

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435764 (CHEMBL2392692) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566635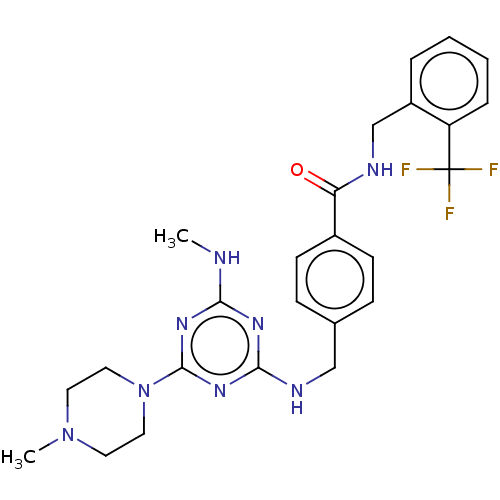 (CHEMBL4875337) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435758 (CHEMBL2392693) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435756 (CHEMBL2392695) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435755 (CHEMBL2392696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435757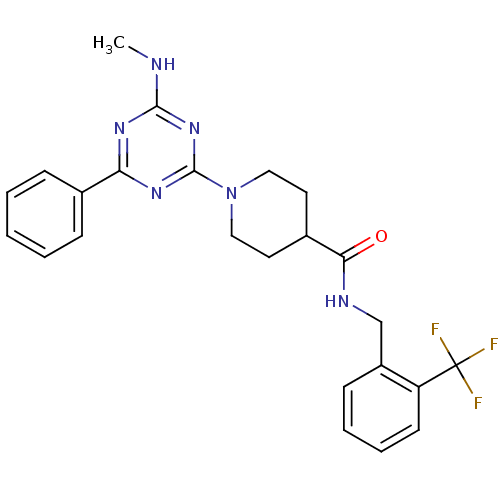 (CHEMBL2392694) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566638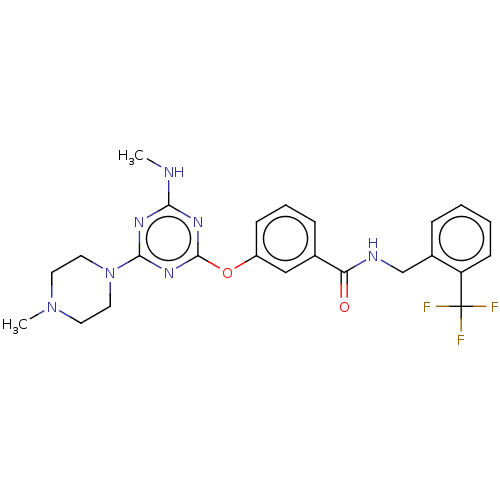 (CHEMBL4862566) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435745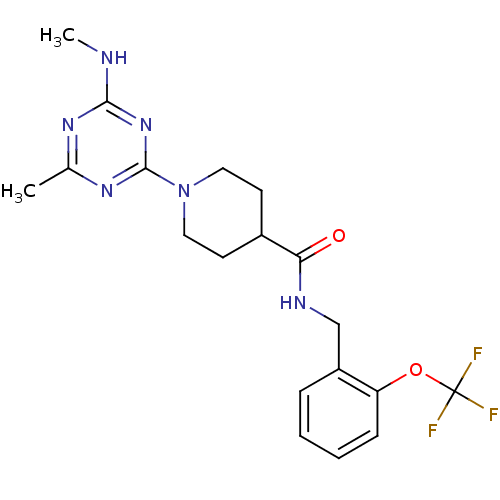 (CHEMBL2392706) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435754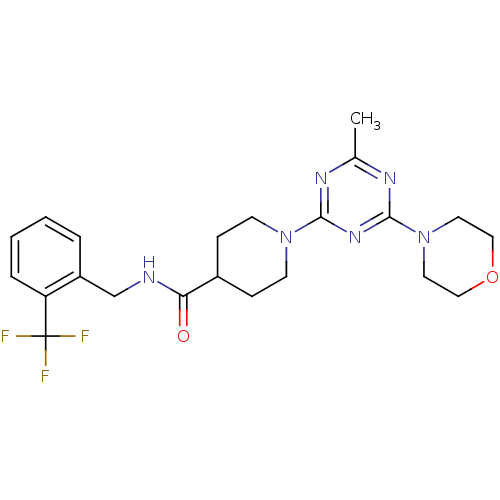 (CHEMBL2392697) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435753 (CHEMBL2392698) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435753 (CHEMBL2392698) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435765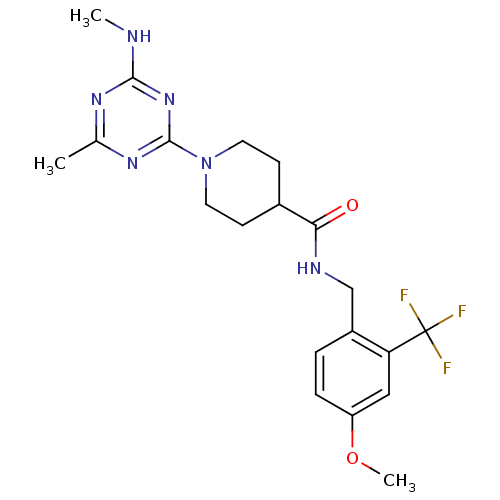 (CHEMBL2392691) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50435764 (CHEMBL2392692) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of rat soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prior ... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435765 (CHEMBL2392691) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36462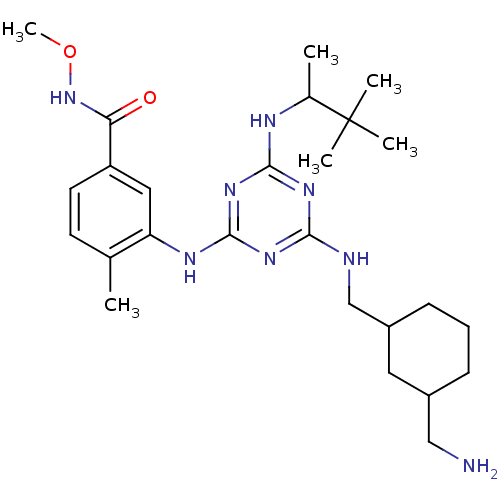 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36463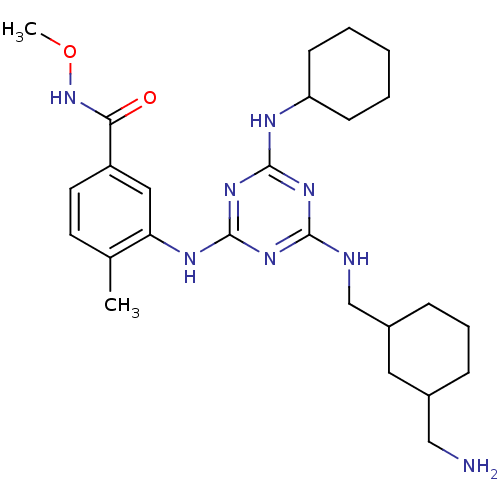 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435760 (CHEMBL2392714) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435764 (CHEMBL2392692) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435745 (CHEMBL2392706) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435754 (CHEMBL2392697) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539700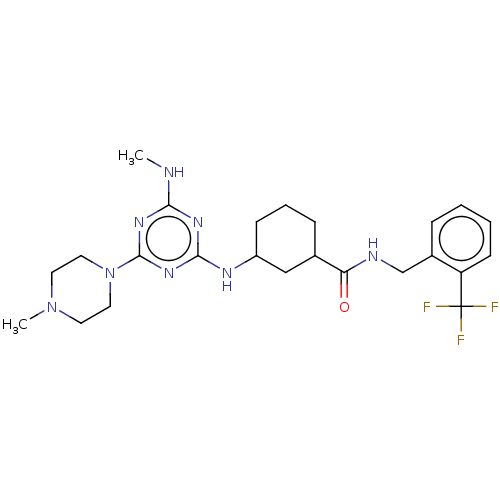 (CHEMBL4637413) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566636 (CHEMBL4870025) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435738 (CHEMBL2392690) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435759 (CHEMBL2392715) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435750 (CHEMBL2392701) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122453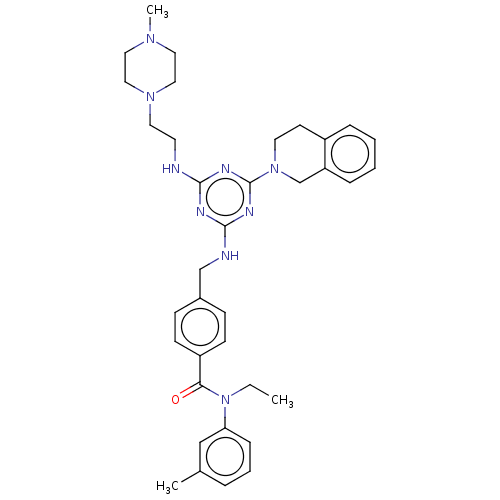 (CHEMBL3622491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566637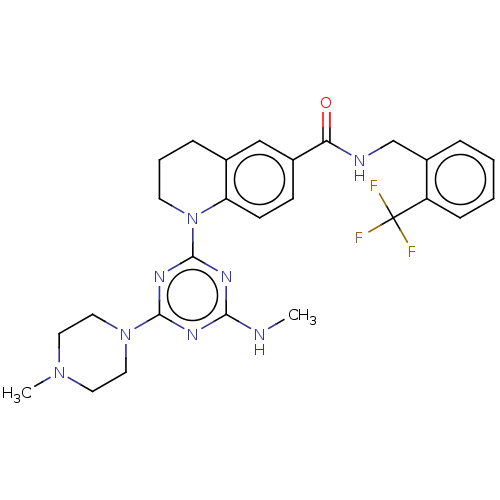 (CHEMBL4871885) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122451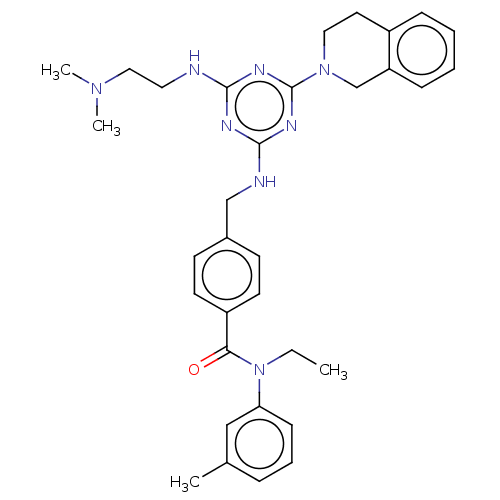 (CHEMBL3622492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566632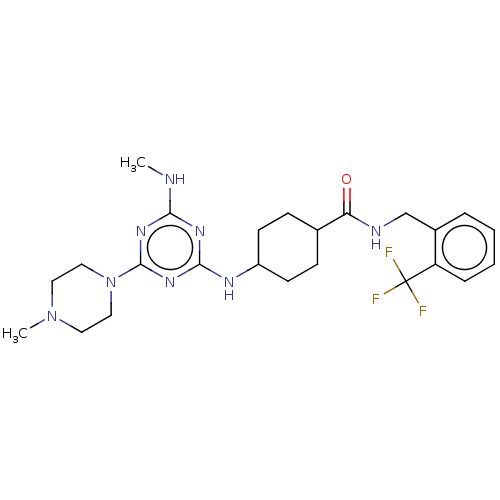 (CHEMBL4850549) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122449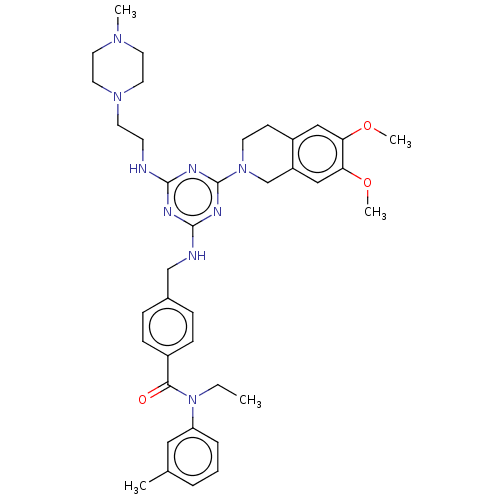 (CHEMBL3622483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435747 (CHEMBL2392704) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435755 (CHEMBL2392696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435763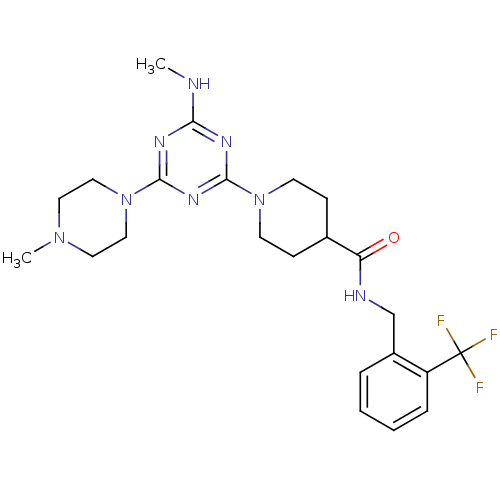 (CHEMBL2392711) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122484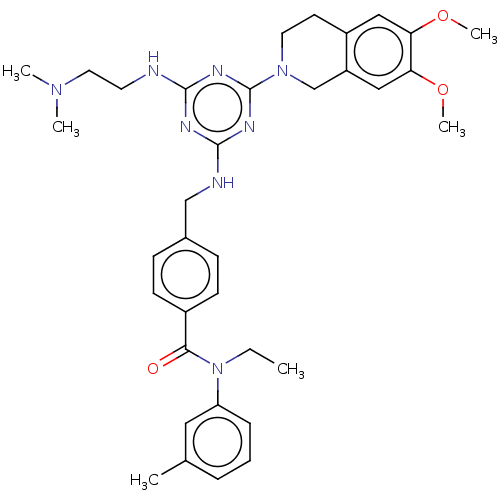 (CHEMBL3622484) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36464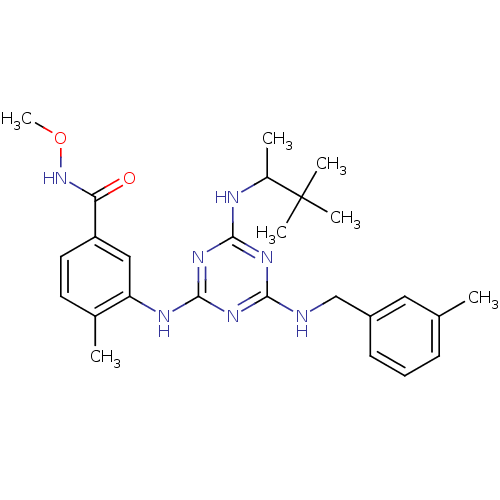 (3-(4-(3,3-Dimethylbutan-2-ylamino)-6-(3-methylbenz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50021695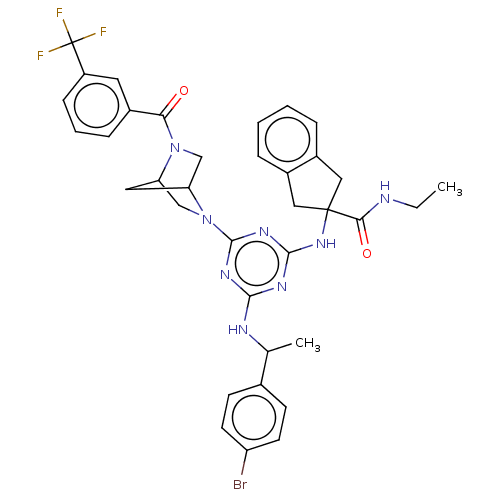 (CHEMBL3298195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at C-terminal 6xHis-tagged LFA-1 LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of interaction with IC... | Bioorg Med Chem 22: 2353-65 (2014) Article DOI: 10.1016/j.bmc.2014.01.050 BindingDB Entry DOI: 10.7270/Q2Q81FN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435756 (CHEMBL2392695) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566631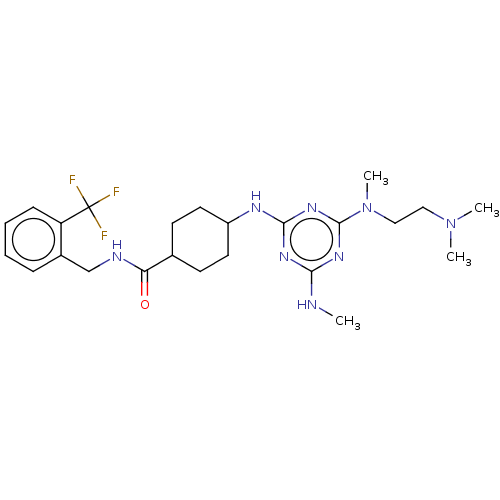 (CHEMBL4852295) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156750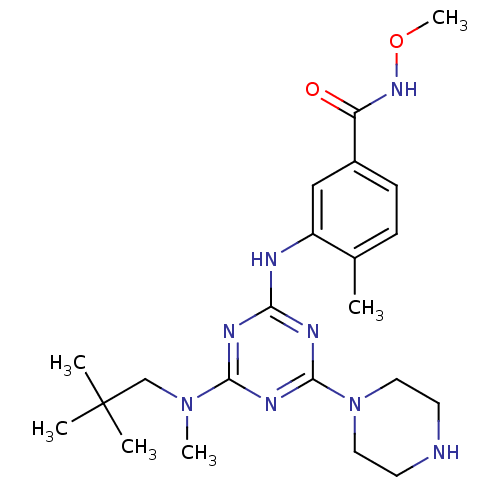 (CHEMBL376506 | DEL-A, 5 | N-methoxy-4-methyl-3-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566629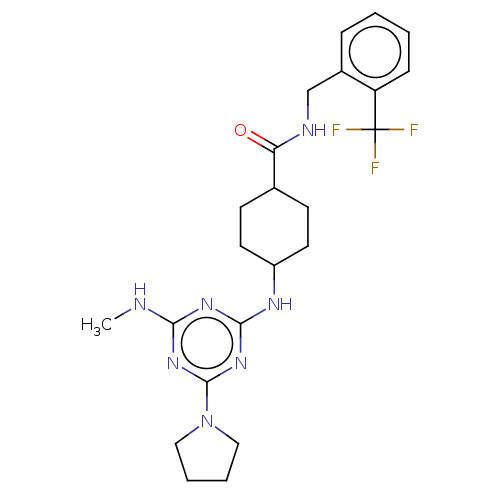 (CHEMBL4846972) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435762 (CHEMBL2392712) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50021698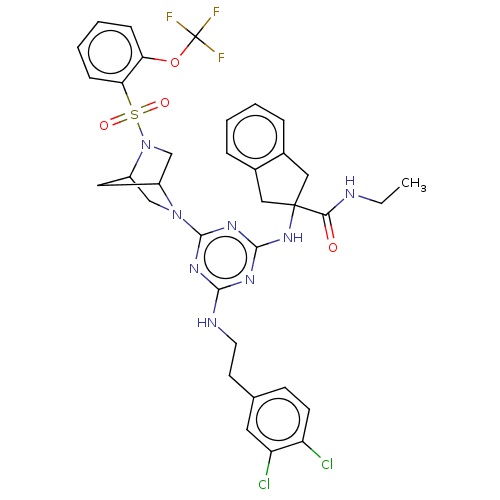 (CHEMBL3298198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at C-terminal 6xHis-tagged LFA-1 LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of interaction with IC... | Bioorg Med Chem 22: 2353-65 (2014) Article DOI: 10.1016/j.bmc.2014.01.050 BindingDB Entry DOI: 10.7270/Q2Q81FN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122485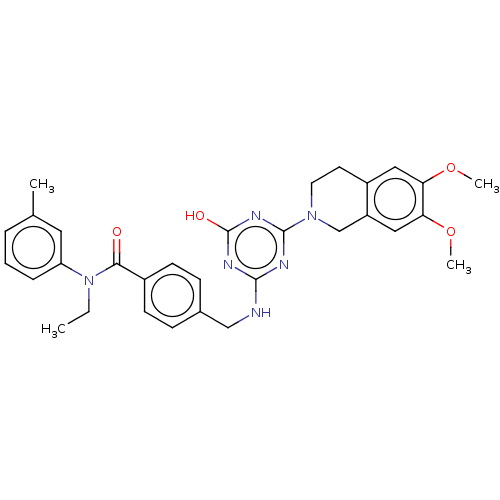 (CHEMBL3622482) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122452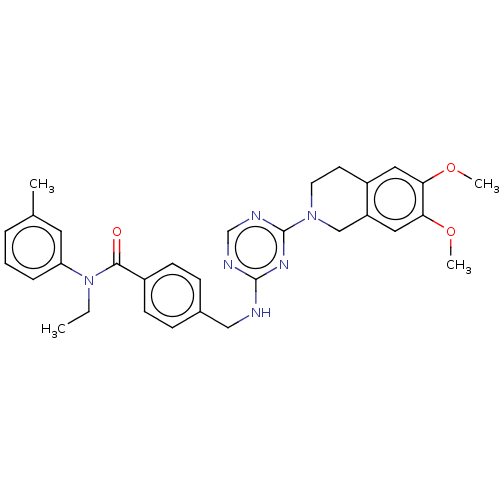 (CHEMBL3622490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50021697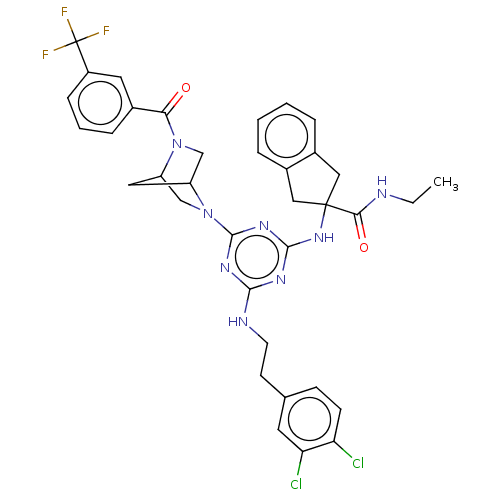 (CHEMBL3298197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at C-terminal 6xHis-tagged LFA-1 LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of interaction with IC... | Bioorg Med Chem 22: 2353-65 (2014) Article DOI: 10.1016/j.bmc.2014.01.050 BindingDB Entry DOI: 10.7270/Q2Q81FN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566620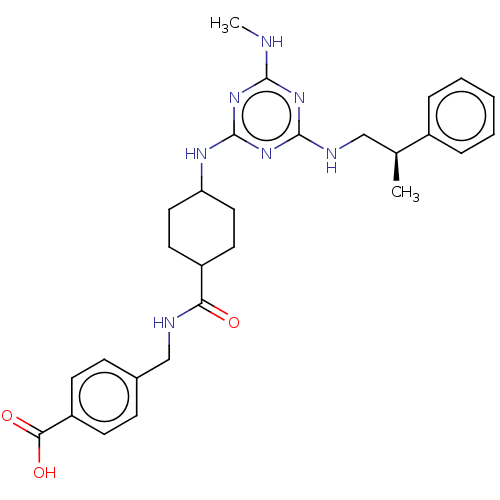 (CHEMBL4850535) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566617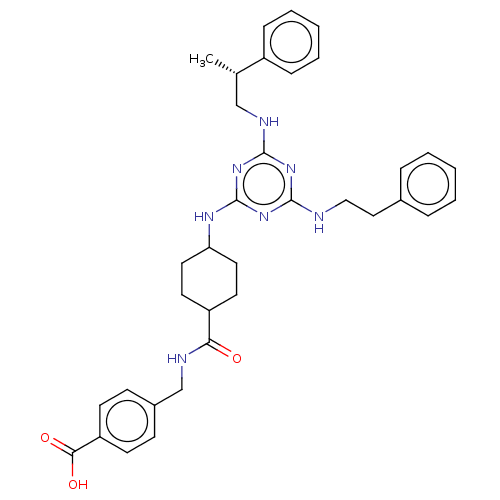 (CHEMBL4851061) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566618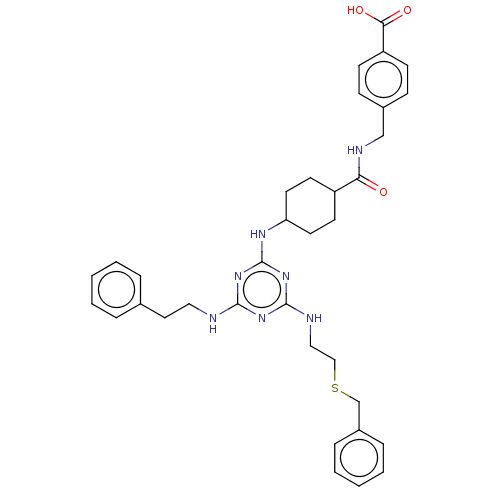 (CHEMBL4868985) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50021694 (CHEMBL3298661) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at C-terminal 6xHis-tagged LFA-1 LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of interaction with IC... | Bioorg Med Chem 22: 2353-65 (2014) Article DOI: 10.1016/j.bmc.2014.01.050 BindingDB Entry DOI: 10.7270/Q2Q81FN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566623 (CHEMBL4853666) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |