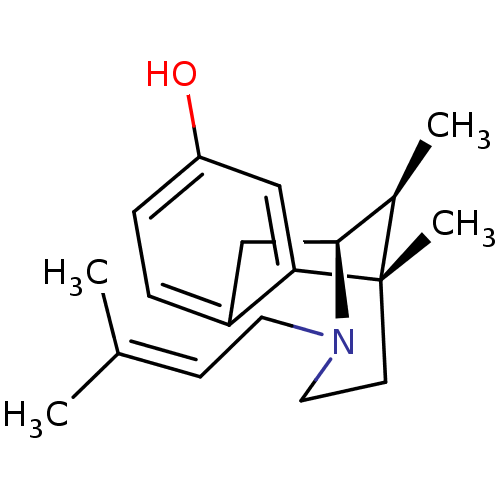
((+)-(6R,11S)-6,11-dimethyl-3-(3-methyl-but-2-enyl)...)Show SMILES [#6]-[#6@@H]1-[#6@@H]-2-[#6]-c3ccc(-[#8])cc3[C@@]1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](/[#6])-[#6] |r,TLB:16:15:10.4.3:1| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 receptor from guinea pig brain membrane |
J Med Chem 54: 5858-67 (2011)
Article DOI: 10.1021/jm200591t
BindingDB Entry DOI: 10.7270/Q2KK9C5Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50351992
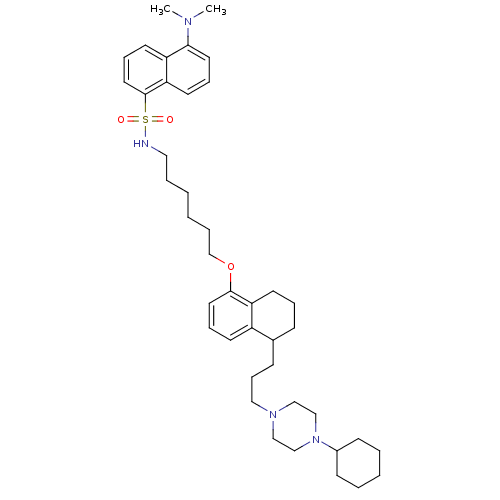
(CHEMBL1822599)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCCCCOc1cccc2C(CCCN3CCN(CC3)C3CCCCC3)CCCc12 Show InChI InChI=1S/C41H60N4O3S/c1-43(2)39-23-11-22-38-36(39)20-13-25-41(38)49(46,47)42-26-8-3-4-9-32-48-40-24-12-19-35-33(15-10-21-37(35)40)16-14-27-44-28-30-45(31-29-44)34-17-6-5-7-18-34/h11-13,19-20,22-25,33-34,42H,3-10,14-18,21,26-32H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 receptor from guinea pig brain membrane |
J Med Chem 54: 5858-67 (2011)
Article DOI: 10.1021/jm200591t
BindingDB Entry DOI: 10.7270/Q2KK9C5Z |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50351993
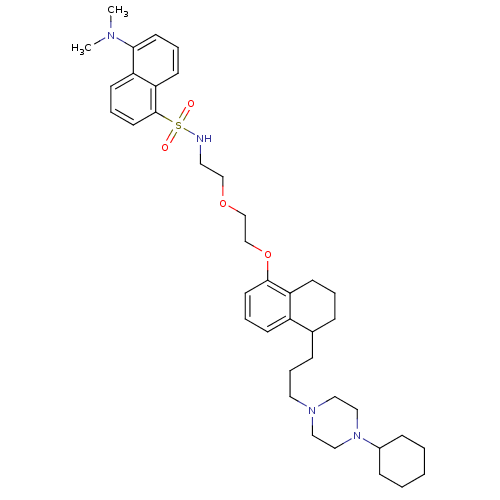
(CHEMBL1822600)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCOCCOc1cccc2C(CCCN3CCN(CC3)C3CCCCC3)CCCc12 Show InChI InChI=1S/C39H56N4O4S/c1-41(2)37-19-7-18-36-34(37)16-9-21-39(36)48(44,45)40-22-28-46-29-30-47-38-20-8-15-33-31(11-6-17-35(33)38)12-10-23-42-24-26-43(27-25-42)32-13-4-3-5-14-32/h7-9,15-16,18-21,31-32,40H,3-6,10-14,17,22-30H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 receptor from guinea pig brain membrane |
J Med Chem 54: 5858-67 (2011)
Article DOI: 10.1021/jm200591t
BindingDB Entry DOI: 10.7270/Q2KK9C5Z |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50351990
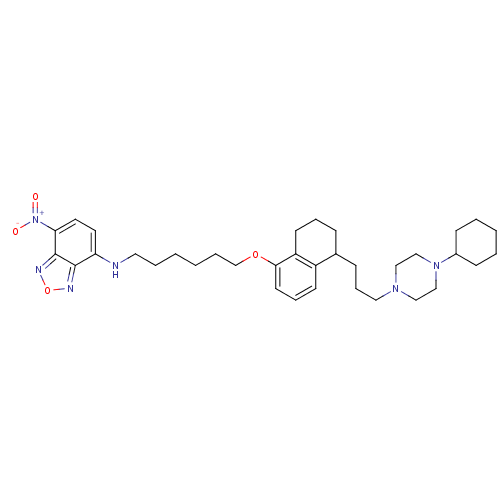
(CHEMBL1822597)Show SMILES [O-][N+](=O)c1ccc(NCCCCCCOc2cccc3C(CCCN4CCN(CC4)C4CCCCC4)CCCc23)c2nonc12 Show InChI InChI=1S/C35H50N6O4/c42-41(43)32-19-18-31(34-35(32)38-45-37-34)36-20-6-1-2-7-26-44-33-17-9-15-29-27(11-8-16-30(29)33)12-10-21-39-22-24-40(25-23-39)28-13-4-3-5-14-28/h9,15,17-19,27-28,36H,1-8,10-14,16,20-26H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 receptor from guinea pig brain membrane |
J Med Chem 54: 5858-67 (2011)
Article DOI: 10.1021/jm200591t
BindingDB Entry DOI: 10.7270/Q2KK9C5Z |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50351991
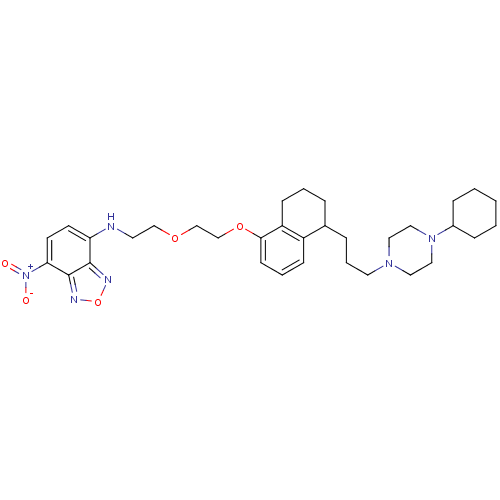
(CHEMBL1822598)Show SMILES [O-][N+](=O)c1ccc(NCCOCCOc2cccc3C(CCCN4CCN(CC4)C4CCCCC4)CCCc23)c2nonc12 Show InChI InChI=1S/C33H46N6O5/c40-39(41)30-15-14-29(32-33(30)36-44-35-32)34-16-22-42-23-24-43-31-13-5-11-27-25(7-4-12-28(27)31)8-6-17-37-18-20-38(21-19-37)26-9-2-1-3-10-26/h5,11,13-15,25-26,34H,1-4,6-10,12,16-24H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 receptor from guinea pig brain membrane |
J Med Chem 54: 5858-67 (2011)
Article DOI: 10.1021/jm200591t
BindingDB Entry DOI: 10.7270/Q2KK9C5Z |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50351988

(CHEMBL1822595)Show SMILES COc1cccc2C(CCCN3CCN(CC3)C3CCN(CCCCCCNc4ccc([N+]([O-])=O)c5[n-][o+]nc45)CC3)CCCc12 Show InChI InChI=1S/C35H51N7O4/c1-45-33-13-7-11-29-27(9-6-12-30(29)33)10-8-20-40-23-25-41(26-24-40)28-16-21-39(22-17-28)19-5-3-2-4-18-36-31-14-15-32(42(43)44)35-34(31)37-46-38-35/h7,11,13-15,27-28,36H,2-6,8-10,12,16-26H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 receptor from guinea pig brain membrane |
J Med Chem 54: 5858-67 (2011)
Article DOI: 10.1021/jm200591t
BindingDB Entry DOI: 10.7270/Q2KK9C5Z |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50351989

(CHEMBL1822596)Show SMILES COc1cccc2C(CCCN3CCN(CC3)C3CCN(CCCCCCNS(=O)(=O)c4cccc5c(cccc45)N(C)C)CC3)CCCc12 Show InChI InChI=1S/C41H61N5O3S/c1-43(2)39-19-9-18-38-36(39)16-11-21-41(38)50(47,48)42-24-6-4-5-7-25-44-27-22-34(23-28-44)46-31-29-45(30-32-46)26-12-14-33-13-8-17-37-35(33)15-10-20-40(37)49-3/h9-11,15-16,18-21,33-34,42H,4-8,12-14,17,22-32H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 receptor from guinea pig brain membrane |
J Med Chem 54: 5858-67 (2011)
Article DOI: 10.1021/jm200591t
BindingDB Entry DOI: 10.7270/Q2KK9C5Z |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50437324
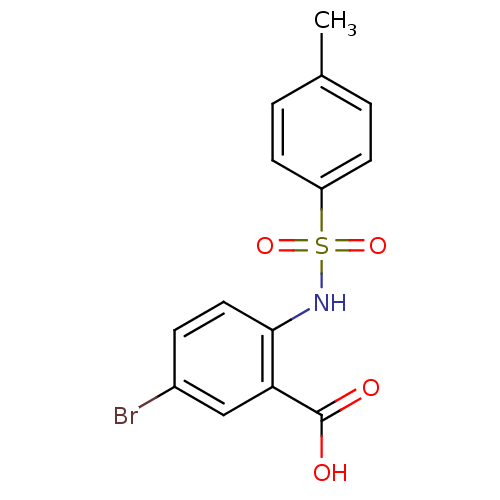
(CHEMBL2407597)Show InChI InChI=1S/C14H12BrNO4S/c1-9-2-5-11(6-3-9)21(19,20)16-13-7-4-10(15)8-12(13)14(17)18/h2-8,16H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM76305
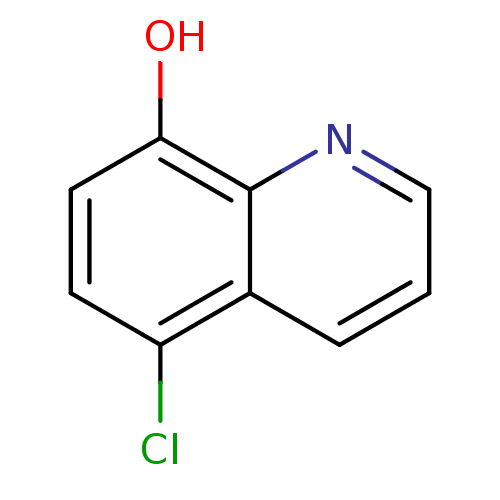
(5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...)Show InChI InChI=1S/C9H6ClNO/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM64987
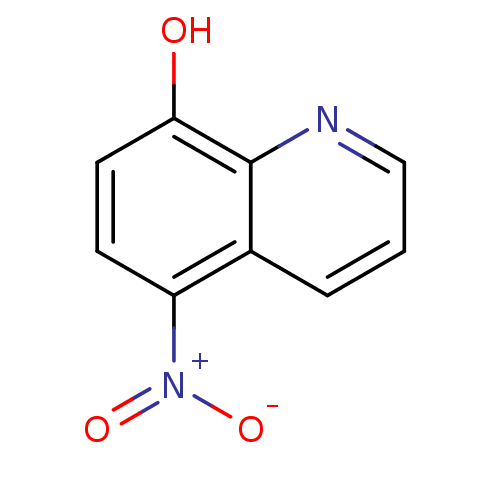
(5-nitro-8-quinolinol | 5-nitroquinolin-8-ol | 8-HY...)Show InChI InChI=1S/C9H6N2O3/c12-8-4-3-7(11(13)14)6-2-1-5-10-9(6)8/h1-5,12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM32203

(8-quinolinol | CHEMBL310555 | US10005735, Table 1....)Show InChI InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM76305

(5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...)Show InChI InChI=1S/C9H6ClNO/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM32203

(8-quinolinol | CHEMBL310555 | US10005735, Table 1....)Show InChI InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50065785

(2-Methyl-quinolin-8-ol | CHEMBL316892)Show InChI InChI=1S/C10H9NO/c1-7-5-6-8-3-2-4-9(12)10(8)11-7/h2-6,12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50437326
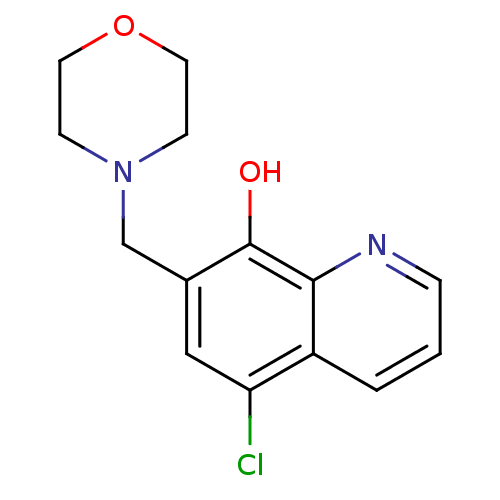
(CHEMBL2407599)Show InChI InChI=1S/C14H15ClN2O2/c15-12-8-10(9-17-4-6-19-7-5-17)14(18)13-11(12)2-1-3-16-13/h1-3,8,18H,4-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50437325
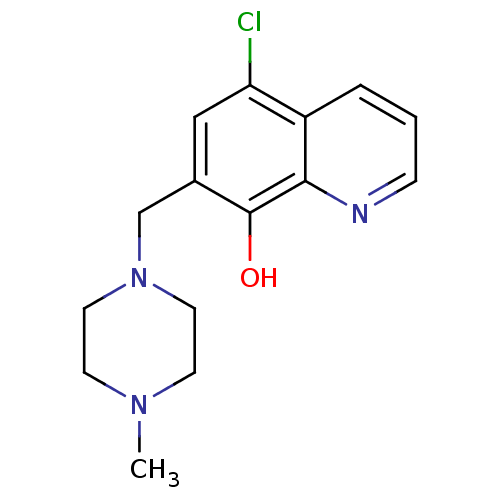
(CHEMBL1368981)Show InChI InChI=1S/C15H18ClN3O/c1-18-5-7-19(8-6-18)10-11-9-13(16)12-3-2-4-17-14(12)15(11)20/h2-4,9,20H,5-8,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50437327
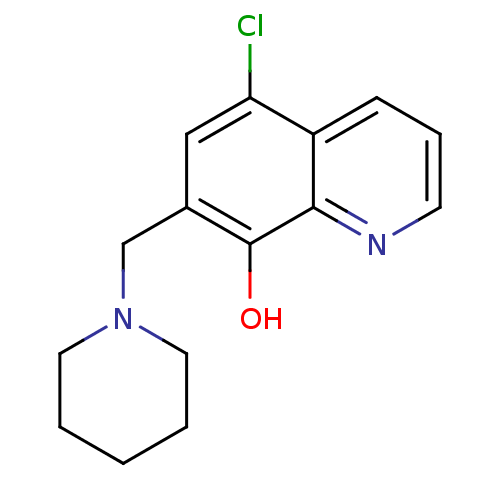
(CHEMBL2023325)Show InChI InChI=1S/C15H17ClN2O/c16-13-9-11(10-18-7-2-1-3-8-18)15(19)14-12(13)5-4-6-17-14/h4-6,9,19H,1-3,7-8,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM32203

(8-quinolinol | CHEMBL310555 | US10005735, Table 1....)Show InChI InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM76305

(5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...)Show InChI InChI=1S/C9H6ClNO/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM64987

(5-nitro-8-quinolinol | 5-nitroquinolin-8-ol | 8-HY...)Show InChI InChI=1S/C9H6N2O3/c12-8-4-3-7(11(13)14)6-2-1-5-10-9(6)8/h1-5,12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50211290
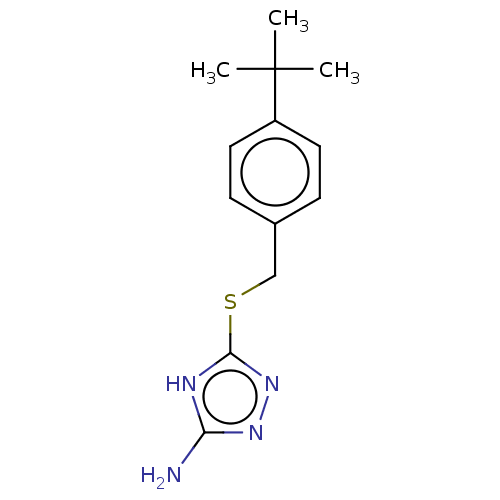
(CHEMBL2407096)Show InChI InChI=1S/C13H18N4S/c1-13(2,3)10-6-4-9(5-7-10)8-18-12-15-11(14)16-17-12/h4-7H,8H2,1-3H3,(H3,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50211290

(CHEMBL2407096)Show InChI InChI=1S/C13H18N4S/c1-13(2,3)10-6-4-9(5-7-10)8-18-12-15-11(14)16-17-12/h4-7H,8H2,1-3H3,(H3,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50065785

(2-Methyl-quinolin-8-ol | CHEMBL316892)Show InChI InChI=1S/C10H9NO/c1-7-5-6-8-3-2-4-9(12)10(8)11-7/h2-6,12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM76305

(5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...)Show InChI InChI=1S/C9H6ClNO/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... |
Bioorg Med Chem 25: 813-824 (2017)
Article DOI: 10.1016/j.bmc.2016.11.013
BindingDB Entry DOI: 10.7270/Q2125VV2 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50437328
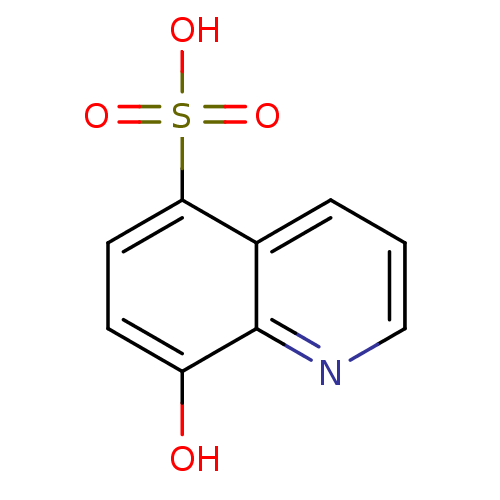
(CHEMBL2407598)Show InChI InChI=1S/C9H7NO4S/c11-7-3-4-8(15(12,13)14)6-2-1-5-10-9(6)7/h1-5,11H,(H,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50065785

(2-Methyl-quinolin-8-ol | CHEMBL316892)Show InChI InChI=1S/C10H9NO/c1-7-5-6-8-3-2-4-9(12)10(8)11-7/h2-6,12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM32203

(8-quinolinol | CHEMBL310555 | US10005735, Table 1....)Show InChI InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50437328

(CHEMBL2407598)Show InChI InChI=1S/C9H7NO4S/c11-7-3-4-8(15(12,13)14)6-2-1-5-10-9(6)7/h1-5,11H,(H,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50065785

(2-Methyl-quinolin-8-ol | CHEMBL316892)Show InChI InChI=1S/C10H9NO/c1-7-5-6-8-3-2-4-9(12)10(8)11-7/h2-6,12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 2 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Salmonella typhimurium (strain LT2 / SGSC1412 / AT...) | BDBM50502707

(CHEMBL4458954)Show InChI InChI=1S/C16H12N4O3/c21-14-13(15(22)23)9-18-16(20-14)19-12-3-1-10(2-4-12)11-5-7-17-8-6-11/h1-9H,(H,22,23)(H2,18,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of Salmonella typhimurium IspF by malachite green dye based assay |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126660
BindingDB Entry DOI: 10.7270/Q20C501R |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Salmonella typhimurium (strain LT2 / SGSC1412 / AT...) | BDBM50502708

(CHEMBL3222163)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C/c3cc(Br)c(O)c(Br)c3)c(=O)n2C1c1cccs1 |c:5,t:8| Show InChI InChI=1S/C21H16Br2N2O4S2/c1-3-29-20(28)16-10(2)24-21-25(17(16)14-5-4-6-30-14)19(27)15(31-21)9-11-7-12(22)18(26)13(23)8-11/h4-9,17,26H,3H2,1-2H3/b15-9- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of Salmonella typhimurium IspF by malachite green dye based assay |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126660
BindingDB Entry DOI: 10.7270/Q20C501R |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50437326

(CHEMBL2407599)Show InChI InChI=1S/C14H15ClN2O2/c15-12-8-10(9-17-4-6-19-7-5-17)14(18)13-11(12)2-1-3-16-13/h1-3,8,18H,4-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50437327

(CHEMBL2023325)Show InChI InChI=1S/C15H17ClN2O/c16-13-9-11(10-18-7-2-1-3-8-18)15(19)14-12(13)5-4-6-17-14/h4-6,9,19H,1-3,7-8,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50437325

(CHEMBL1368981)Show InChI InChI=1S/C15H18ClN3O/c1-18-5-7-19(8-6-18)10-11-9-13(16)12-3-2-4-17-14(12)15(11)20/h2-4,9,20H,5-8,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
ACS Med Chem Lett 4: (2013)
Article DOI: 10.1021/ml400034m
BindingDB Entry DOI: 10.7270/Q2XW4M72 |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM31916
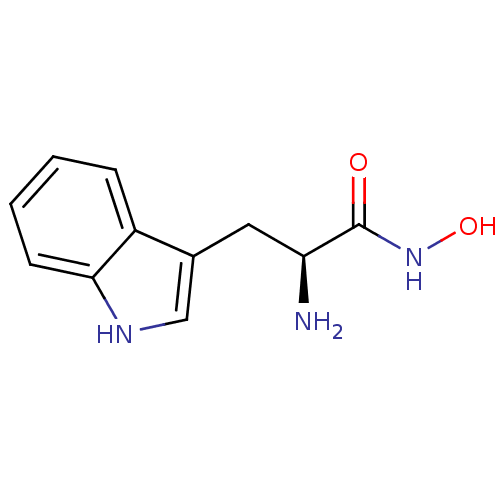
(Tryptophanhydroxamate, 13)Show InChI InChI=1S/C11H13N3O2/c12-9(11(15)14-16)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13,16H,5,12H2,(H,14,15)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 8.48E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Burkholderia pseudomallei IspF at pH 6 measured by isothermal titration calorimetry method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128273
BindingDB Entry DOI: 10.7270/Q2VT1WTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443772

(CHEMBL1230597)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3ccncc3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C15H17N5O5/c16-10-3-6-20(15(24)19-10)14-12(22)11(21)9(25-14)7-18-13(23)8-1-4-17-5-2-8/h1-6,9,11-12,14,21-22H,7H2,(H,18,23)(H2,16,19,24)/t9-,11-,12-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM31916

(Tryptophanhydroxamate, 13)Show InChI InChI=1S/C11H13N3O2/c12-9(11(15)14-16)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13,16H,5,12H2,(H,14,15)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Burkholderia pseudomallei IspF at pH 8 measured by isothermal titration calorimetry method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128273
BindingDB Entry DOI: 10.7270/Q2VT1WTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443771
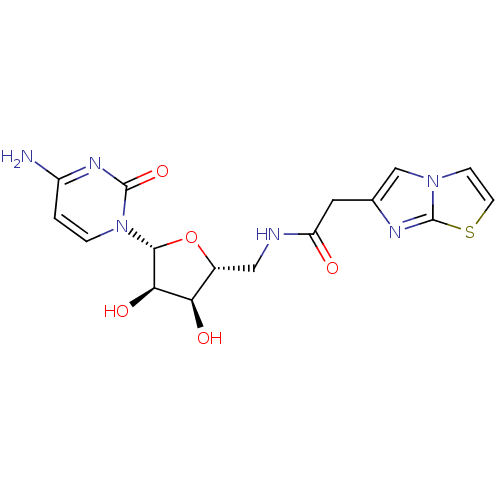
(CHEMBL3094103)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)Cc3cn4ccsc4n3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C16H18N6O5S/c17-10-1-2-22(15(26)20-10)14-13(25)12(24)9(27-14)6-18-11(23)5-8-7-21-3-4-28-16(21)19-8/h1-4,7,9,12-14,24-25H,5-6H2,(H,18,23)(H2,17,20,26)/t9-,12-,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443773
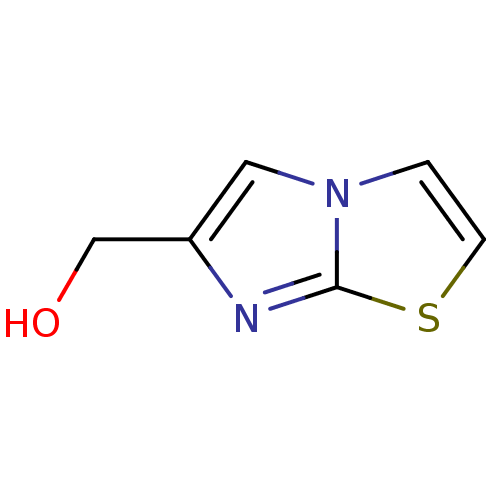
(CHEMBL1230528)Show InChI InChI=1S/C6H6N2OS/c9-4-5-3-8-1-2-10-6(8)7-5/h1-3,9H,4H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Escherichia coli (strain K12)) | BDBM31916

(Tryptophanhydroxamate, 13)Show InChI InChI=1S/C11H13N3O2/c12-9(11(15)14-16)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13,16H,5,12H2,(H,14,15)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Escherichia coli IspF at pH 7.4 measured by isothermal titration calorimetry method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128273
BindingDB Entry DOI: 10.7270/Q2VT1WTJ |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443770

(CHEMBL3094104)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3cn4ccsc4n3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C15H16N6O5S/c16-9-1-2-21(14(25)19-9)13-11(23)10(22)8(26-13)5-17-12(24)7-6-20-3-4-27-15(20)18-7/h1-4,6,8,10-11,13,22-23H,5H2,(H,17,24)(H2,16,19,25)/t8-,10-,11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443774
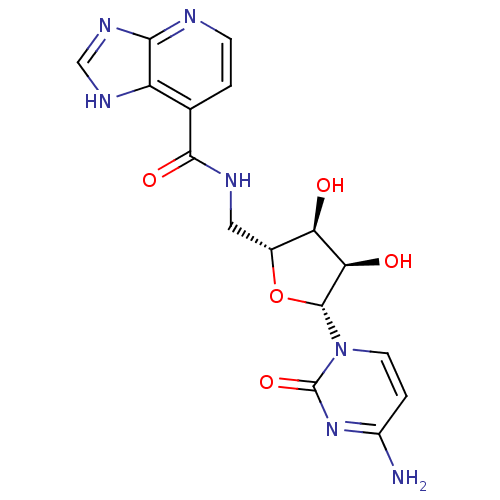
(CHEMBL3094106)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3ccnc4nc[nH]c34)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C16H17N7O5/c17-9-2-4-23(16(27)22-9)15-12(25)11(24)8(28-15)5-19-14(26)7-1-3-18-13-10(7)20-6-21-13/h1-4,6,8,11-12,15,24-25H,5H2,(H,19,26)(H2,17,22,27)(H,18,20,21)/t8-,11-,12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50194153

(5'-CDP | CDP | CHEMBL425252 | Cytidine | Cytidine ...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 Show InChI InChI=1S/C9H15N3O11P2/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(22-8)3-21-25(19,20)23-24(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50043799
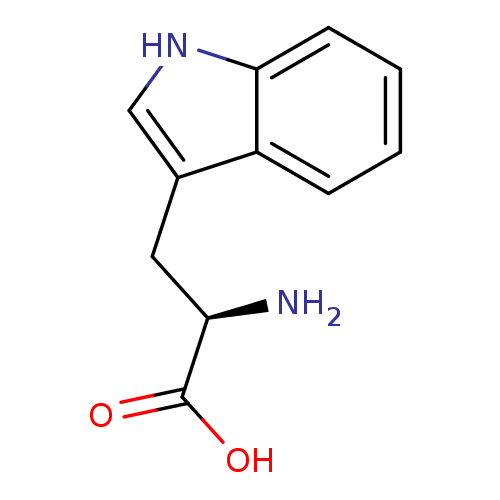
((2R)-2-amino-3-(1H-indol-3-yl)propanoic acid | (R)...)Show InChI InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Burkholderia pseudomallei IspF at pH 7.4 measured by isothermal titration calorimetry method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128273
BindingDB Entry DOI: 10.7270/Q2VT1WTJ |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM21974
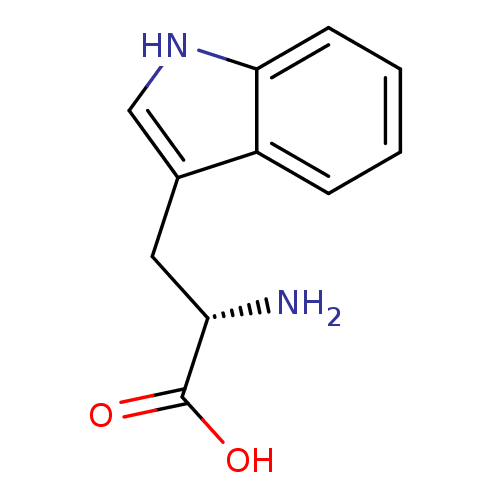
((2S)-2-amino-3-(1H-indol-3-yl)propanoic acid | CHE...)Show InChI InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Burkholderia pseudomallei IspF at pH 7.4 measured by isothermal titration calorimetry method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128273
BindingDB Entry DOI: 10.7270/Q2VT1WTJ |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
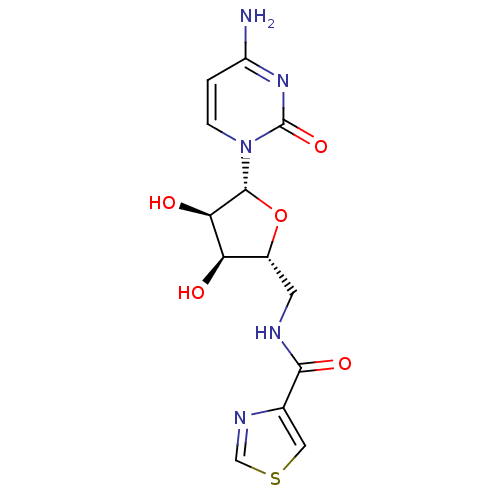
(Burkholderia pseudomallei (strain K96243)) | BDBM50443775
(CHEMBL3094105)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3cscn3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C13H15N5O5S/c14-8-1-2-18(13(22)17-8)12-10(20)9(19)7(23-12)3-15-11(21)6-4-24-5-16-6/h1-2,4-5,7,9-10,12,19-20H,3H2,(H,15,21)(H2,14,17,22)/t7-,9-,10-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM31916

(Tryptophanhydroxamate, 13)Show InChI InChI=1S/C11H13N3O2/c12-9(11(15)14-16)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13,16H,5,12H2,(H,14,15)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Burkholderia pseudomallei IspF at pH 7.4 measured by isothermal titration calorimetry method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128273
BindingDB Entry DOI: 10.7270/Q2VT1WTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50566954
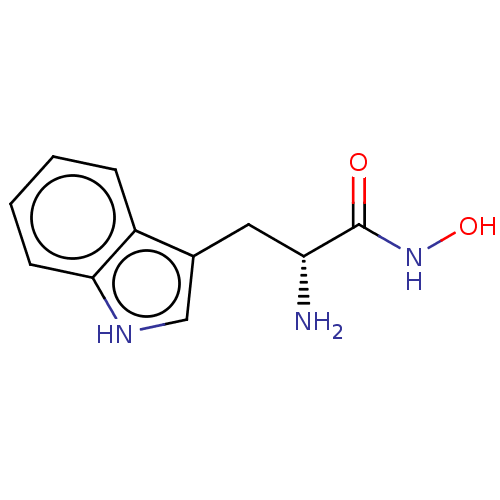
(CHEMBL4874588) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Burkholderia pseudomallei IspF at pH 7.4 measured by isothermal titration calorimetry method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128273
BindingDB Entry DOI: 10.7270/Q2VT1WTJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data