

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260806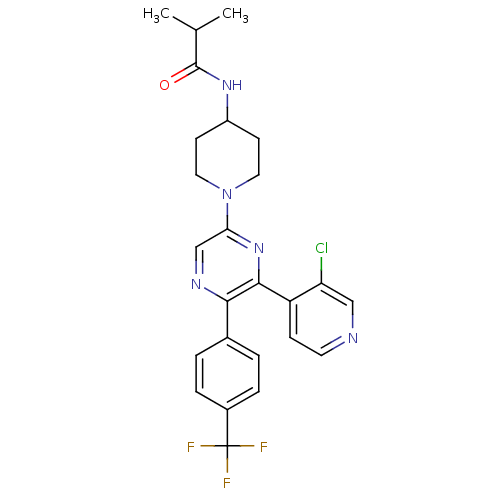 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017233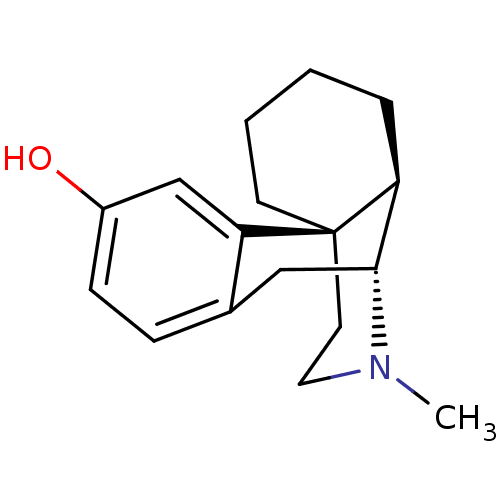 (CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260767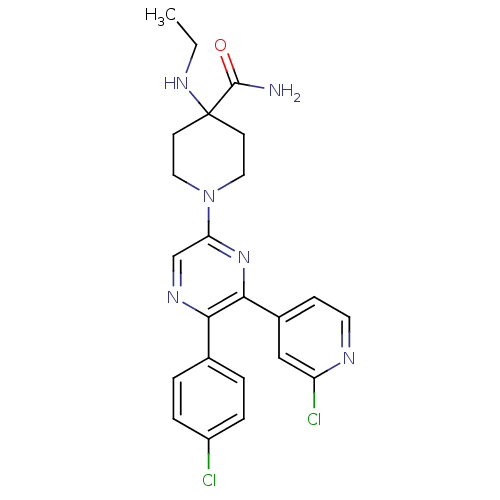 (1-(5-(4-chlorophenyl)-6-(2-chloropyridin-4-yl)pyra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260805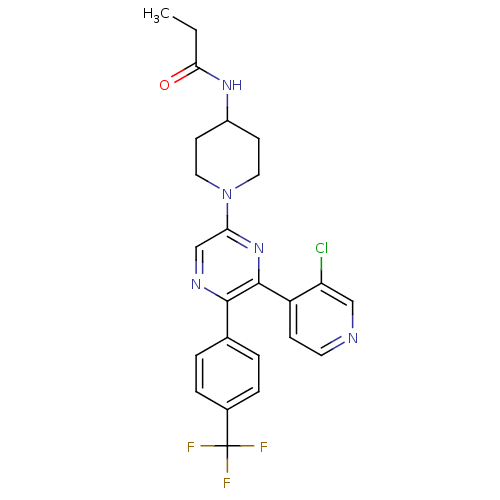 (CHEMBL524804 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260681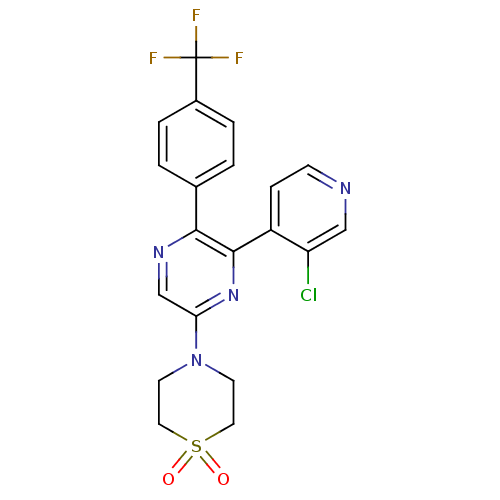 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260768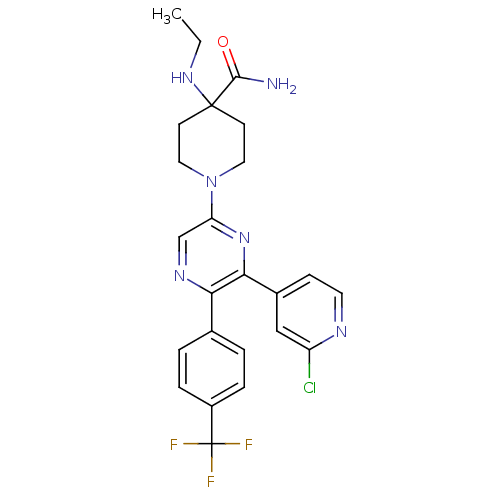 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017231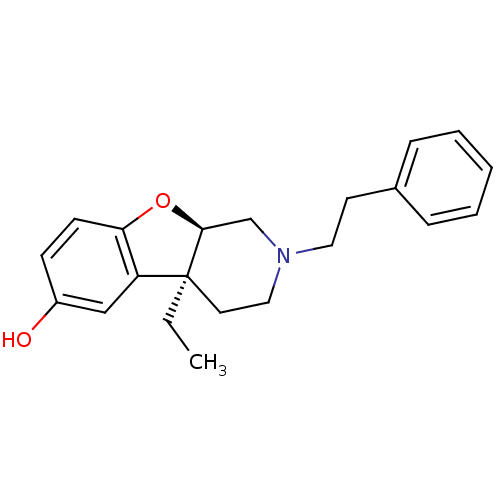 ((4aS,9aR)-4a-Ethyl-2-phenethyl-1,2,3,4,4a,9a-hexah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260682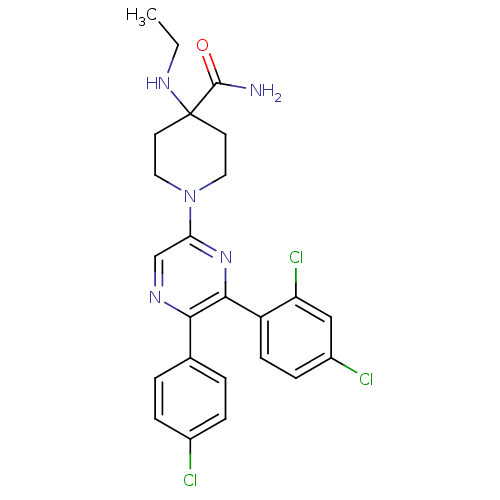 (1-(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260803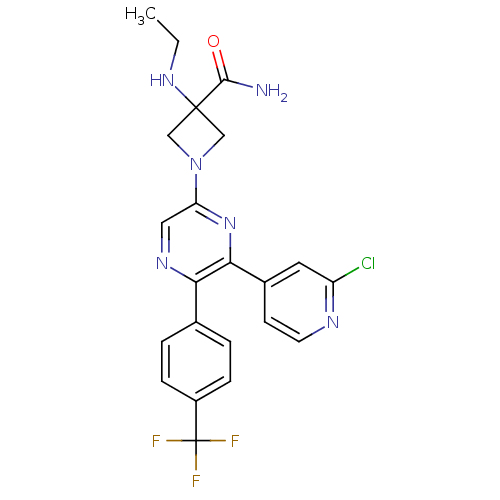 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017232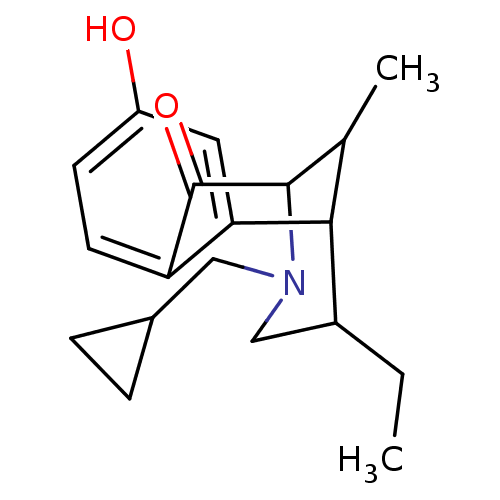 (3-Cyclopropylmethyl-5-ethyl-8-hydroxy-11-methyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50094569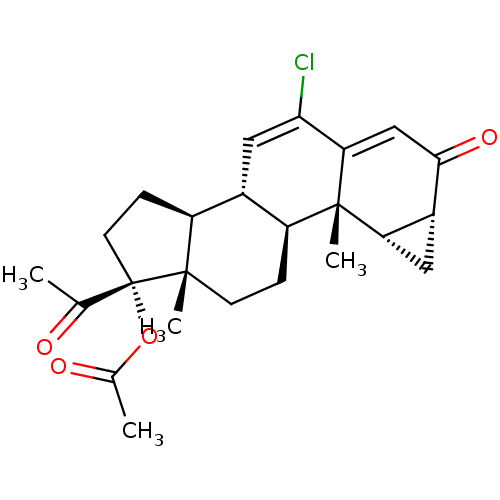 (6-chloro-3,20-dioxo-1beta,2beta-dihydro-3'H-cyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Adenosine A1 receptor binding was measured in adenosine deaminase (ADA) pretreated rat cortical membranes using [3H]cyclohexyladenosine in the presen... | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260718 (1-(6-(2-chlorophenyl)-5-(4-chlorophenyl)pyrazin-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260717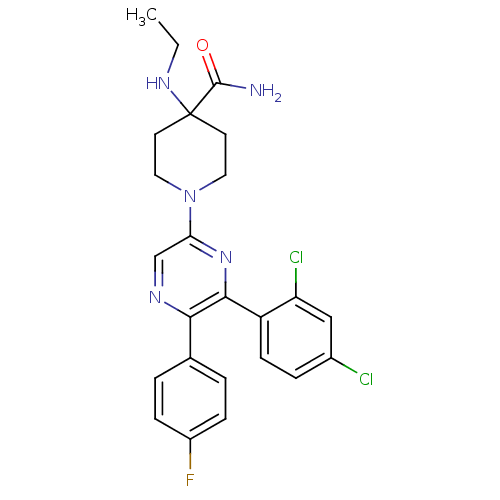 (1-(6-(2,4-dichlorophenyl)-5-(4-fluorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Mus musculus (Mouse)) | BDBM50017233 (CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse sigma opioid receptor | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260804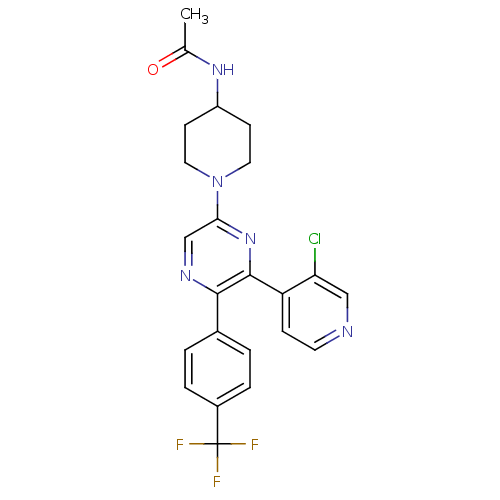 (CHEMBL497556 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260803 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM81970 (CAS_108-91-8 | CHA | Cyclohexylamines) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-Geigy Corporation Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 47-55 (1989) BindingDB Entry DOI: 10.7270/Q2B856MX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Mus musculus (Mouse)) | BDBM50017232 (3-Cyclopropylmethyl-5-ethyl-8-hydroxy-11-methyl-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse sigma opioid receptor | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260720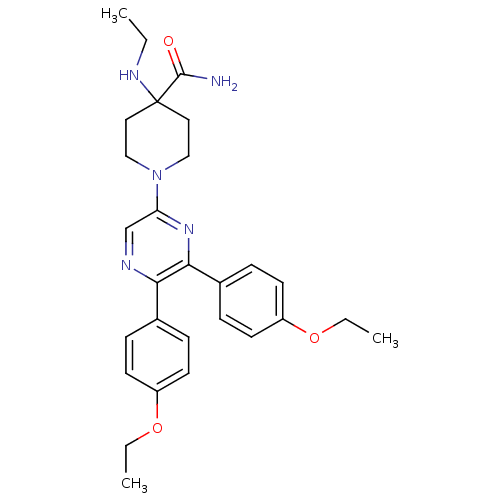 (1-(5,6-bis(4-ethoxyphenyl)pyrazin-2-yl)-4-(ethylam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017238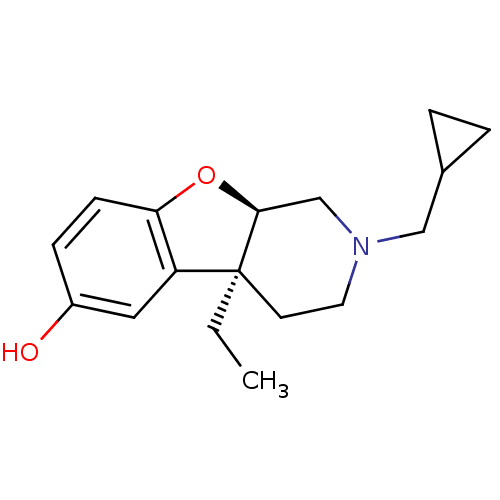 ((4aS,9aR)-2-Cyclopropylmethyl-4a-ethyl-1,2,3,4,4a,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50017232 (3-Cyclopropylmethyl-5-ethyl-8-hydroxy-11-methyl-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor kappa | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50015655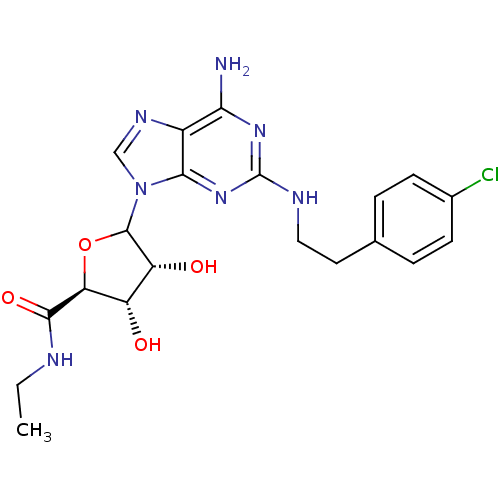 ((2S,3S,4R)-5-{(R)-6-Amino-2-[2-(4-chloro-phenyl)-e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50015648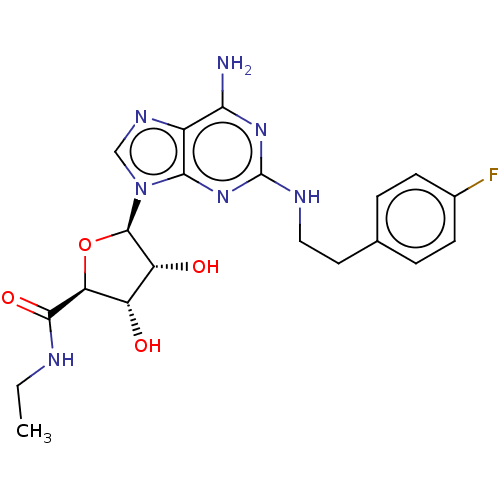 ((2S,3S,4R)-5-{(R)-6-Amino-2-[2-(4-fluoro-phenyl)-e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Mus musculus (Mouse)) | BDBM50017231 ((4aS,9aR)-4a-Ethyl-2-phenethyl-1,2,3,4,4a,9a-hexah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse sigma opioid receptor | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50015649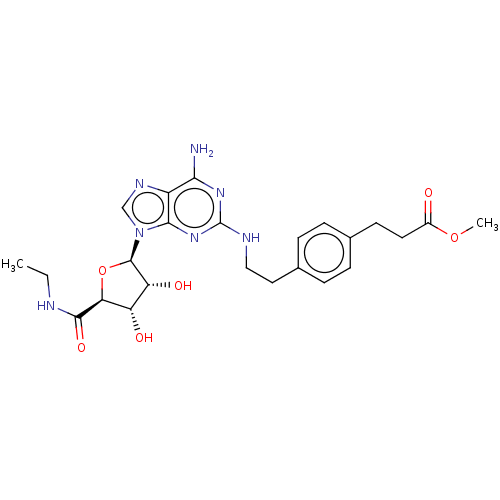 (3-(4-{2-[(R)-6-Amino-9-((3R,4S,5S)-5-ethylcarbamoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50385958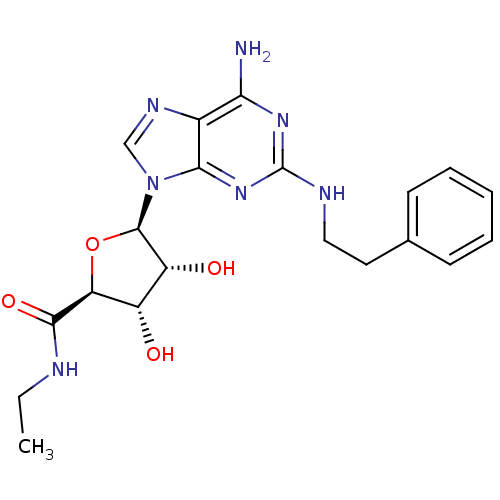 (CHEMBL2042298) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260766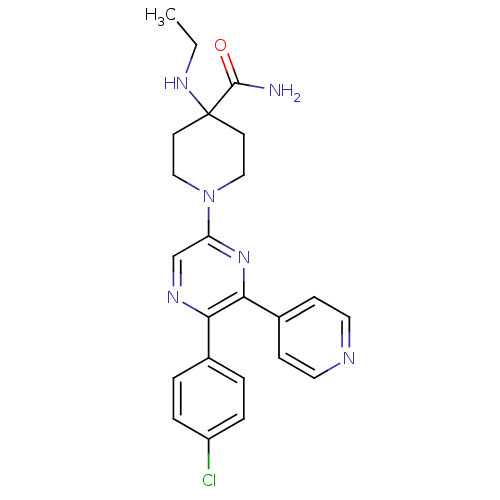 (1-(5-(4-chlorophenyl)-6-(pyridin-4-yl)pyrazin-2-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50017233 (CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor kappa | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-Geigy Corporation Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 47-55 (1989) BindingDB Entry DOI: 10.7270/Q2B856MX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-Geigy Corporation Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 47-55 (1989) BindingDB Entry DOI: 10.7270/Q2B856MX | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50015653 ((2S,3S,4R)-5-((R)-6-Amino-2-phenethylamino-purin-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-Geigy Corporation Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 47-55 (1989) BindingDB Entry DOI: 10.7270/Q2B856MX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50015657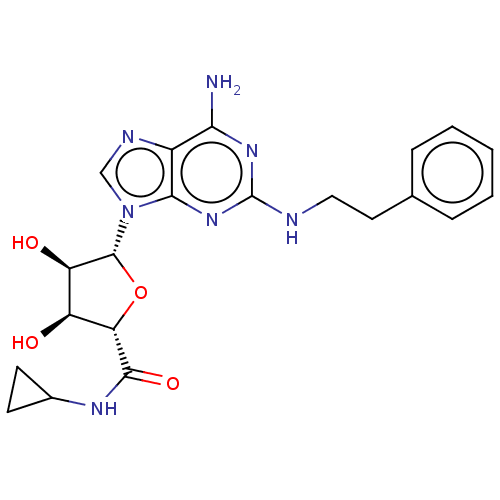 ((2S,3S,4R)-5-((R)-6-Amino-2-phenethylamino-purin-9...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260719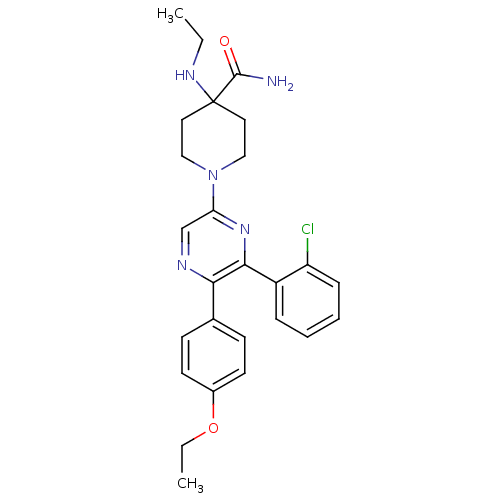 (1-(6-(2-chlorophenyl)-5-(4-ethoxyphenyl)pyrazin-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017234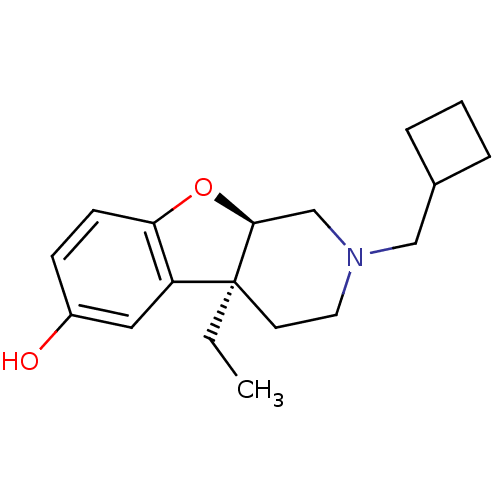 ((4aS,9aR)-2-Cyclobutylmethyl-4a-ethyl-1,2,3,4,4a,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017236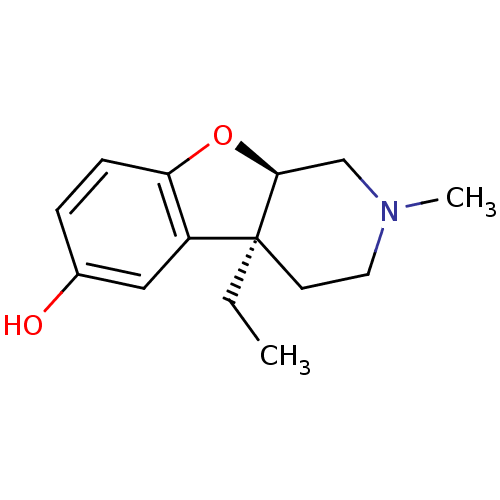 ((4aS,9aR)-4a-Ethyl-2-methyl-1,2,3,4,4a,9a-hexahydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 21.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-Geigy Corporation Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 47-55 (1989) BindingDB Entry DOI: 10.7270/Q2B856MX | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017237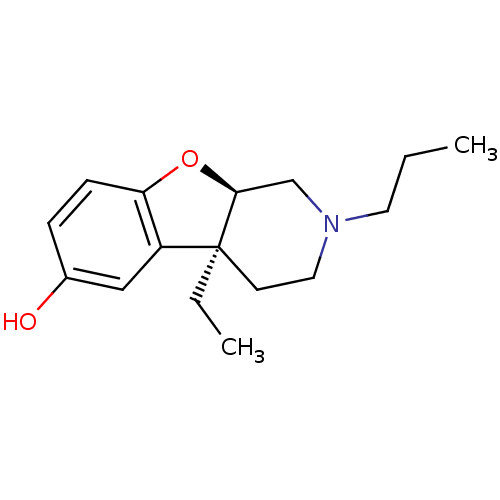 ((4aS,9aR)-4a-Ethyl-2-propyl-1,2,3,4,4a,9a-hexahydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017239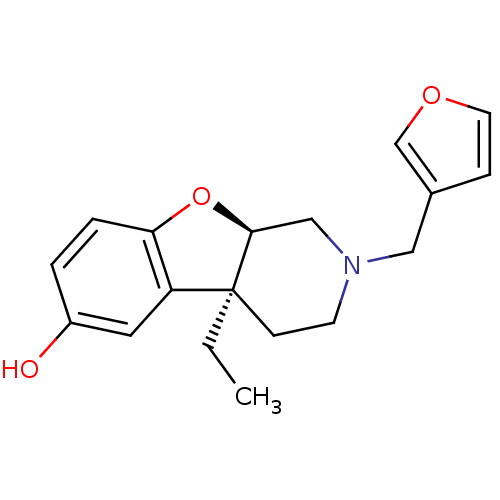 ((4aS,9aR)-4a-Ethyl-2-furan-3-ylmethyl-1,2,3,4,4a,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260765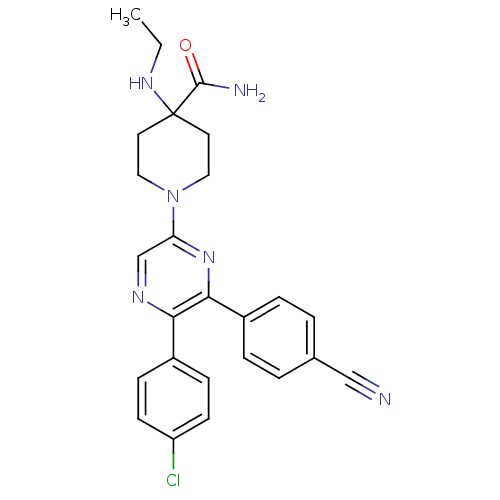 (1-(5-(4-chlorophenyl)-6-(4-cyanophenyl)pyrazin-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50017238 ((4aS,9aR)-2-Cyclopropylmethyl-4a-ethyl-1,2,3,4,4a,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor kappa | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50015650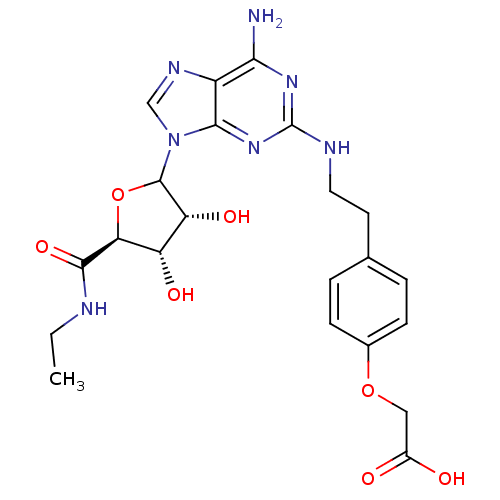 ((4-{2-[(R)-6-Amino-9-((3R,4S,5S)-5-ethylcarbamoyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50015654 ((4-{2-[(R)-6-Amino-9-((3R,4S,5S)-5-ethylcarbamoyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding to Adenosine A2 receptor was measured in ADA-pretreated rat striatal membranes using [3H]-CGS- 21680 | J Med Chem 33: 1919-24 (1990) BindingDB Entry DOI: 10.7270/Q2VD701M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50017240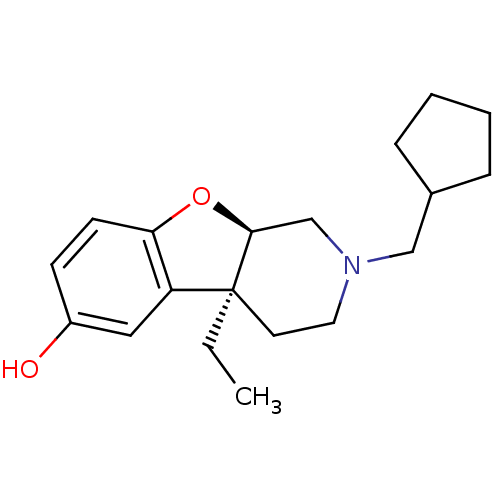 ((4aS,9aR)-2-Cyclopentylmethyl-4a-ethyl-1,2,3,4,4a,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse opioid receptor mu | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Mus musculus (Mouse)) | BDBM50017238 ((4aS,9aR)-2-Cyclopropylmethyl-4a-ethyl-1,2,3,4,4a,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Binding affinity for mouse sigma opioid receptor | J Med Chem 32: 2221-6 (1989) BindingDB Entry DOI: 10.7270/Q2Q240T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 315 total ) | Next | Last >> |