

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057433 (CHEMBL278806 | [2-(5-Fluoro-chroman-8-yloxy)-ethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057432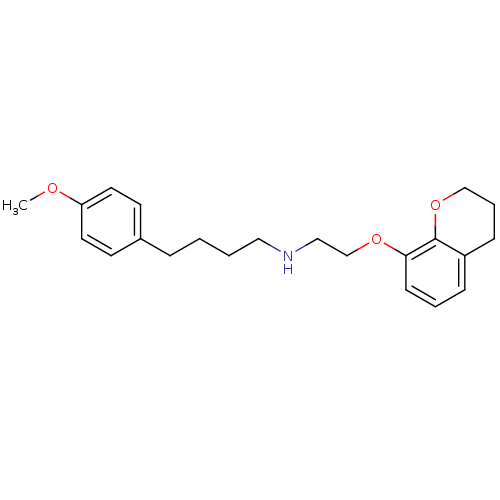 (CHEMBL25215 | [2-(Chroman-8-yloxy)-ethyl]-[4-(4-me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065562 (CHEMBL96279 | [2-(6-Fluoro-2,2-dimethyl-chroman-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065578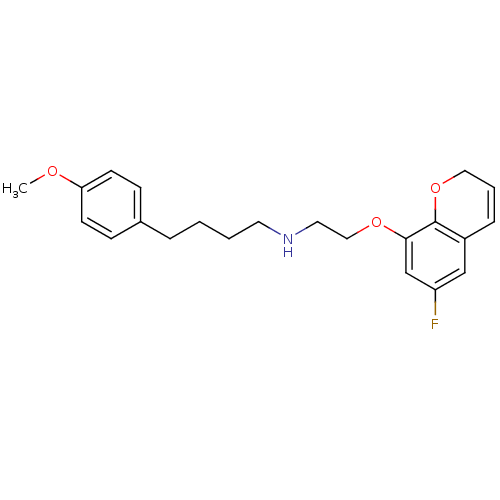 (CHEMBL419147 | [2-(6-Fluoro-2H-chromen-8-yloxy)-et...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065555 (CHEMBL96578 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM85052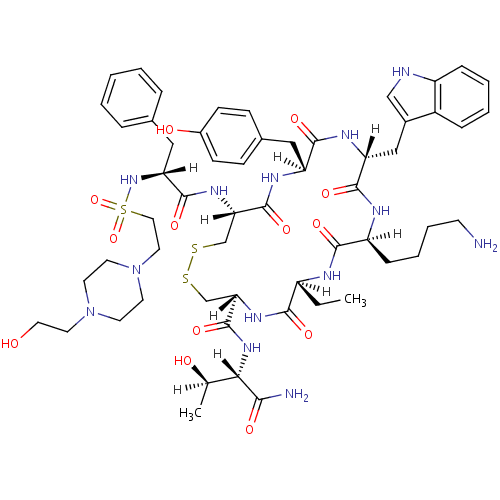 (BIM 23197 | BIM-23197) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]T3 from recombinant thyroid hormone receptor alpha (unknown origin) expressed in sf9 cells by scintillation proximity assay | Proc Natl Acad Sci U S A 104: 15490-5 (2007) Article DOI: 10.1073/pnas.0702759104 BindingDB Entry DOI: 10.7270/Q2JW8DPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50019568 (Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065573 (CHEMBL96222 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057434 (8-{2-[4-(4-Methoxy-phenyl)-butylamino]-ethoxy}-chr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065578 (CHEMBL419147 | [2-(6-Fluoro-2H-chromen-8-yloxy)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065573 (CHEMBL96222 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50370683 (CHEMBL1169543) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM85051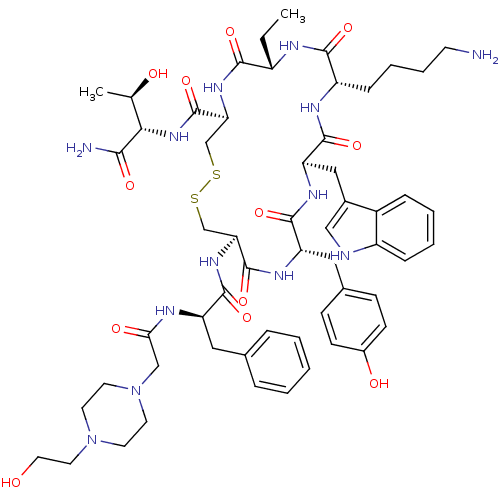 (BIM-23190) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057429 (CHEMBL22328 | [2-(7-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18869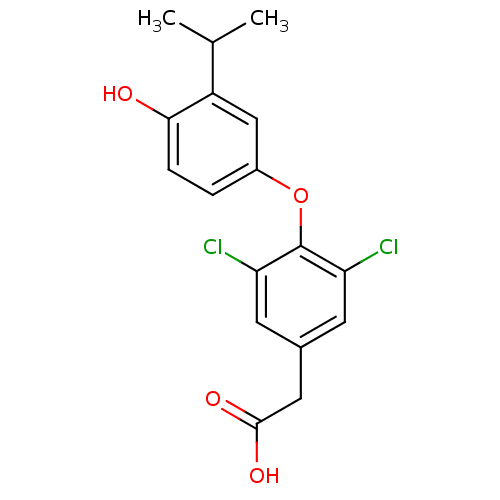 (2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay | Proc Natl Acad Sci U S A 104: 15490-5 (2007) Article DOI: 10.1073/pnas.0702759104 BindingDB Entry DOI: 10.7270/Q2JW8DPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50063839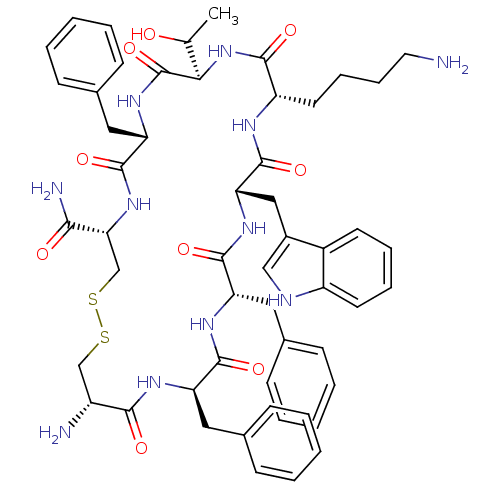 (BIM 23268 | CHEMBL263606 | H-cyclo[DCys-Phe-Phe-DT...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM82465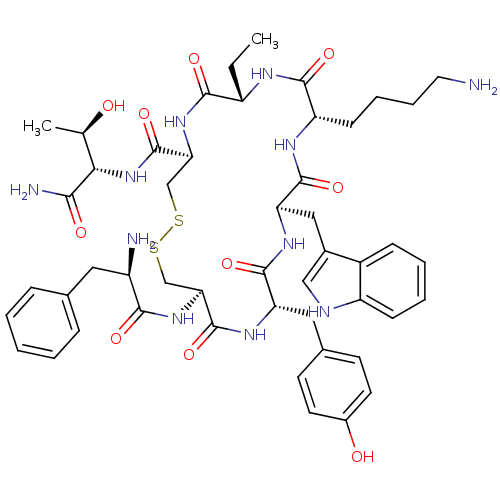 (BIM 23023 | BIM-23023 | D-Phe-L-Cys(1)-L-Tyr-D-Trp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065562 (CHEMBL96279 | [2-(6-Fluoro-2,2-dimethyl-chroman-8-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057428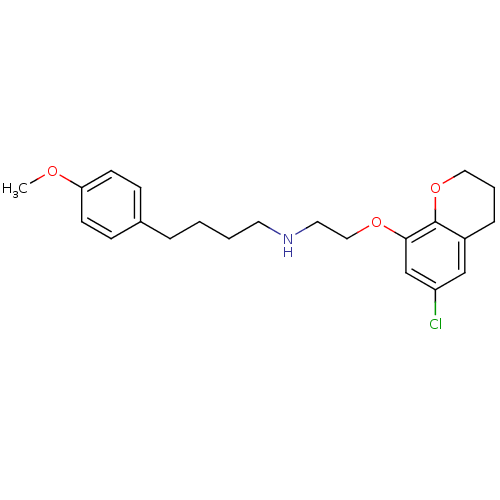 (CHEMBL22682 | [2-(6-Chloro-chroman-8-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay | Proc Natl Acad Sci U S A 104: 15490-5 (2007) Article DOI: 10.1073/pnas.0702759104 BindingDB Entry DOI: 10.7270/Q2JW8DPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50059090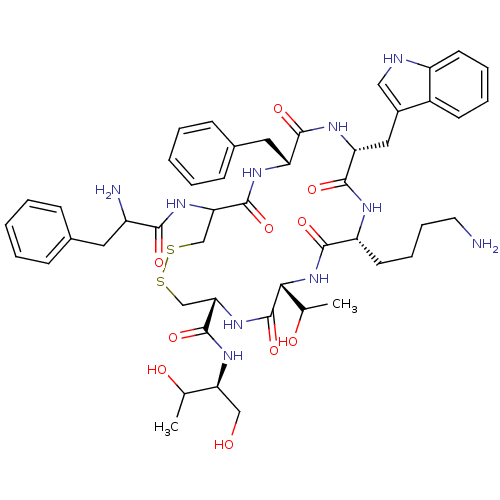 (10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057432 (CHEMBL25215 | [2-(Chroman-8-yloxy)-ethyl]-[4-(4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated against Dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand; ND = Not determined | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065563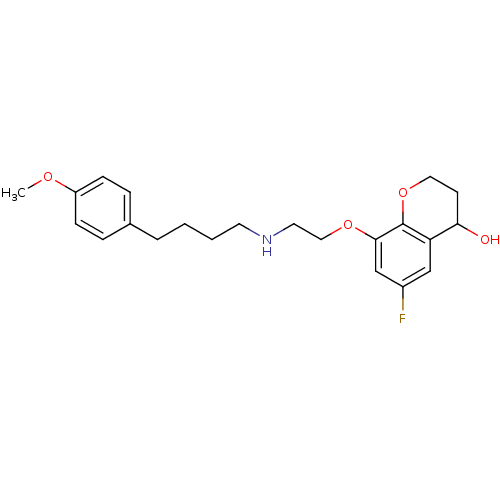 (6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065563 (6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM82470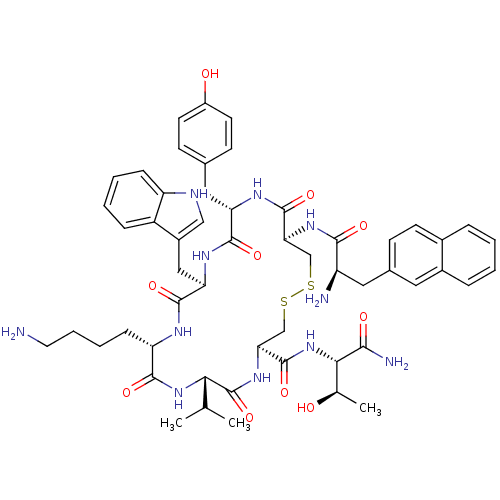 (3-(2-Naphtyl)-D-Ala-L-Cys(1)-L-Tyr-D-Trp-L-Lys-L-V...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057431 (CHEMBL21475 | [4-(4-Methoxy-phenyl)-butyl]-[2-(6-m...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50258672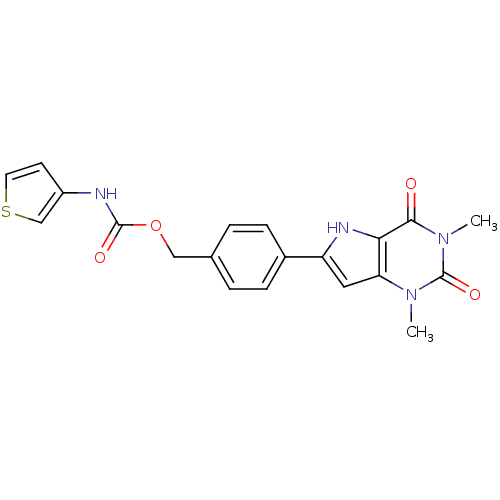 (CHEMBL513681 | [4-(2,3,4,5-Tetrahydro-1,3-dimethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Estadual de Feira de Santana Curated by ChEMBL | Assay Description Antagonist activity at Adenosine receptor subtype 2B in human RBC assessed as change in erythrocyte morphology incubated for 3 hrs with occasional ge... | Eur J Med Chem 136: 487-496 (2017) Article DOI: 10.1016/j.ejmech.2017.05.035 BindingDB Entry DOI: 10.7270/Q2RJ4N1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50370683 (CHEMBL1169543) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50019568 (Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175574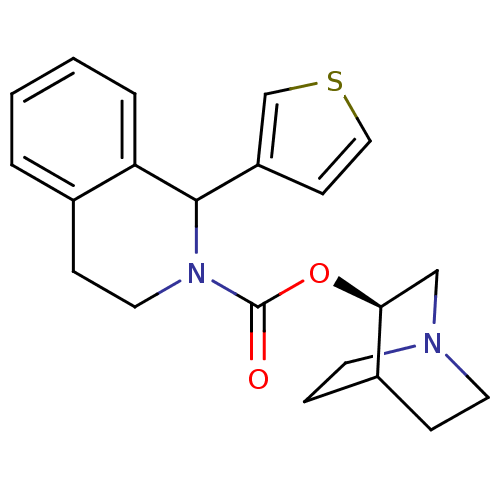 ((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50282641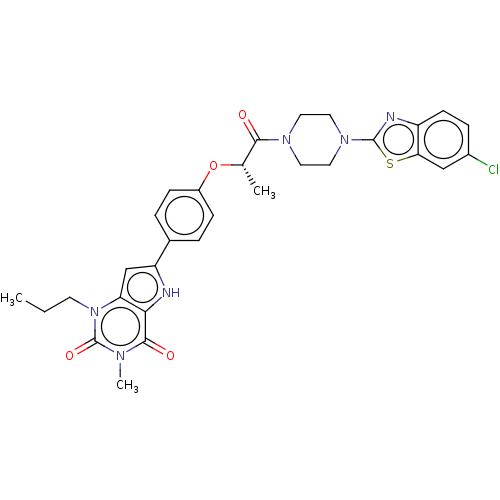 (CHEMBL4160732) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Estadual de Feira de Santana Curated by ChEMBL | Assay Description Antagonist activity at Adenosine receptor subtype 2B in human RBC assessed as change in erythrocyte morphology incubated for 3 hrs with occasional ge... | Eur J Med Chem 136: 487-496 (2017) Article DOI: 10.1016/j.ejmech.2017.05.035 BindingDB Entry DOI: 10.7270/Q2RJ4N1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin receptor 1 (Homo sapiens (Human)) | BDBM50563230 (CHEMBL4754949) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Eu3+-labelled H2 relaxin from human RXFP1 expressed in human HEK-293T cells in presence of 10 % FCS by competition binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01533 BindingDB Entry DOI: 10.7270/Q2XW4PHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM82253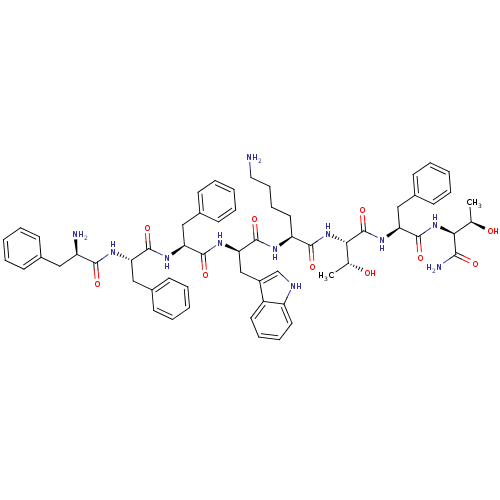 (BIM 23052 | CAS_133073-82-2) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50282638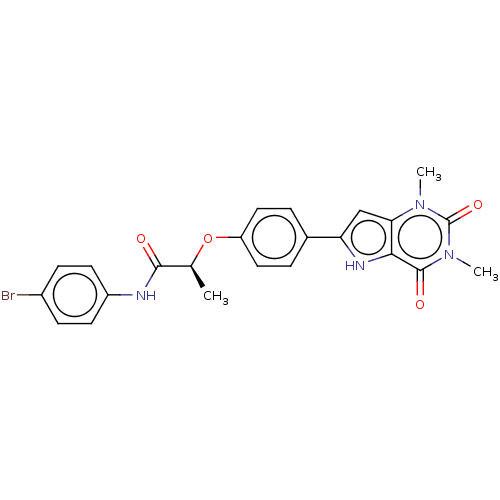 (CHEMBL4175851) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Estadual de Feira de Santana Curated by ChEMBL | Assay Description Antagonist activity at Adenosine receptor subtype 2B in human RBC assessed as change in erythrocyte morphology incubated for 3 hrs with occasional ge... | Eur J Med Chem 136: 487-496 (2017) Article DOI: 10.1016/j.ejmech.2017.05.035 BindingDB Entry DOI: 10.7270/Q2RJ4N1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057428 (CHEMBL22682 | [2-(6-Chloro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175570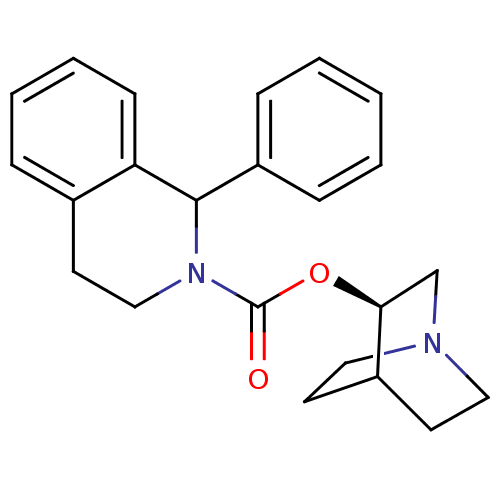 ((S)-1-Phenyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50019568 (Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175581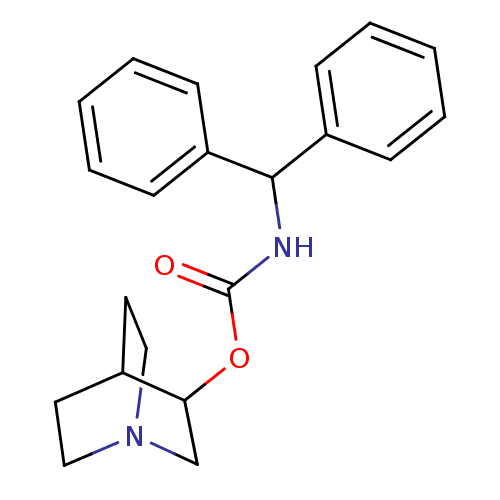 (Benzhydryl-carbamic acid 1-aza-bicyclo[2.2.2]oct-3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50019568 (Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute Curated by PDSP Ki Database | J Clin Invest 99: 789-98 (1997) Article DOI: 10.1172/JCI119225 BindingDB Entry DOI: 10.7270/Q2H70DCK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057434 (8-{2-[4-(4-Methoxy-phenyl)-butylamino]-ethoxy}-chr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057431 (CHEMBL21475 | [4-(4-Methoxy-phenyl)-butyl]-[2-(6-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057435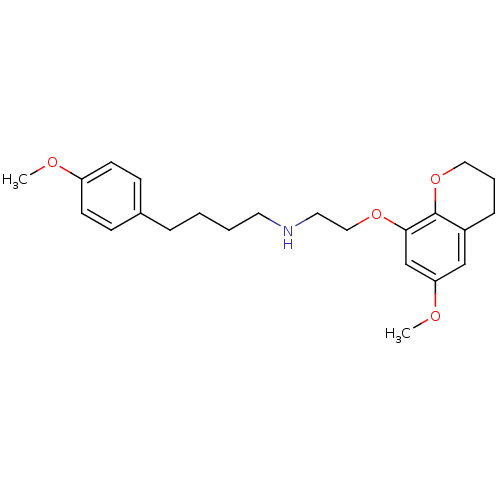 (CHEMBL25663 | [2-(6-Methoxy-chroman-8-yloxy)-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro radioligand binding assay on Dopamine receptor D2 of rat striatum using [3H]- spiperone | J Med Chem 40: 1252-7 (1997) Article DOI: 10.1021/jm960760d BindingDB Entry DOI: 10.7270/Q20R9Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175575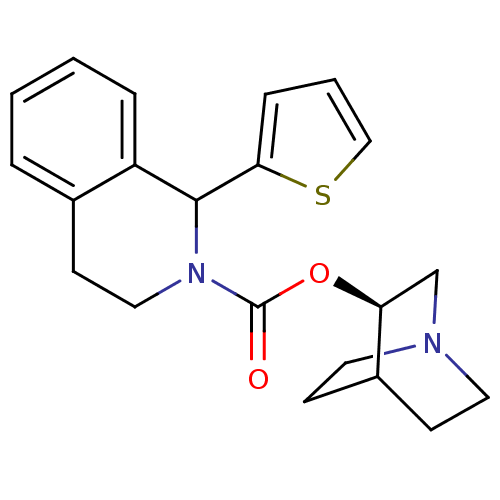 (1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50282631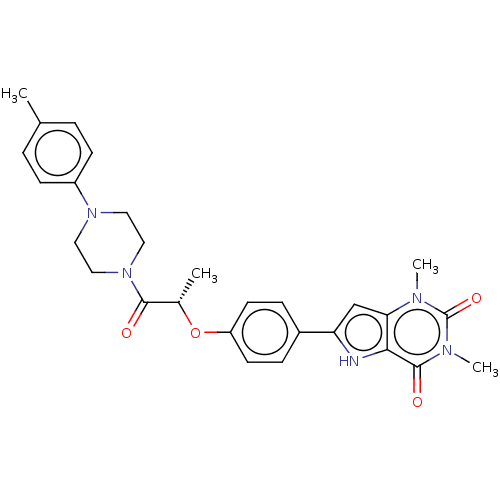 (CHEMBL4175454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Estadual de Feira de Santana Curated by ChEMBL | Assay Description Antagonist activity at Adenosine receptor subtype 2B in human RBC assessed as change in erythrocyte morphology incubated for 3 hrs with occasional ge... | Eur J Med Chem 136: 487-496 (2017) Article DOI: 10.1016/j.ejmech.2017.05.035 BindingDB Entry DOI: 10.7270/Q2RJ4N1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2730 total ) | Next | Last >> |