

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM160666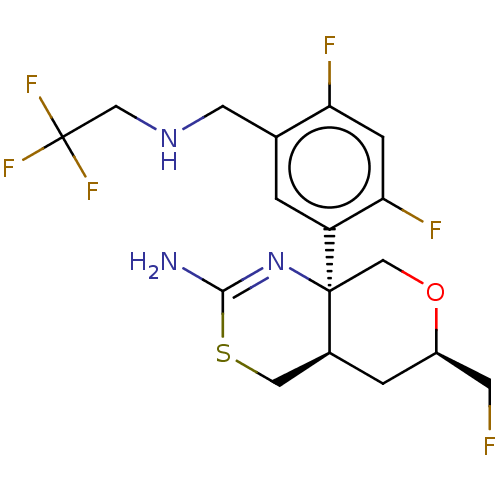 (US9045498, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259964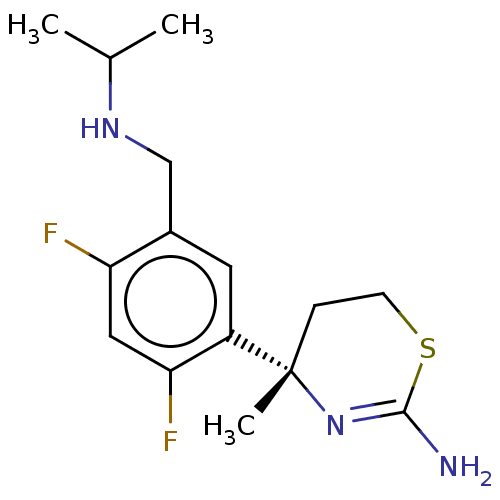 (CHEMBL4083698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259968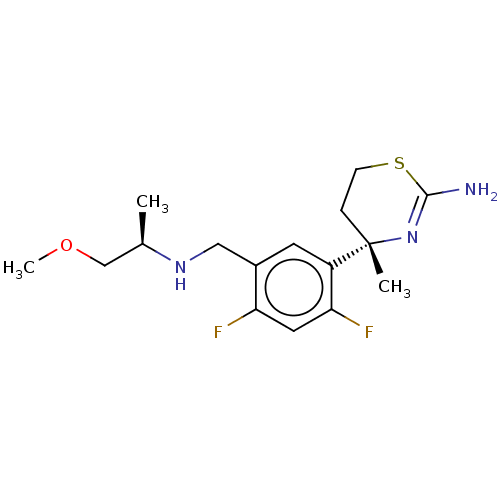 (CHEMBL4084653) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259974 (CHEMBL4102593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259966 (CHEMBL4092406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259963 (CHEMBL4061944) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259959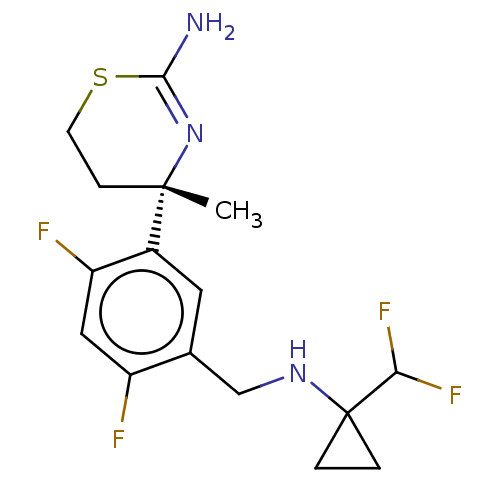 (CHEMBL4062728) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259971 (CHEMBL4064148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259961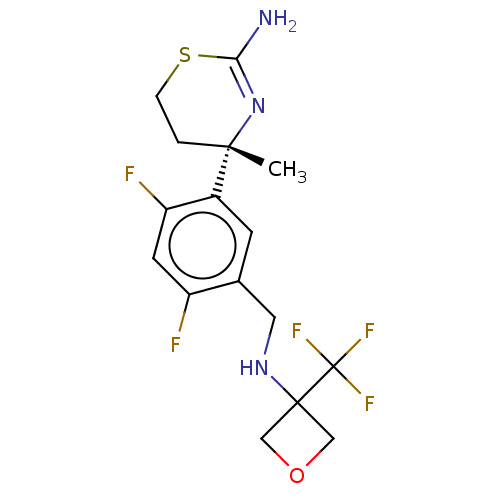 (CHEMBL4080380) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259973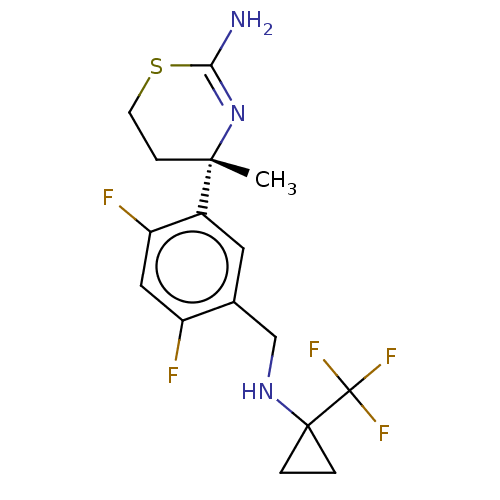 (CHEMBL4098403) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259965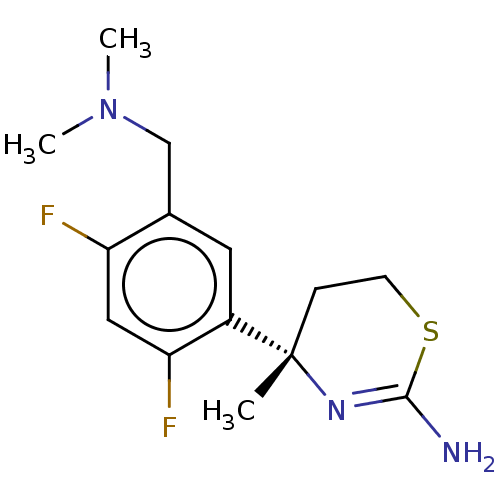 (CHEMBL4100140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259960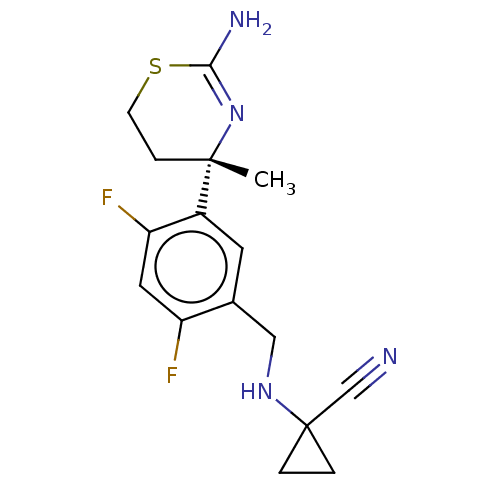 (CHEMBL4077211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259957 (CHEMBL4085715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259954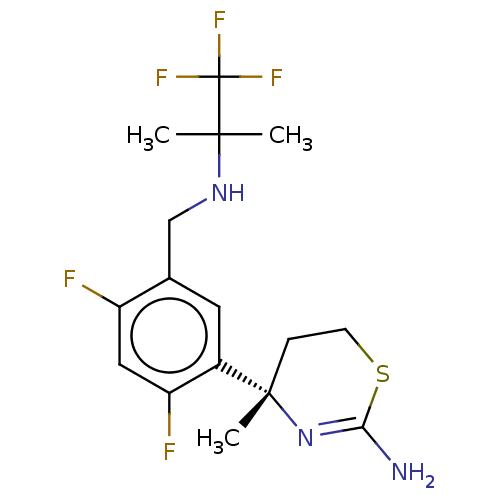 (CHEMBL4094377) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259975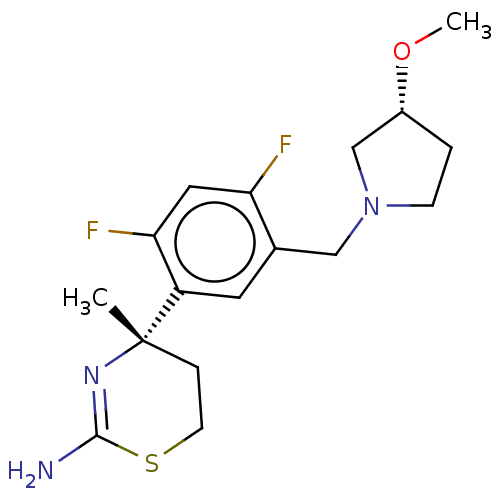 (CHEMBL4073296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259967 (CHEMBL4064708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259950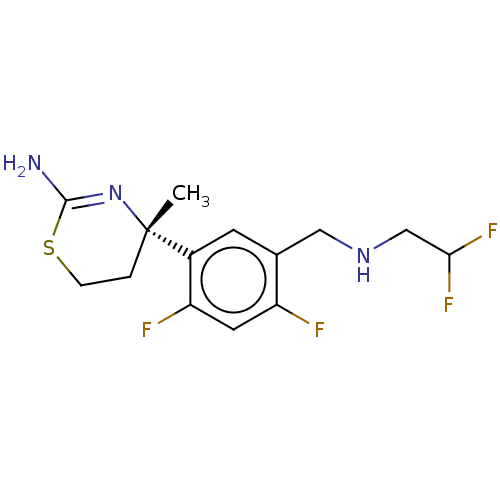 (CHEMBL4066885) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259949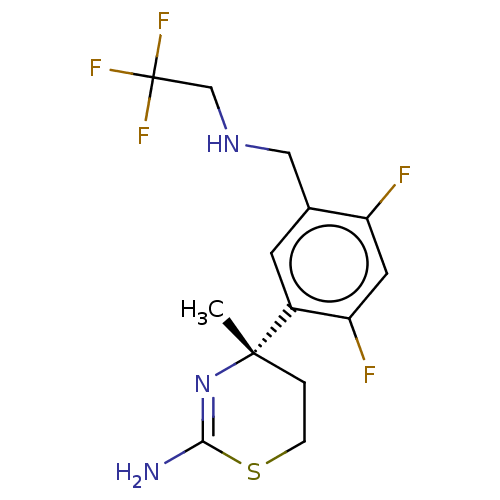 (CHEMBL4086633) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259970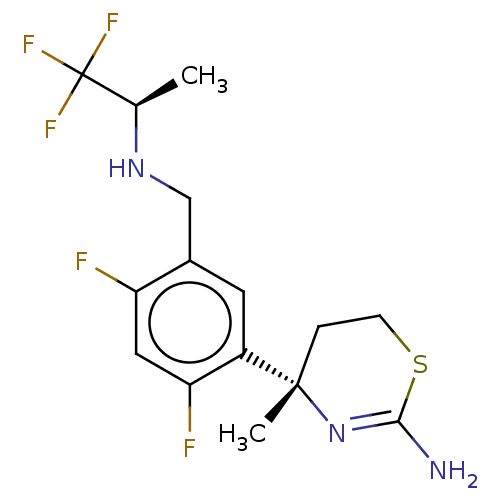 (CHEMBL4061602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259973 (CHEMBL4098403) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM160666 (US9045498, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50259973 (CHEMBL4098403) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM160666 (US9045498, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259959 (CHEMBL4062728) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259955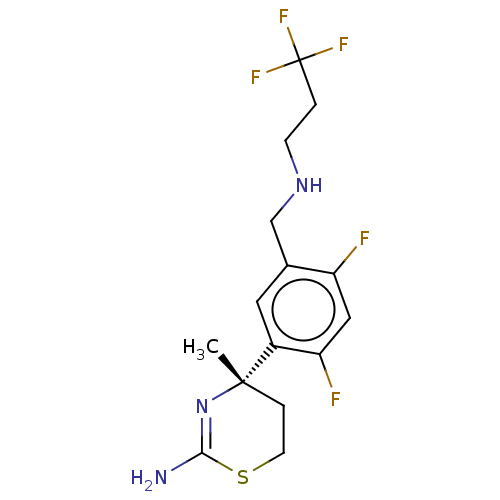 (CHEMBL4084170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50259973 (CHEMBL4098403) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 405 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259954 (CHEMBL4094377) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259961 (CHEMBL4080380) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259960 (CHEMBL4077211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 952 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50259959 (CHEMBL4062728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259969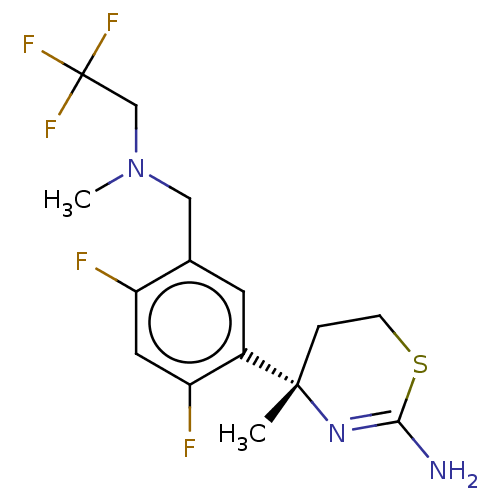 (CHEMBL4104527) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50259954 (CHEMBL4094377) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259949 (CHEMBL4086633) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259976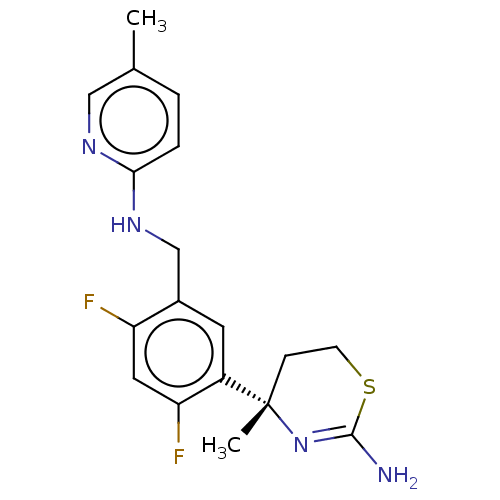 (CHEMBL4091480) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50259961 (CHEMBL4080380) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259970 (CHEMBL4061602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259974 (CHEMBL4102593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells at -80 mV holding potential after 5 mins by patch clamp assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50259973 (CHEMBL4098403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells at -80 mV holding potential after 5 mins by patch clamp assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259968 (CHEMBL4084653) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM160666 (US9045498, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells at -80 mV holding potential after 5 mins by patch clamp assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259950 (CHEMBL4066885) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50259949 (CHEMBL4086633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells at -80 mV holding potential after 5 mins by patch clamp assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50259970 (CHEMBL4061602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50259949 (CHEMBL4086633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259966 (CHEMBL4092406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |