

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-lipoxygenase (Bos taurus) | BDBM50060871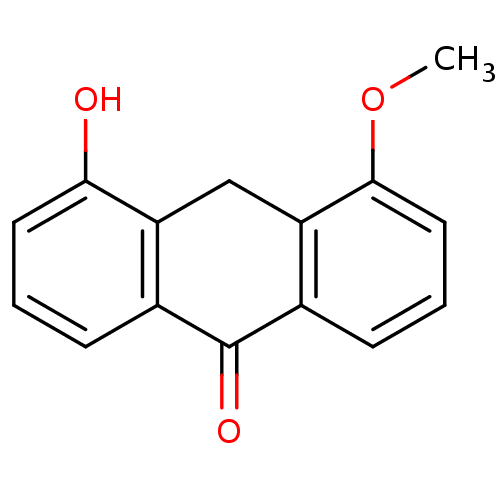 (4-Hydroxy-5-methoxy-10H-anthracen-9-one | CHEMBL12...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052464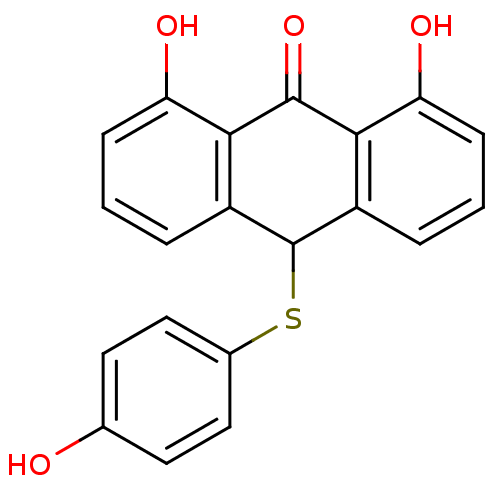 (1,8-Dihydroxy-10-(4-hydroxy-phenylsulfanyl)-10H-an...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060860 (9(10H)-anthracenone | 9,10-dihydro-9-oxoanthracene...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052466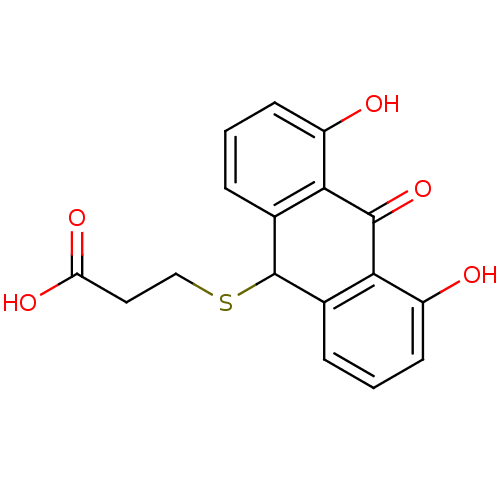 (3-(4,5-Dihydroxy-10-oxo-9,10-dihydro-anthracen-9-y...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052462 (10-(2-Amino-phenylsulfanyl)-1,8-dihydroxy-10H-anth...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052457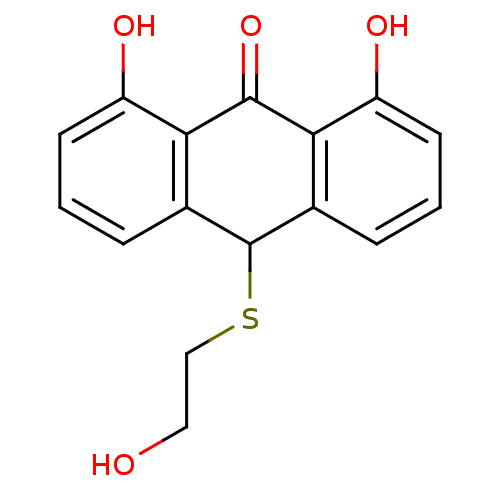 (1,8-Dihydroxy-10-(2-hydroxy-ethylsulfanyl)-10H-ant...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX12 (Mus musculus) | BDBM50052464 (1,8-Dihydroxy-10-(4-hydroxy-phenylsulfanyl)-10H-an...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 12-LO (12-lipoxygenase) as an inhibitor of 12(S)-HETE biosynthesis in mouse epidermal homogenates | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060855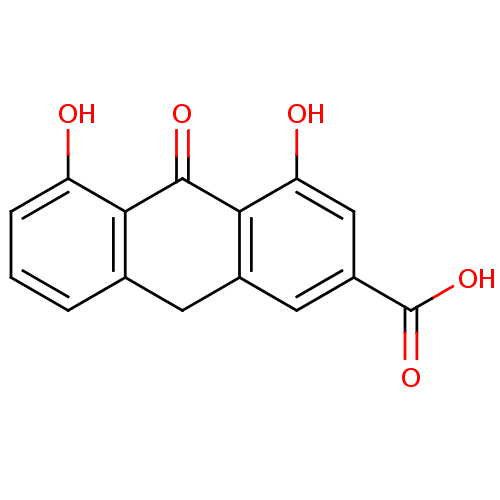 (4,5-Dihydroxy-10-oxo-9,10-dihydro-anthracene-2-car...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX12 (Mus musculus) | BDBM50052450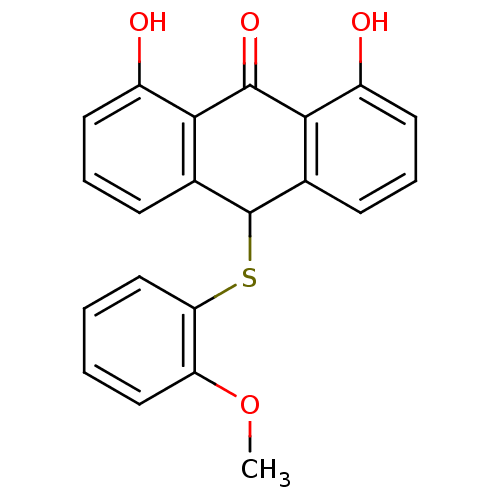 (1,8-Dihydroxy-10-(2-methoxy-phenylsulfanyl)-10H-an...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 12-LO (12-lipoxygenase) as an inhibitor of 12(S)-HETE biosynthesis in mouse epidermal homogenates | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052470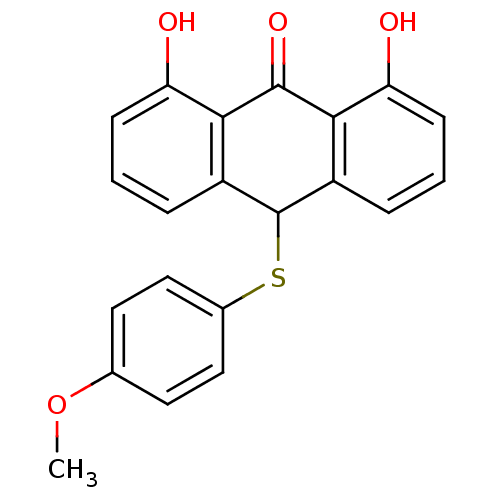 (1,8-Dihydroxy-10-(4-methoxy-phenylsulfanyl)-10H-an...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060898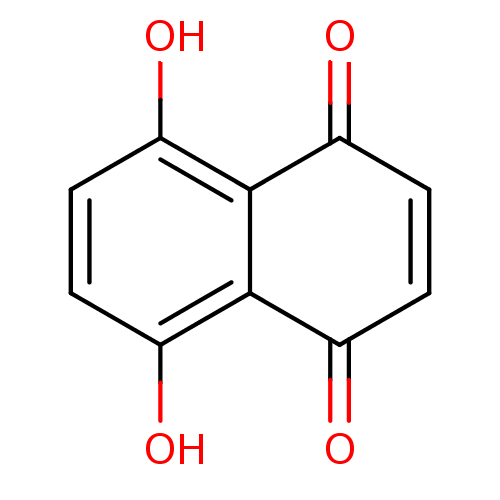 (5,8-Dihydroxy-[1,4]naphthoquinone | 5,8-dihydroxy-...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor ligand superfamily member 11 (Mus musculus) | BDBM50108929 (CHEMBL3596956) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant mouse RANKL-induced TRAP activity in mouse RAW264.7 cells using 4-nitrophenyl phosphate as TRAP substrate preincubated for ... | Bioorg Med Chem 23: 4522-32 (2015) Article DOI: 10.1016/j.bmc.2015.06.007 BindingDB Entry DOI: 10.7270/Q2V126KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060891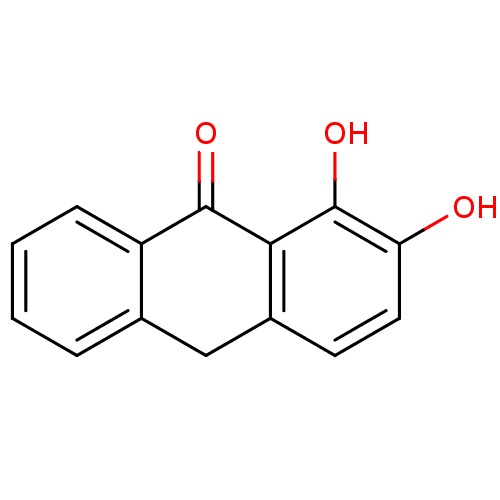 (1,2-Dihydroxy-10H-anthracen-9-one | CHEMBL123692) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060858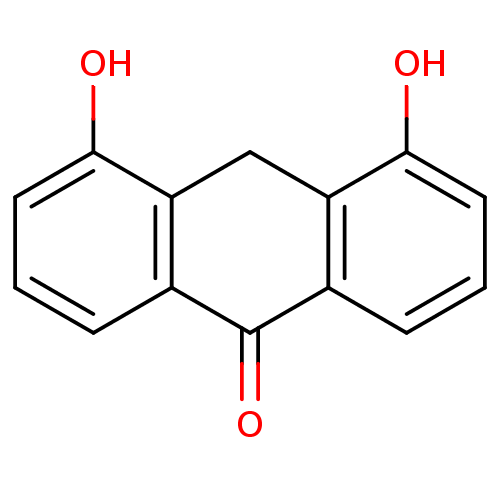 (4,5-Dihydroxy-10H-anthracen-9-one | CHEMBL124951) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50031472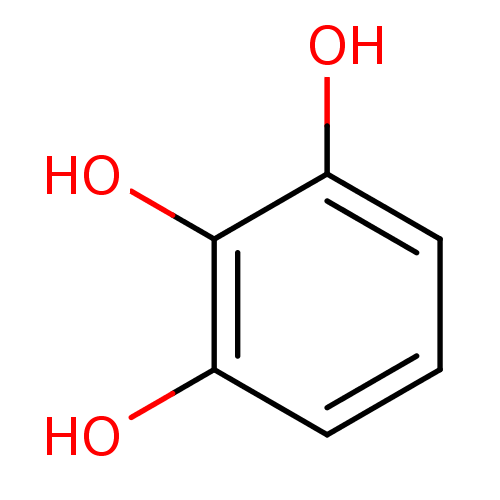 (1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060872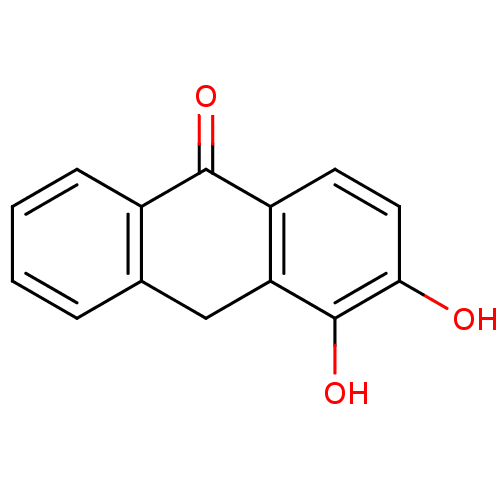 (3,4-Dihydroxy-10H-anthracen-9-one | CHEMBL121693) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060881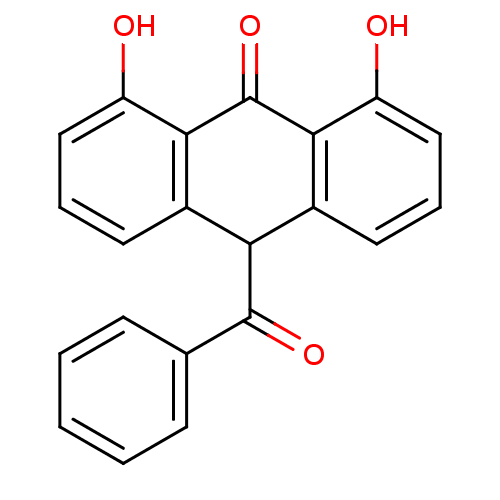 (10-Benzoyl-1,8-dihydroxy-10H-anthracen-9-one | CHE...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060867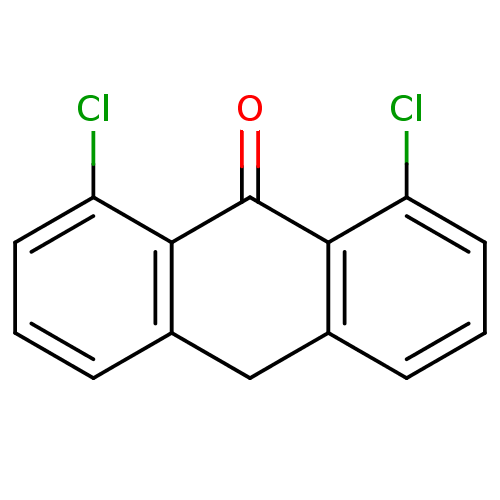 (1,8-Dichloro-10H-anthracen-9-one | CHEMBL122022) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor ligand superfamily member 11 (Mus musculus) | BDBM50108928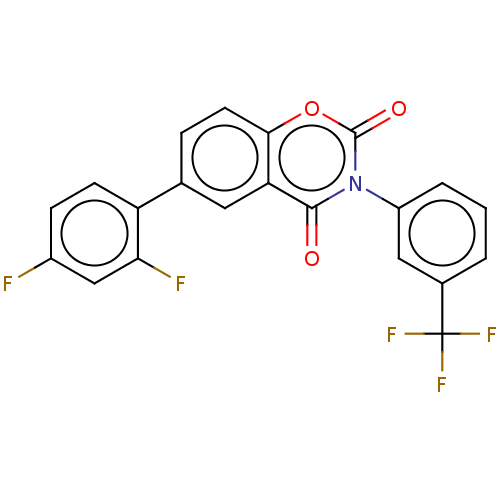 (CHEMBL3596954) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant mouse RANKL-induced TRAP activity in mouse RAW264.7 cells using 4-nitrophenyl phosphate as TRAP substrate preincubated for ... | Bioorg Med Chem 23: 4522-32 (2015) Article DOI: 10.1016/j.bmc.2015.06.007 BindingDB Entry DOI: 10.7270/Q2V126KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor ligand superfamily member 11 (Mus musculus) | BDBM50108927 (CHEMBL3596952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant mouse RANKL-induced TRAP activity in mouse RAW264.7 cells using 4-nitrophenyl phosphate as TRAP substrate preincubated for ... | Bioorg Med Chem 23: 4522-32 (2015) Article DOI: 10.1016/j.bmc.2015.06.007 BindingDB Entry DOI: 10.7270/Q2V126KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052456 (1,8-Dihydroxy-10-(2-hydroxy-phenylsulfanyl)-10H-an...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50428824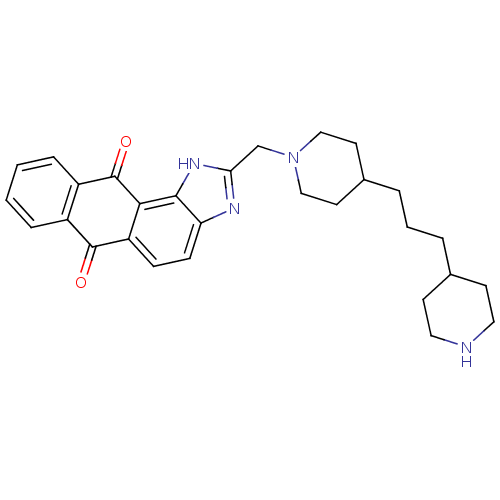 (CHEMBL2336662) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Inhibition of TERT activity in human H1299 cells homogenates incubated for 5 mins by TRAP assay | Eur J Med Chem 60: 29-41 (2013) Article DOI: 10.1016/j.ejmech.2012.11.032 BindingDB Entry DOI: 10.7270/Q20P11DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX12 (Mus musculus) | BDBM50052462 (10-(2-Amino-phenylsulfanyl)-1,8-dihydroxy-10H-anth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 12-LO (12-lipoxygenase) as an inhibitor of 12(S)-HETE biosynthesis in mouse epidermal homogenates | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052450 (1,8-Dihydroxy-10-(2-methoxy-phenylsulfanyl)-10H-an...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060901 (1,8-Dihydroxy-2-propionyl-10H-anthracen-9-one | CH...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060895 (Acetic acid 8-hydroxy-9-oxo-9,10-dihydro-anthracen...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060884 (1,8-Dihydroxy-9-oxo-9,10-dihydro-anthracene-2-carb...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50068320 (2,7-Bis[3-(pyrrolidino)propionamido]anthraquinone ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Inhibition of G-quadruplex-induced human TERT in H1299 cells by TRAP assay | Bioorg Med Chem 16: 6976-86 (2008) Article DOI: 10.1016/j.bmc.2008.05.072 BindingDB Entry DOI: 10.7270/Q27944HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060859 (4,5-Dihydroxy-10-oxo-9,10-dihydro-anthracene-9-car...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052459 (10-(4-Amino-phenylsulfanyl)-1,8-dihydroxy-10H-anth...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052460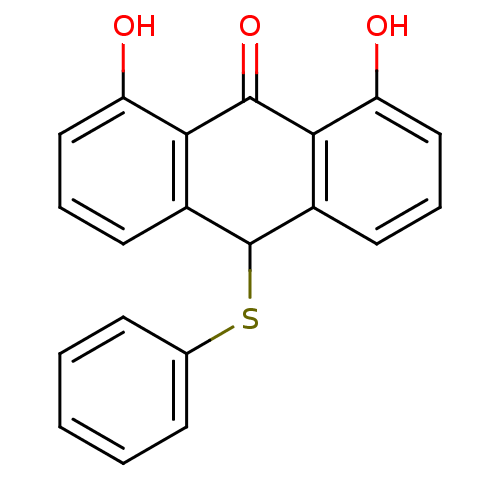 (1,8-Dihydroxy-10-phenylsulfanyl-10H-anthracen-9-on...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060899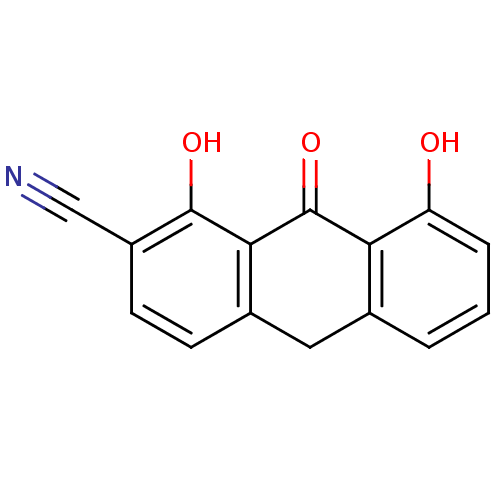 (1,8-Dihydroxy-9-oxo-9,10-dihydro-anthracene-2-carb...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060856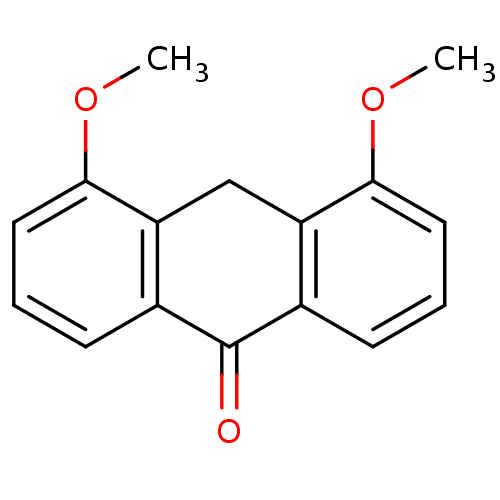 (4,5-Dimethoxy-10H-anthracen-9-one | CHEMBL125822) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50068321 (2,7-Bis[3-(piperidino)propionamido]anthraquinone |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Inhibition of G-quadruplex-induced human TERT in H1299 cells by TRAP assay | Bioorg Med Chem 16: 6976-86 (2008) Article DOI: 10.1016/j.bmc.2008.05.072 BindingDB Entry DOI: 10.7270/Q27944HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052471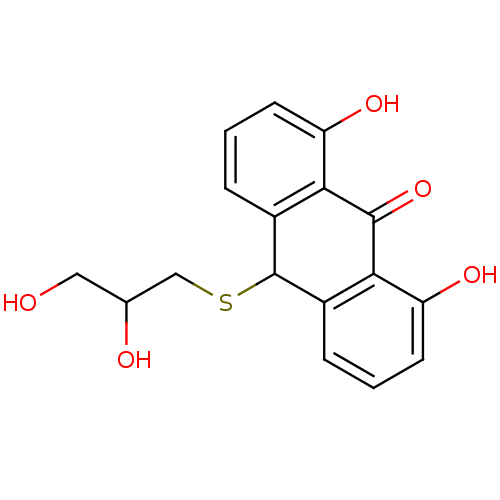 (10-(2,3-Dihydroxy-propylsulfanyl)-1,8-dihydroxy-10...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060868 (Acetic acid 8-acetoxy-9-oxo-9,10-dihydro-anthracen...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50428823 (CHEMBL2336666 | NSC-749235) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Inhibition of TERT activity in human H1299 cells homogenates incubated for 5 mins by TRAP assay | Eur J Med Chem 60: 29-41 (2013) Article DOI: 10.1016/j.ejmech.2012.11.032 BindingDB Entry DOI: 10.7270/Q20P11DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50125944 (CHEMBL3627832) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant topoisomerase 2 alpha assessed as blocking of supercoiled pHOT DNA relaxation incubated for 45 mins at 37 degC by eth... | Eur J Med Chem 103: 615-27 (2015) Article DOI: 10.1016/j.ejmech.2014.09.050 BindingDB Entry DOI: 10.7270/Q2D79D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50125944 (CHEMBL3627832) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant topoisomerase 1 assessed as blocking of supercoiled pHOT DNA relaxation incubated for 25 mins at 37 degC by ethidium ... | Eur J Med Chem 103: 615-27 (2015) Article DOI: 10.1016/j.ejmech.2014.09.050 BindingDB Entry DOI: 10.7270/Q2D79D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060873 (1,5-Dihydroxy-10H-anthracen-9-one | CHEMBL333452) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50428822 (CHEMBL2336667) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center Curated by ChEMBL | Assay Description Inhibition of TERT activity in human H1299 cells homogenates incubated for 5 mins by TRAP assay | Eur J Med Chem 60: 29-41 (2013) Article DOI: 10.1016/j.ejmech.2012.11.032 BindingDB Entry DOI: 10.7270/Q20P11DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060900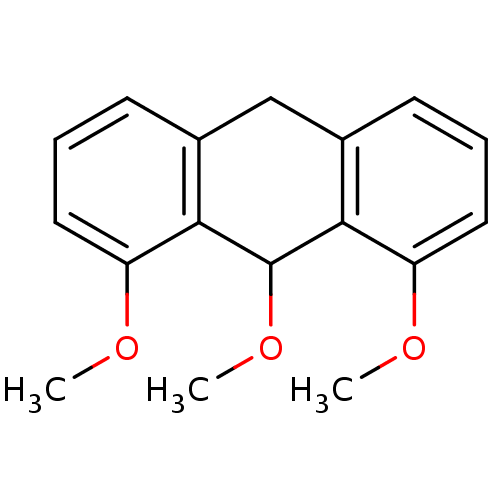 (1,8,9-Trimethoxy-9,10-dihydro-anthracene | CHEMBL1...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor ligand superfamily member 11 (Mus musculus) | BDBM50108930 (CHEMBL3335186) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant mouse RANKL-induced TRAP activity in mouse RAW264.7 cells using 4-nitrophenyl phosphate as TRAP substrate preincubated for ... | Bioorg Med Chem 23: 4522-32 (2015) Article DOI: 10.1016/j.bmc.2015.06.007 BindingDB Entry DOI: 10.7270/Q2V126KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060886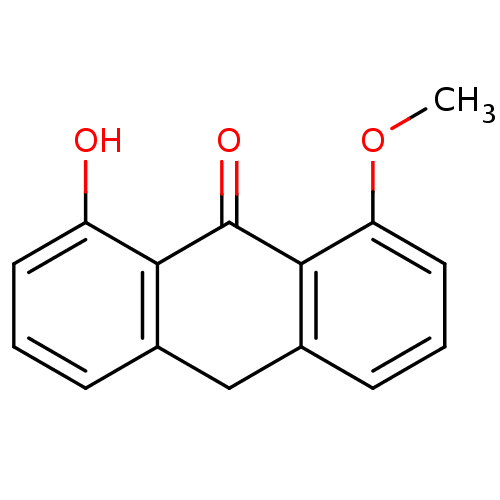 (1-Hydroxy-8-methoxy-10H-anthracen-9-one | CHEMBL12...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060887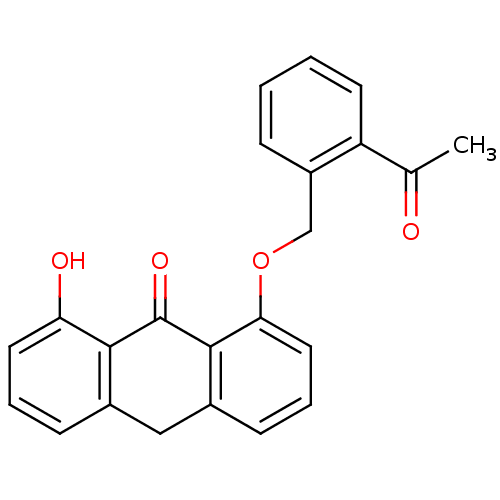 (1-(2-Acetyl-benzyloxy)-8-hydroxy-10H-anthracen-9-o...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060879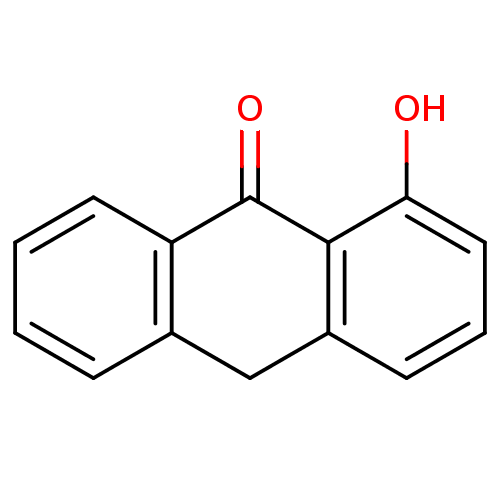 (1-Hydroxy-10H-anthracen-9-one | CHEMBL123161) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052469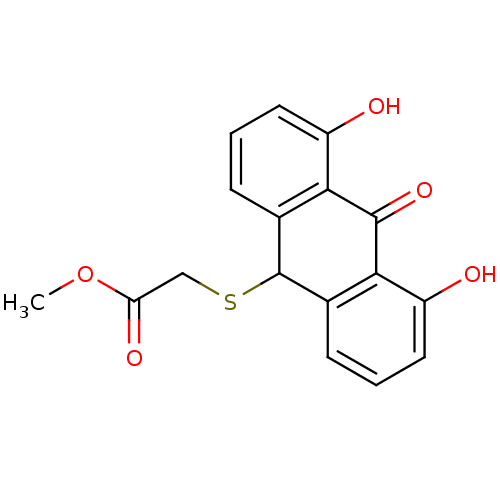 ((4,5-Dihydroxy-10-oxo-9,10-dihydro-anthracen-9-yls...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50060878 (1,3,8-Trihydroxy-6-methyl-10H-anthracen-9-one | CH...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052473 (1,8-Dihydroxy-10-(3-methoxy-phenylsulfanyl)-10H-an...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase (Bos taurus) | BDBM50052458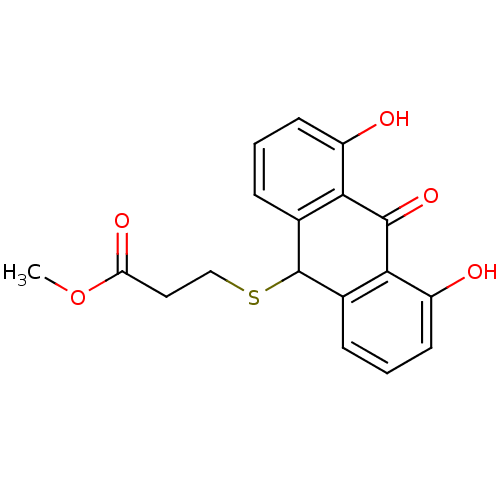 (3-(4,5-Dihydroxy-10-oxo-9,10-dihydro-anthracen-9-y...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Regensburg Curated by ChEMBL | Assay Description Tested for inhibition of 5-lipoxygenase as inhibition of 5-HETE and LTB4 biosynthesis in bovine PMNL | J Med Chem 39: 3132-8 (1996) Article DOI: 10.1021/jm960259l BindingDB Entry DOI: 10.7270/Q2MK6BZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 122 total ) | Next | Last >> |