 Found 84 hits with Last Name = 'ivy carroll' and Initial = 'f'
Found 84 hits with Last Name = 'ivy carroll' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86812

(CAS_45263784 | NSC_45263784 | rac-2-(6-fluoro-5-(4...)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:11:16:19.20:22| Show InChI InChI=1S/C17H16FN3O2/c18-17-15(10-1-4-13(5-2-10)21(22)23)7-11(9-19-17)14-8-12-3-6-16(14)20-12/h1-2,4-5,7,9,12,14,16,20H,3,6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50100717
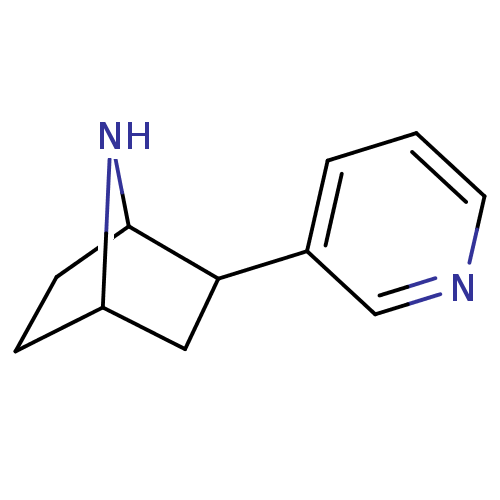
(2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86815
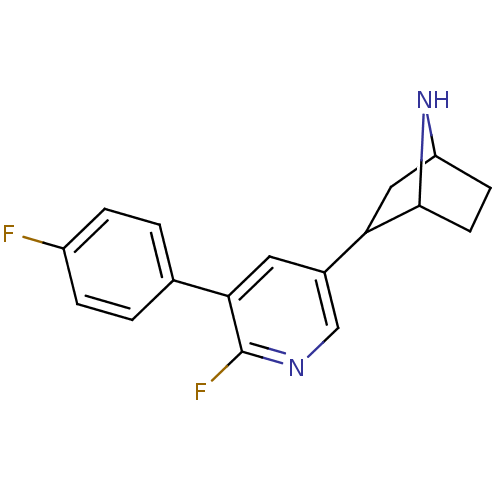
(CAS_45266019 | NSC_45266019 | rac-2-(6-fluoro-5-(4...)Show SMILES Fc1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H16F2N2/c18-12-3-1-10(2-4-12)15-7-11(9-20-17(15)19)14-8-13-5-6-16(14)21-13/h1-4,7,9,13-14,16,21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86816

(CAS_45263769 | NSC_45263769 | rac-2-(5-(4-chloroph...)Show SMILES Fc1ncc(cc1-c1ccc(Cl)cc1)C1CC2CCC1N2 |TLB:4:14:17.18:20| Show InChI InChI=1S/C17H16ClFN2/c18-12-3-1-10(2-4-12)15-7-11(9-20-17(15)19)14-8-13-5-6-16(14)21-13/h1-4,7,9,13-14,16,21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86810
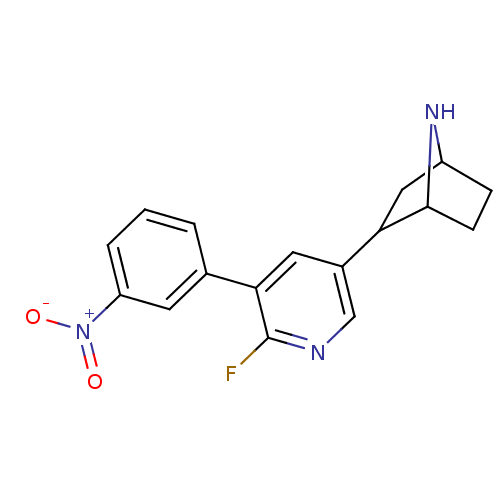
(CAS_45266065 | NSC_45266065 | rac-2-(6-fluoro-5-(3...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:11:16:19.20:22| Show InChI InChI=1S/C17H16FN3O2/c18-17-15(10-2-1-3-13(6-10)21(22)23)7-11(9-19-17)14-8-12-4-5-16(14)20-12/h1-3,6-7,9,12,14,16,20H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86805
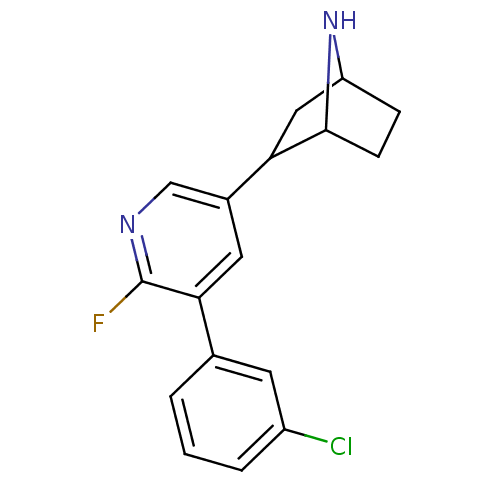
(CAS_45263788 | NSC_45263788 | US9150581, RTI-7527-...)Show SMILES Fc1ncc(cc1-c1cccc(Cl)c1)C1CC2CCC1N2 |TLB:4:14:17.18:20| Show InChI InChI=1S/C17H16ClFN2/c18-12-3-1-2-10(6-12)15-7-11(9-20-17(15)19)14-8-13-4-5-16(14)21-13/h1-3,6-7,9,13-14,16,21H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86819

(CAS_45263772 | NSC_45263772 | rac-2-(6-fluoro-5-(3...)Show SMILES Fc1cccc(c1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H16F2N2/c18-12-3-1-2-10(6-12)15-7-11(9-20-17(15)19)14-8-13-4-5-16(14)21-13/h1-3,6-7,9,13-14,16,21H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86817
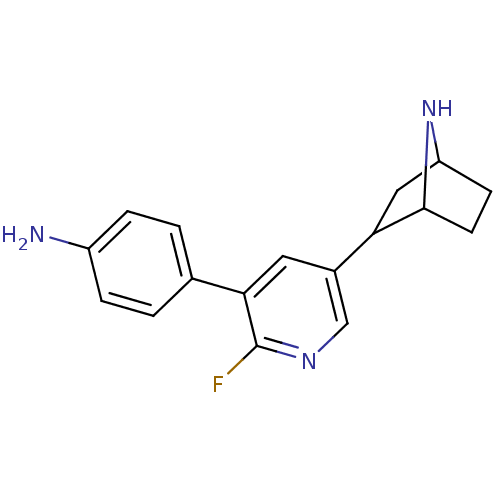
(CAS_45263779 | NSC_45263779 | rac-4-(5-(7-aza-bicy...)Show SMILES Nc1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H18FN3/c18-17-15(10-1-3-12(19)4-2-10)7-11(9-20-17)14-8-13-5-6-16(14)21-13/h1-4,7,9,13-14,16,21H,5-6,8,19H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86807
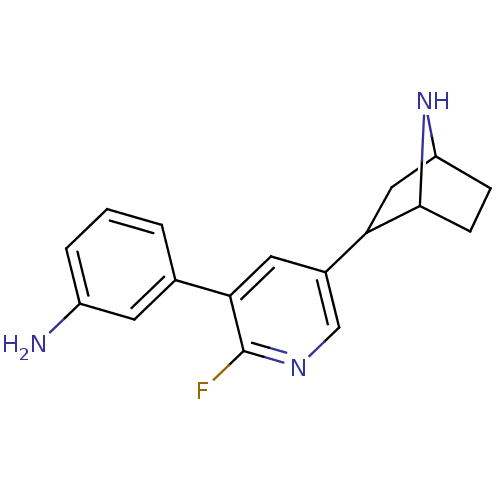
(CAS_45263775 | NSC_45263775 | rac-3-(5-(7-aza-bicy...)Show SMILES Nc1cccc(c1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H18FN3/c18-17-15(10-2-1-3-12(19)6-10)7-11(9-20-17)14-8-13-4-5-16(14)21-13/h1-3,6-7,9,13-14,16,21H,4-5,8,19H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86818

(CAS_45266054 | NSC_45266054 | rac-2-(6-fluoro-5-(3...)Show SMILES COc1cccc(c1)-c1cc(cnc1F)C1CC2CCC1N2 |THB:10:15:18.19:21| Show InChI InChI=1S/C18H19FN2O/c1-22-14-4-2-3-11(7-14)16-8-12(10-20-18(16)19)15-9-13-5-6-17(15)21-13/h2-4,7-8,10,13,15,17,21H,5-6,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86809

(3'-(4-fluorophenyl)deschloroepibatidine | CAS_...)Show SMILES Fc1ccc(cc1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H17FN2/c18-14-3-1-11(2-4-14)12-7-13(10-19-9-12)16-8-15-5-6-17(16)20-15/h1-4,7,9-10,15-17,20H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86820

(CAS_45263774 | NSC_45263774 | rac-3'-(3-chloro...)Show SMILES Clc1cccc(c1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H17ClN2/c18-14-3-1-2-11(7-14)12-6-13(10-19-9-12)16-8-15-4-5-17(16)20-15/h1-3,6-7,9-10,15-17,20H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86822

(CAS_45263767 | NSC_45263767 | rac-3'-(3-nitrop...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1cncc(c1)C1CC2CCC1N2 |TLB:13:15:18.19:21| Show InChI InChI=1S/C17H17N3O2/c21-20(22)15-3-1-2-11(7-15)12-6-13(10-18-9-12)16-8-14-4-5-17(16)19-14/h1-3,6-7,9-10,14,16-17,19H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298610
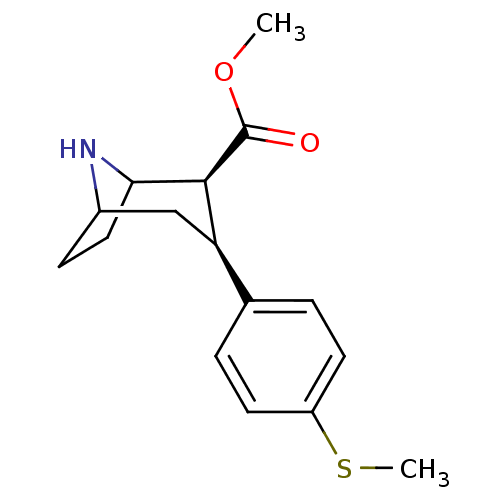
(3beta-(4-Methylthiophenyl)nortropane-2beta-carboxy...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC)cc1)N2 |r,TLB:11:10:19:7.6,THB:2:4:19:7.6| Show InChI InChI=1S/C16H21NO2S/c1-19-16(18)15-13(9-11-5-8-14(15)17-11)10-3-6-12(20-2)7-4-10/h3-4,6-7,11,13-15,17H,5,8-9H2,1-2H3/t11?,13-,14?,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86821
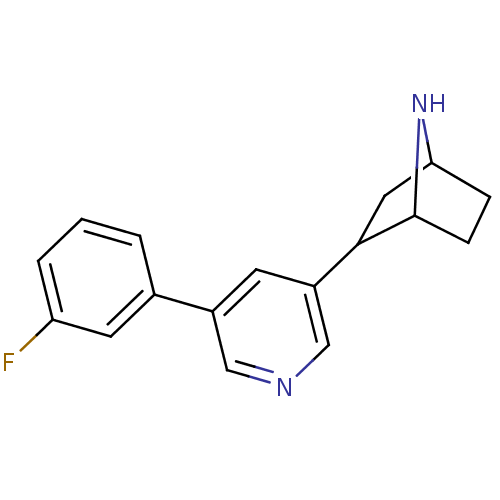
(CAS_45263766 | NSC_45263766 | rac-3'-(3-fluoro...)Show SMILES Fc1cccc(c1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H17FN2/c18-14-3-1-2-11(7-14)12-6-13(10-19-9-12)16-8-15-4-5-17(16)20-15/h1-3,6-7,9-10,15-17,20H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86811

(3-(4-chlorophenyl)deschloroepibatidine | CAS_44452...)Show SMILES Clc1ccc(cc1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H17ClN2/c18-14-3-1-11(2-4-14)12-7-13(10-19-9-12)16-8-15-5-6-17(16)20-15/h1-4,7,9-10,15-17,20H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86806
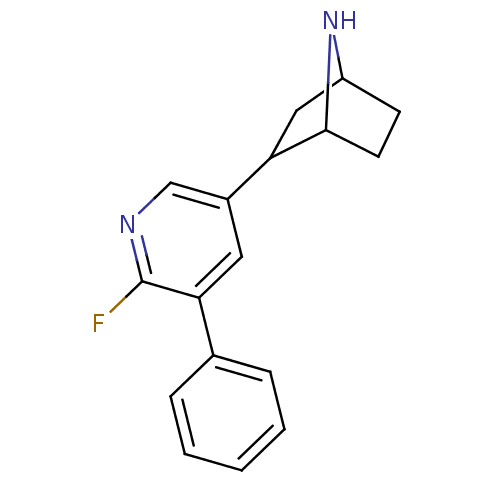
(CAS_45263762 | NSC_45263762 | US9150581, RTI-7527-...)Show SMILES Fc1ncc(cc1-c1ccccc1)C1CC2CCC1N2 |TLB:4:13:16.17:19| Show InChI InChI=1S/C17H17FN2/c18-17-15(11-4-2-1-3-5-11)8-12(10-19-17)14-9-13-6-7-16(14)20-13/h1-5,8,10,13-14,16,20H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86814

(CAS_45266060 | NSC_45266060 | rac-3'-(4-nitrop...)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1cncc(c1)C1CC2CCC1N2 |TLB:13:15:18.19:21| Show InChI InChI=1S/C17H17N3O2/c21-20(22)15-4-1-11(2-5-15)12-7-13(10-18-9-12)16-8-14-3-6-17(16)19-14/h1-2,4-5,7,9-10,14,16-17,19H,3,6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86813

(CAS_45266021 | NSC_45266021 | rac-3-(4-aminophenyl...)Show SMILES Nc1ccc(cc1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H19N3/c18-14-3-1-11(2-4-14)12-7-13(10-19-9-12)16-8-15-5-6-17(16)20-15/h1-4,7,9-10,15-17,20H,5-6,8,18H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86823

(CAS_45263792 | NSC_45263792 | rac-3-(3-methoxyphen...)Show SMILES COc1cccc(c1)-c1cncc(c1)C1CC2CCC1N2 |THB:12:14:17.18:20| Show InChI InChI=1S/C18H20N2O/c1-21-16-4-2-3-12(8-16)13-7-14(11-19-10-13)17-9-15-5-6-18(17)20-15/h2-4,7-8,10-11,15,17-18,20H,5-6,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86824
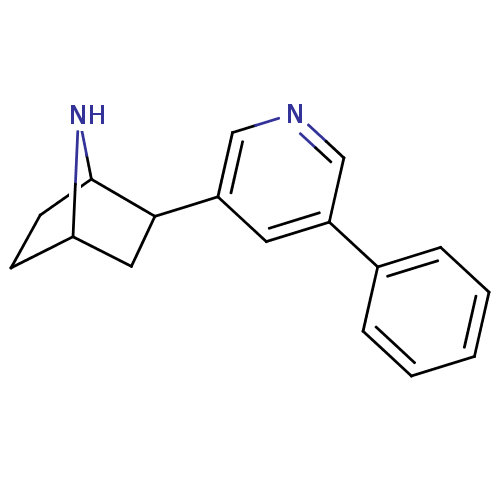
(CAS_45266048 | NSC_45266048 | rac-3-phenyldeschlor...)Show SMILES C1CC2NC1CC2c1cncc(c1)-c1ccccc1 |TLB:7:6:0.1:3| Show InChI InChI=1S/C17H18N2/c1-2-4-12(5-3-1)13-8-14(11-18-10-13)16-9-15-6-7-17(16)19-15/h1-5,8,10-11,15-17,19H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298606
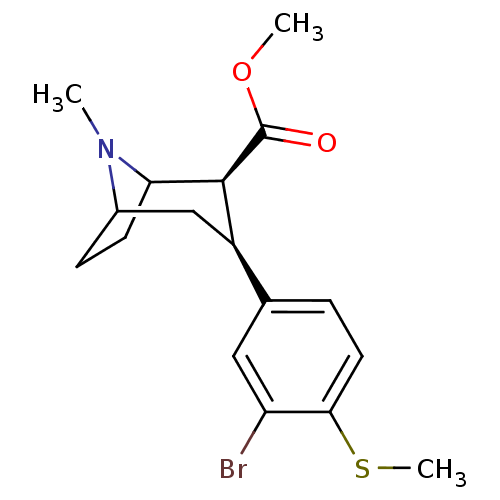
(3beta-(3-Bromo-4-methylthiophenyl)tropane-2beta-ca...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC)c(Br)c1)N2C |r,TLB:11:10:20:7.6,THB:2:4:20:7.6| Show InChI InChI=1S/C17H22BrNO2S/c1-19-11-5-6-14(19)16(17(20)21-2)12(9-11)10-4-7-15(22-3)13(18)8-10/h4,7-8,11-12,14,16H,5-6,9H2,1-3H3/t11?,12-,14?,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298602
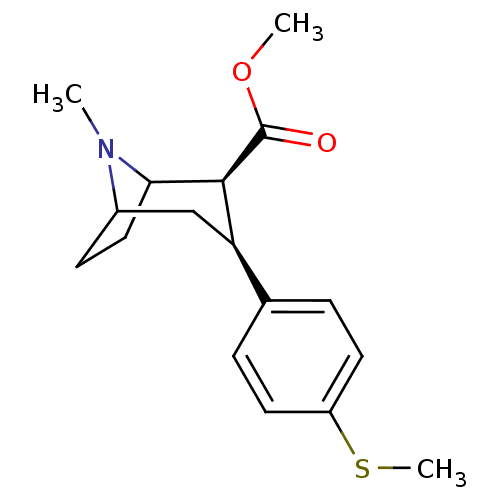
(3beta-(4-Methylthiophenyl)tropane-2beta-carboxylic...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC)cc1)N2C |r,TLB:11:10:19:7.6,THB:2:4:19:7.6| Show InChI InChI=1S/C17H23NO2S/c1-18-12-6-9-15(18)16(17(19)20-2)14(10-12)11-4-7-13(21-3)8-5-11/h4-5,7-8,12,14-16H,6,9-10H2,1-3H3/t12?,14-,15?,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50198443
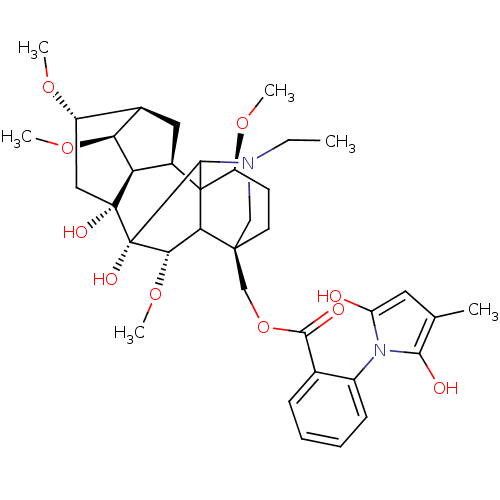
(CHEMBL223494 | [(2R,3R,4S,5R,6S,8R,9R,13S,16S,18S)...)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)C34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,44.48,42.46,36.39,39.43,wD:35.37,31.32,29.31,32.34,25.27,TLB:26:25:47:48.3.2,45:44:28:4.3.2,45:44:36.29.35:48,37:36:44.47:48,38:36:44.47:48,5:4:44.42:28,28:29:32:36.39.38,THB:2:48:44.47:36.29.35,1:2:44.42:28,1:2:24.23.25:47,4:47:36.29.35:48,23:4:44.42:28,25:28:44.42:4.3.2,36:42:28:4.3.2,42:48:24.23.25:47,29:28:44.42:4.3.2,30:29:44.47:48,(-6.09,1.27,;-5.33,-.06,;-3.79,-.06,;-5,-2.01,;-3.5,-1.62,;-3.5,-3.16,;-4.84,-3.93,;-5.98,-4.95,;-7.41,-4.44,;-5.66,-6.46,;-4.2,-6.93,;-3.88,-8.44,;-5.03,-9.47,;-6.49,-8.99,;-6.79,-7.48,;-8.26,-7,;-9.51,-7.9,;-9.51,-9.44,;-10.75,-7,;-10.26,-5.54,;-11.17,-4.29,;-8.73,-5.53,;-8.47,-4.09,;-4.82,-.86,;-4.82,.68,;-3.5,1.46,;-3.95,3.19,;-5.94,3.32,;-2.18,.68,;-.92,1.67,;-1.04,3.14,;.32,3.71,;1.28,2.6,;2.81,2.74,;3.7,1.47,;.53,1.34,;1.18,-.03,;2.5,-.8,;4.95,.06,;4.95,3.78,;6.03,4.88,;7.51,4.48,;.58,-1.42,;1.58,-2.58,;-.95,-1.81,;-1.27,-3.33,;-.12,-4.34,;-2.18,-.86,;-2.77,2.27,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22-,24+,25+,27-,28+,29?,30+,33?,34+,35-,36?,37+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoMLA from alpha7 nAChR in rat cerebral cortex |
Bioorg Med Chem 15: 678-85 (2006)
Article DOI: 10.1016/j.bmc.2006.10.061
BindingDB Entry DOI: 10.7270/Q20K287P |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86808
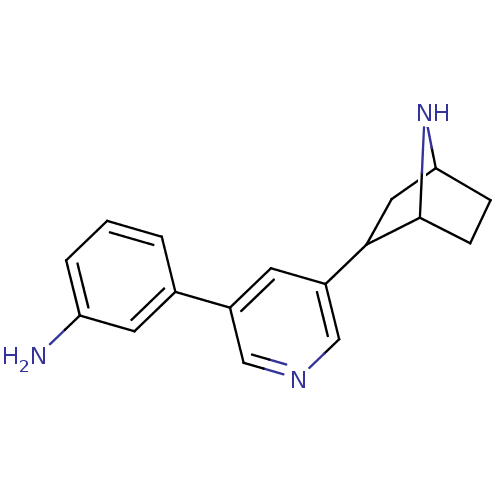
(CAS_45263778 | NSC_45263778 | rac-3'-(3-aminop...)Show SMILES Nc1cccc(c1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H19N3/c18-14-3-1-2-11(7-14)12-6-13(10-19-9-12)16-8-15-4-5-17(16)20-15/h1-3,6-7,9-10,15-17,20H,4-5,8,18H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298611
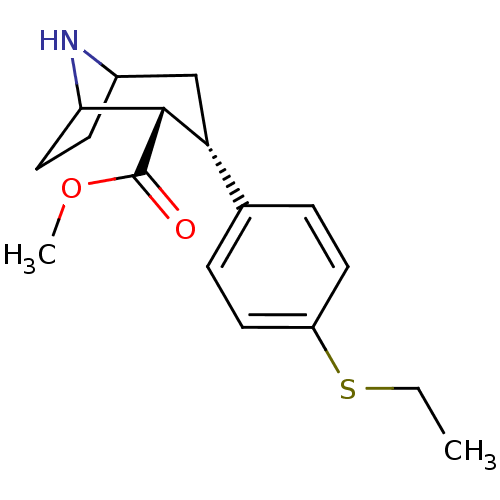
(3beta-(4-Ethylthiophenyl)nortropane-2beta-carboxyl...)Show SMILES CCSc1ccc(cc1)[C@H]1CC2CCC(N2)[C@H]1C(=O)OC |r,TLB:6:9:15:12.13,THB:17:16:15:12.13| Show InChI InChI=1S/C17H23NO2S/c1-3-21-13-7-4-11(5-8-13)14-10-12-6-9-15(18-12)16(14)17(19)20-2/h4-5,7-8,12,14-16,18H,3,6,9-10H2,1-2H3/t12?,14-,15?,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50198446
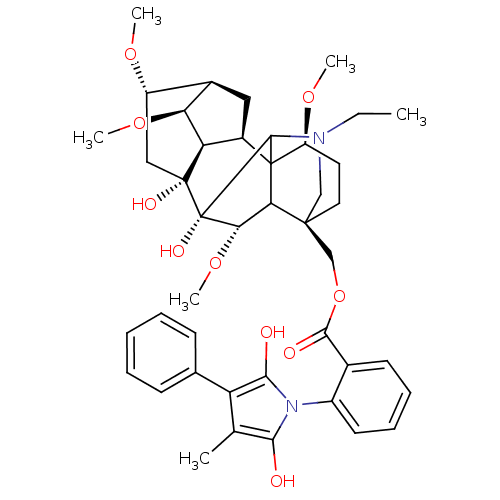
(CHEMBL413306 | phenyllycaconitine)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)c(C)c(c3O)-c3ccccc3)CC[C@H](OC)C34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,50.55,48.53,42.46,45.50,wD:41.44,37.39,35.38,38.41,31.34,TLB:51:50:34:4.3.2,51:50:42.35.41:54,32:31:53:54.3.2,43:42:50.53:54,44:42:50.53:54,5:4:50.48:34,34:35:38:42.45.44,THB:1:2:50.48:34,1:2:30.29.31:53,2:54:50.53:42.35.41,4:53:42.35.41:54,29:4:50.48:34,31:34:50.48:4.3.2,42:48:34:4.3.2,48:54:30.29.31:53,35:34:50.48:4.3.2,36:35:50.53:54,(12.79,-18.76,;13.55,-20.1,;15.09,-20.1,;13.87,-22.05,;15.37,-21.66,;15.38,-23.2,;14.04,-23.96,;12.89,-24.99,;11.47,-24.48,;13.22,-26.5,;14.67,-26.97,;15,-28.48,;13.84,-29.5,;12.39,-29.03,;12.08,-27.52,;10.62,-27.04,;9.37,-27.94,;9.37,-29.48,;8.13,-27.03,;6.67,-27.5,;8.61,-25.58,;10.14,-25.57,;10.41,-24.13,;8.17,-24.1,;9.24,-22.98,;8.8,-21.5,;7.3,-21.14,;6.24,-22.27,;6.68,-23.74,;14.05,-20.9,;14.05,-19.36,;15.37,-18.58,;14.92,-16.85,;12.94,-16.72,;16.7,-19.36,;17.96,-18.37,;17.84,-16.9,;19.19,-16.33,;20.16,-17.44,;21.69,-17.3,;22.57,-18.57,;19.4,-18.7,;20.06,-20.07,;21.38,-20.84,;23.83,-19.98,;23.83,-16.26,;24.91,-15.16,;26.39,-15.55,;19.46,-21.45,;20.46,-22.62,;17.93,-21.85,;17.61,-23.36,;18.76,-24.38,;16.7,-20.9,;16.11,-17.77,)| Show InChI InChI=1S/C43H54N2O10/c1-7-44-21-40(22-55-38(48)25-15-11-12-16-28(25)45-36(46)23(2)31(37(45)47)24-13-9-8-10-14-24)18-17-30(52-4)42-27-19-26-29(51-3)20-41(49,32(27)33(26)53-5)43(50,39(42)44)35(54-6)34(40)42/h8-16,26-27,29-30,32-35,39,46-47,49-50H,7,17-22H2,1-6H3/t26-,27-,29+,30+,32-,33+,34?,35+,39?,40+,41-,42?,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoMLA from alpha7 nAChR in rat cerebral cortex |
Bioorg Med Chem 15: 678-85 (2006)
Article DOI: 10.1016/j.bmc.2006.10.061
BindingDB Entry DOI: 10.7270/Q20K287P |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50198445
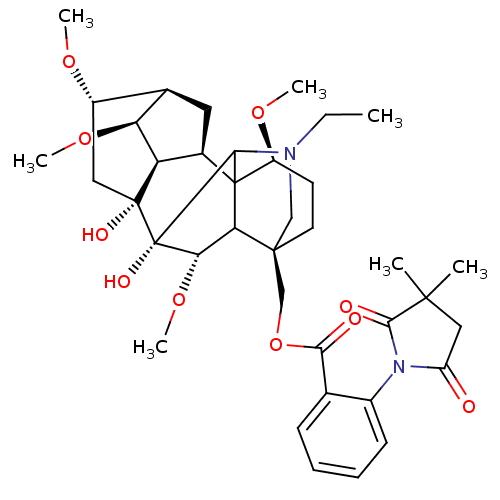
(2,2-dimethyllycaconitine | CHEMBL387363)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3N3C(=O)CC(C)(C)C3=O)CC[C@H](OC)C34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |TLB:46:45:29:4.3.2,46:45:37.30.36:49,27:26:48:49.3.2,38:37:45.48:49,39:37:45.48:49,5:4:45.43:29,29:30:33:37.40.39,THB:1:2:45.43:29,1:2:25.24.26:48,2:49:45.48:37.30.36,4:48:37.30.36:49,24:4:45.43:29,26:29:45.43:4.3.2,37:43:29:4.3.2,43:49:25.24.26:48,30:29:45.43:4.3.2,31:30:45.48:49| Show InChI InChI=1S/C38H52N2O10/c1-8-39-18-35(19-50-31(42)20-11-9-10-12-23(20)40-26(41)17-34(2,3)33(40)43)14-13-25(47-5)37-22-15-21-24(46-4)16-36(44,27(22)28(21)48-6)38(45,32(37)39)30(49-7)29(35)37/h9-12,21-22,24-25,27-30,32,44-45H,8,13-19H2,1-7H3/t21-,22-,24+,25+,27-,28+,29?,30+,32?,35+,36-,37?,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoMLA from alpha7 nAChR in rat cerebral cortex |
Bioorg Med Chem 15: 678-85 (2006)
Article DOI: 10.1016/j.bmc.2006.10.061
BindingDB Entry DOI: 10.7270/Q20K287P |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50198442

((R)-methyllycaconitine | CHEMBL387362)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)C34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,44.48,42.46,36.39,39.43,wD:35.37,31.32,29.31,32.34,25.27,TLB:45:44:28:4.3.2,45:44:36.29.35:48,26:25:47:48.3.2,37:36:44.47:48,38:36:44.47:48,5:4:44.42:28,28:29:32:36.39.38,THB:1:2:44.42:28,1:2:24.23.25:47,2:48:44.47:36.29.35,4:47:36.29.35:48,23:4:44.42:28,25:28:44.42:4.3.2,36:42:28:4.3.2,42:48:24.23.25:47,29:28:44.42:4.3.2,30:29:44.47:48,(14.18,3.38,;14.94,2.04,;16.48,2.04,;15.27,.09,;16.77,.48,;16.77,-1.06,;15.43,-1.82,;14.29,-2.85,;12.86,-2.34,;14.61,-4.36,;16.07,-4.83,;16.39,-6.34,;15.24,-7.36,;13.78,-6.89,;13.48,-5.38,;12.01,-4.9,;10.76,-5.8,;10.76,-7.34,;9.52,-4.89,;10.01,-3.44,;8.47,-3.42,;11.54,-3.43,;11.8,-1.99,;15.45,1.24,;15.45,2.78,;16.77,3.56,;16.32,5.3,;14.33,5.42,;18.09,2.78,;19.35,3.78,;19.23,5.25,;20.59,5.82,;21.55,4.7,;23.08,4.84,;23.97,3.57,;20.8,3.44,;21.45,2.07,;22.77,1.3,;25.22,2.16,;25.22,5.88,;26.3,6.98,;27.78,6.59,;20.85,.69,;21.85,-.48,;19.32,.29,;19,-1.22,;20.15,-2.24,;18.1,1.24,;17.5,4.37,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22-,24+,25+,27-,28+,29?,30+,33?,34+,35-,36?,37+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoMLA from alpha7 nAChR in rat cerebral cortex |
Bioorg Med Chem 15: 678-85 (2006)
Article DOI: 10.1016/j.bmc.2006.10.061
BindingDB Entry DOI: 10.7270/Q20K287P |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50198444
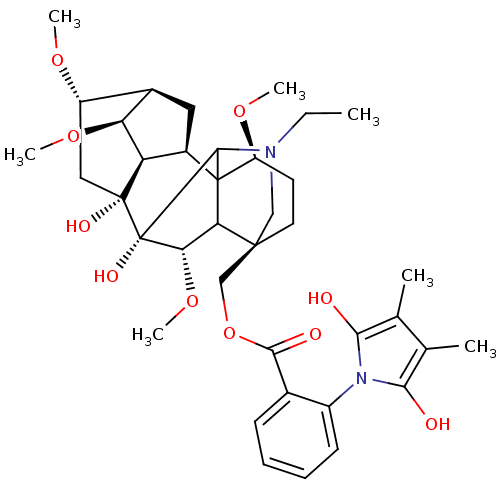
(2,3-dimethyllycaconitine | CHEMBL442048)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)c(C)c(C)c3O)CC[C@H](OC)C34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,45.49,43.47,37.40,40.44,wD:36.38,32.33,30.32,33.35,26.28,TLB:46:45:29:4.3.2,46:45:37.30.36:49,27:26:48:49.3.2,38:37:45.48:49,39:37:45.48:49,5:4:45.43:29,29:30:33:37.40.39,THB:1:2:45.43:29,1:2:25.24.26:48,2:49:45.48:37.30.36,4:48:37.30.36:49,24:4:45.43:29,26:29:45.43:4.3.2,37:43:29:4.3.2,43:49:25.24.26:48,30:29:45.43:4.3.2,31:30:45.48:49,(-5.77,-17.21,;-5.01,-18.54,;-3.47,-18.54,;-4.69,-20.5,;-3.19,-20.1,;-3.18,-21.64,;-4.52,-22.41,;-5.67,-23.44,;-7.09,-22.92,;-5.35,-24.94,;-3.89,-25.41,;-3.56,-26.93,;-4.72,-27.95,;-6.17,-27.48,;-6.48,-25.96,;-7.94,-25.48,;-8.42,-24.01,;-8.15,-22.57,;-9.95,-24.02,;-9.96,-22.48,;-10.43,-25.48,;-11.89,-25.94,;-9.19,-26.38,;-9.19,-27.92,;-4.51,-19.34,;-4.51,-17.8,;-3.19,-17.02,;-3.64,-15.29,;-5.62,-15.17,;-1.86,-17.8,;-.6,-16.81,;-.72,-15.34,;.63,-14.77,;1.6,-15.88,;3.13,-15.75,;4.01,-17.01,;.84,-17.14,;1.5,-18.51,;2.82,-19.28,;5.27,-18.42,;5.27,-14.7,;6.35,-13.61,;7.83,-14,;.9,-19.9,;1.9,-21.06,;-.63,-20.3,;-.95,-21.81,;.2,-22.82,;-1.86,-19.34,;-2.45,-16.21,)| Show InChI InChI=1S/C38H52N2O10/c1-8-39-17-35(18-50-33(43)21-11-9-10-12-24(21)40-31(41)19(2)20(3)32(40)42)14-13-26(47-5)37-23-15-22-25(46-4)16-36(44,27(23)28(22)48-6)38(45,34(37)39)30(49-7)29(35)37/h9-12,22-23,25-30,34,41-42,44-45H,8,13-18H2,1-7H3/t22-,23-,25+,26+,27-,28+,29?,30+,34?,35+,36-,37?,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoMLA from alpha7 nAChR in rat cerebral cortex |
Bioorg Med Chem 15: 678-85 (2006)
Article DOI: 10.1016/j.bmc.2006.10.061
BindingDB Entry DOI: 10.7270/Q20K287P |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298612
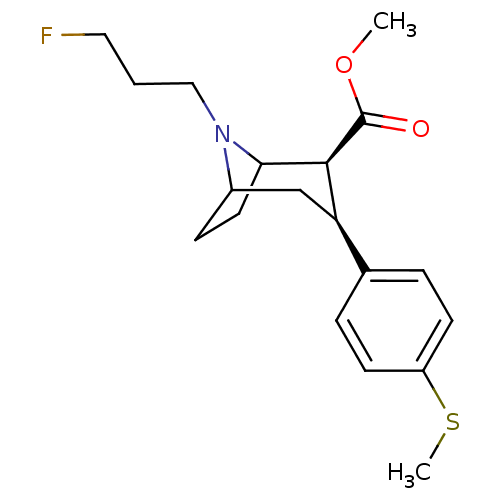
(3beta-(4-Methylthiophenyl)-8-(3-fluoropropyl)nortr...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC)cc1)N2CCCF |r,TLB:11:10:19:7.6,THB:2:4:19:7.6| Show InChI InChI=1S/C19H26FNO2S/c1-23-19(22)18-16(13-4-7-15(24-2)8-5-13)12-14-6-9-17(18)21(14)11-3-10-20/h4-5,7-8,14,16-18H,3,6,9-12H2,1-2H3/t14?,16-,17?,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298607
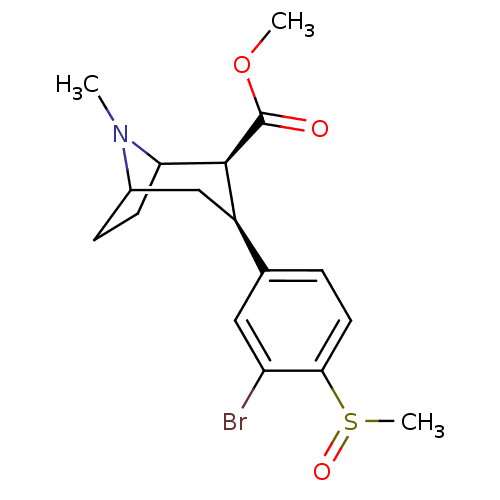
(3beta-(3-bromo-4-methylsulfinylphenyl)tropane-2bet...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(c(Br)c1)S(C)=O)N2C |r,TLB:11:10:21:7.6,THB:2:4:21:7.6| Show InChI InChI=1S/C17H22BrNO3S/c1-19-11-5-6-14(19)16(17(20)22-2)12(9-11)10-4-7-15(23(3)21)13(18)8-10/h4,7-8,11-12,14,16H,5-6,9H2,1-3H3/t11?,12-,14?,16+,23?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298608
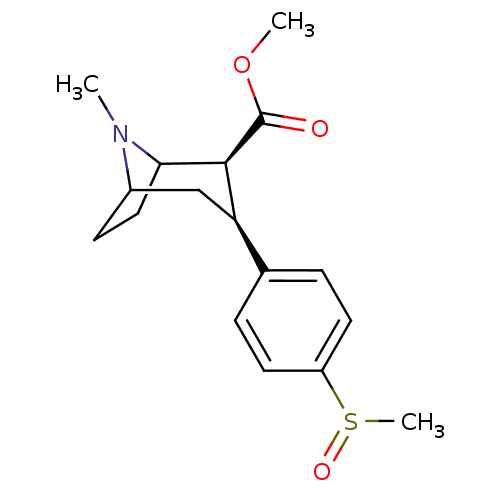
(3beta-(4-methylsulfinylphenyl)tropane-2beta-carbox...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(cc1)S(C)=O)N2C |r,TLB:11:10:20:7.6,THB:2:4:20:7.6| Show InChI InChI=1S/C17H23NO3S/c1-18-12-6-9-15(18)16(17(19)21-2)14(10-12)11-4-7-13(8-5-11)22(3)20/h4-5,7-8,12,14-16H,6,9-10H2,1-3H3/t12?,14-,15?,16+,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298618
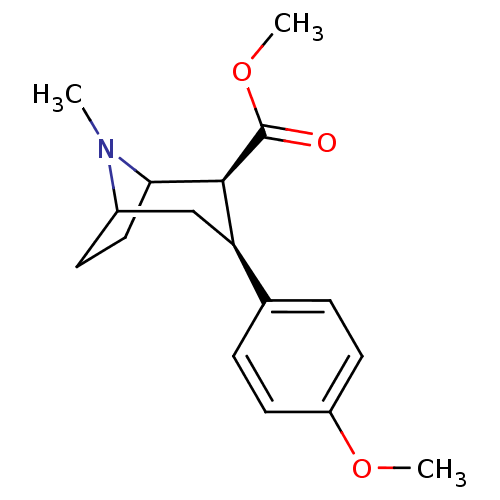
(3beta-(4-methoxyphenyl)tropane-2beta-carboxylic ac...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(OC)cc1)N2C |r,TLB:11:10:19:7.6,THB:2:4:19:7.6| Show InChI InChI=1S/C17H23NO3/c1-18-12-6-9-15(18)16(17(19)21-3)14(10-12)11-4-7-13(20-2)8-5-11/h4-5,7-8,12,14-16H,6,9-10H2,1-3H3/t12?,14-,15?,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298603
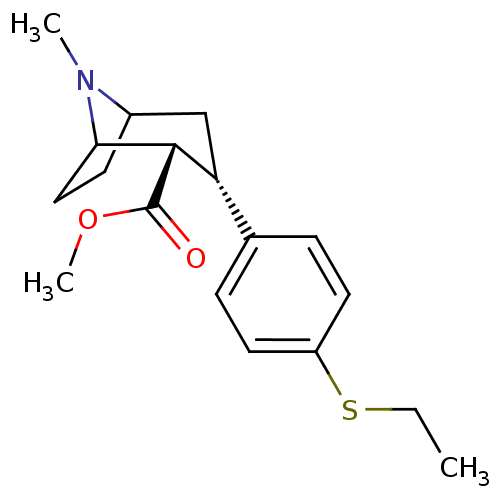
(3beta-(4-Ethylthiophenyl)tropane-2beta-carboxylic ...)Show SMILES CCSc1ccc(cc1)[C@H]1CC2CCC([C@H]1C(=O)OC)N2C |r,TLB:6:9:20:12.13,THB:16:15:20:12.13| Show InChI InChI=1S/C18H25NO2S/c1-4-22-14-8-5-12(6-9-14)15-11-13-7-10-16(19(13)2)17(15)18(20)21-3/h5-6,8-9,13,15-17H,4,7,10-11H2,1-3H3/t13?,15-,16?,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298614
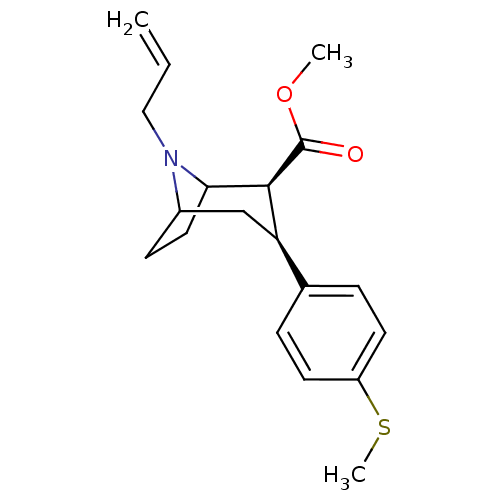
(3beta-(4-Methylthiophenyl)-8-allylnortropane-2beta...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC)cc1)N2CC=C |r,TLB:11:10:19:7.6,THB:2:4:19:7.6| Show InChI InChI=1S/C19H25NO2S/c1-4-11-20-14-7-10-17(20)18(19(21)22-2)16(12-14)13-5-8-15(23-3)9-6-13/h4-6,8-9,14,16-18H,1,7,10-12H2,2-3H3/t14?,16-,17?,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298616
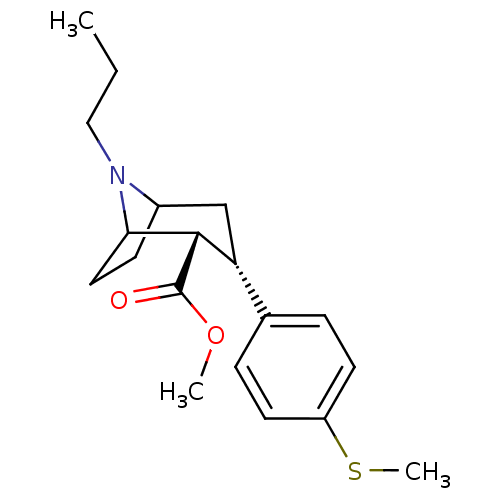
(3beta-(4-Methylthiophenyl)-8-propylnortropane-2bet...)Show SMILES CCCN1C2CCC1[C@H]([C@H](C2)c1ccc(SC)cc1)C(=O)OC |r,TLB:19:8:3:5.6,THB:11:9:3:5.6| Show InChI InChI=1S/C19H27NO2S/c1-4-11-20-14-7-10-17(20)18(19(21)22-2)16(12-14)13-5-8-15(23-3)9-6-13/h5-6,8-9,14,16-18H,4,7,10-12H2,1-3H3/t14?,16-,17?,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298605

(3beta-(4-Trifluoromethylthiophenyl)tropane-2beta-c...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC(F)(F)F)cc1)N2C |r,TLB:11:10:22:7.6,THB:2:4:22:7.6| Show InChI InChI=1S/C17H20F3NO2S/c1-21-11-5-8-14(21)15(16(22)23-2)13(9-11)10-3-6-12(7-4-10)24-17(18,19)20/h3-4,6-7,11,13-15H,5,8-9H2,1-2H3/t11?,13-,14?,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50298610

(3beta-(4-Methylthiophenyl)nortropane-2beta-carboxy...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC)cc1)N2 |r,TLB:11:10:19:7.6,THB:2:4:19:7.6| Show InChI InChI=1S/C16H21NO2S/c1-19-16(18)15-13(9-11-5-8-14(15)17-11)10-3-6-12(20-2)7-4-10/h3-4,6-7,11,13-15,17H,5,8-9H2,1-2H3/t11?,13-,14?,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298604
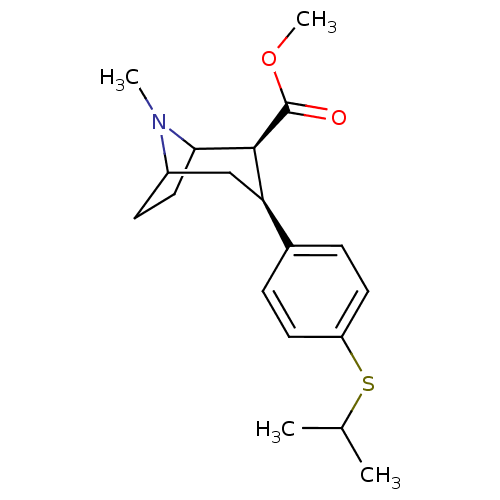
(3beta-(4-Isopropylthiophenyl)tropane-2beta-carboxy...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC(C)C)cc1)N2C |r,TLB:11:10:21:7.6,THB:2:4:21:7.6| Show InChI InChI=1S/C19H27NO2S/c1-12(2)23-15-8-5-13(6-9-15)16-11-14-7-10-17(20(14)3)18(16)19(21)22-4/h5-6,8-9,12,14,16-18H,7,10-11H2,1-4H3/t14?,16-,17?,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50198441

(CHEMBL267682 | cyclohexyllycaconitine)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)c(C)c(C4CCCCC4)c3O)CC[C@H](OC)C34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,50.55,48.53,42.46,45.50,wD:41.44,37.39,35.38,38.41,31.34,TLB:51:50:34:4.3.2,51:50:42.35.41:54,32:31:53:54.3.2,43:42:50.53:54,44:42:50.53:54,5:4:50.48:34,34:35:38:42.45.44,THB:1:2:50.48:34,1:2:30.29.31:53,2:54:50.53:42.35.41,4:53:42.35.41:54,29:4:50.48:34,31:34:50.48:4.3.2,42:48:34:4.3.2,48:54:30.29.31:53,35:34:50.48:4.3.2,36:35:50.53:54,(32.63,-18.97,;33.4,-20.3,;34.94,-20.3,;33.72,-22.26,;35.22,-21.86,;35.22,-23.4,;33.89,-24.17,;32.74,-25.2,;31.31,-24.68,;33.06,-26.7,;34.52,-27.17,;34.84,-28.69,;33.69,-29.71,;32.23,-29.24,;31.93,-27.72,;30.47,-27.24,;29.22,-28.14,;29.22,-29.68,;27.98,-27.24,;26.52,-27.71,;28.46,-25.78,;28.16,-24.27,;29.33,-23.25,;29.03,-21.74,;27.57,-21.24,;26.41,-22.26,;26.71,-23.77,;29.99,-25.77,;30.26,-24.34,;33.9,-21.1,;33.9,-19.56,;35.22,-18.78,;34.77,-17.05,;32.79,-16.93,;36.55,-19.56,;37.81,-18.57,;37.69,-17.1,;39.04,-16.53,;40.01,-17.64,;41.54,-17.51,;42.42,-18.77,;39.25,-18.91,;39.91,-20.27,;41.23,-21.04,;43.68,-20.18,;43.68,-16.46,;44.75,-15.37,;46.24,-15.76,;39.31,-21.66,;40.31,-22.82,;37.77,-22.06,;37.46,-23.57,;38.6,-24.59,;36.55,-21.1,;35.96,-17.97,)| Show InChI InChI=1S/C43H60N2O10/c1-7-44-21-40(22-55-38(48)25-15-11-12-16-28(25)45-36(46)23(2)31(37(45)47)24-13-9-8-10-14-24)18-17-30(52-4)42-27-19-26-29(51-3)20-41(49,32(27)33(26)53-5)43(50,39(42)44)35(54-6)34(40)42/h11-12,15-16,24,26-27,29-30,32-35,39,46-47,49-50H,7-10,13-14,17-22H2,1-6H3/t26-,27-,29+,30+,32-,33+,34?,35+,39?,40+,41-,42?,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoMLA from alpha7 nAChR in rat cerebral cortex |
Bioorg Med Chem 15: 678-85 (2006)
Article DOI: 10.1016/j.bmc.2006.10.061
BindingDB Entry DOI: 10.7270/Q20K287P |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298613
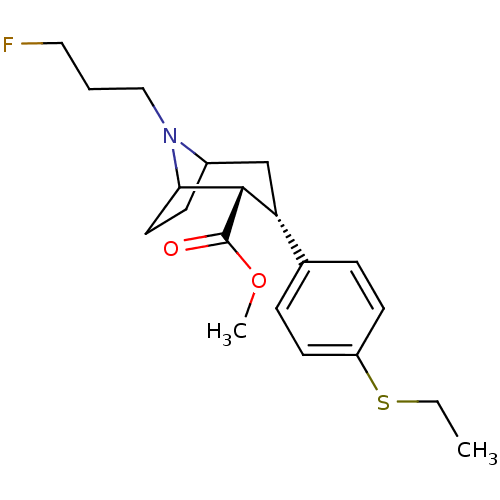
(3beta-(4-Ethylthiophenyl)-8-(3-fluoropropyl)nortro...)Show SMILES CCSc1ccc(cc1)[C@H]1CC2CCC([C@H]1C(=O)OC)N2CCCF |r,TLB:6:9:20:12.13,THB:16:15:20:12.13| Show InChI InChI=1S/C20H28FNO2S/c1-3-25-16-8-5-14(6-9-16)17-13-15-7-10-18(19(17)20(23)24-2)22(15)12-4-11-21/h5-6,8-9,15,17-19H,3-4,7,10-13H2,1-2H3/t15?,17-,18?,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298615

(3beta-(4-Ethylthiophenyl)-8-allylnortropane-2beta-...)Show SMILES CCSc1ccc(cc1)[C@H]1CC2CCC([C@H]1C(=O)OC)N2CC=C |r,TLB:6:9:20:12.13,THB:16:15:20:12.13| Show InChI InChI=1S/C20H27NO2S/c1-4-12-21-15-8-11-18(21)19(20(22)23-3)17(13-15)14-6-9-16(10-7-14)24-5-2/h4,6-7,9-10,15,17-19H,1,5,8,11-13H2,2-3H3/t15?,17-,18?,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50298617
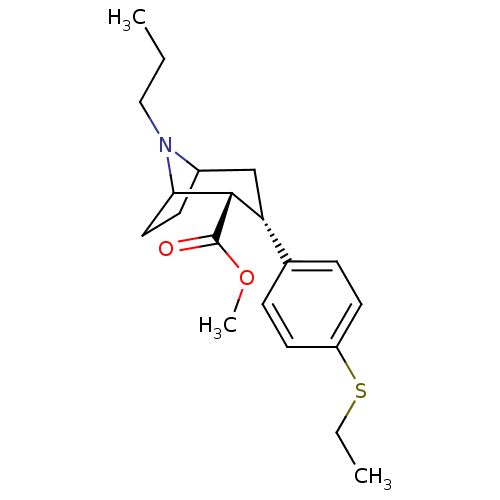
(3beta-(4-Ethylthiophenyl)-8-propylnortropane-2beta...)Show SMILES CCCN1C2CCC1[C@H]([C@H](C2)c1ccc(SCC)cc1)C(=O)OC |r,TLB:20:8:3:5.6,THB:11:9:3:5.6| Show InChI InChI=1S/C20H29NO2S/c1-4-12-21-15-8-11-18(21)19(20(22)23-3)17(13-15)14-6-9-16(10-7-14)24-5-2/h6-7,9-10,15,17-19H,4-5,8,11-13H2,1-3H3/t15?,17-,18?,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50084717
((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1C(=O)Oc1ccccc1)N2C |THB:2:4:20:6.7| Show InChI InChI=1S/C17H21NO4/c1-18-11-8-9-14(18)15(17(20)21-2)13(10-11)16(19)22-12-6-4-3-5-7-12/h3-7,11,13-15H,8-10H2,1-2H3/t11?,13-,14?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5-HTT |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50298611

(3beta-(4-Ethylthiophenyl)nortropane-2beta-carboxyl...)Show SMILES CCSc1ccc(cc1)[C@H]1CC2CCC(N2)[C@H]1C(=O)OC |r,TLB:6:9:15:12.13,THB:17:16:15:12.13| Show InChI InChI=1S/C17H23NO2S/c1-3-21-13-7-4-11(5-8-13)14-10-12-6-9-15(18-12)16(14)17(19)20-2/h4-5,7-8,12,14-16,18H,3,6,9-10H2,1-2H3/t12?,14-,15?,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50298606

(3beta-(3-Bromo-4-methylthiophenyl)tropane-2beta-ca...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1c1ccc(SC)c(Br)c1)N2C |r,TLB:11:10:20:7.6,THB:2:4:20:7.6| Show InChI InChI=1S/C17H22BrNO2S/c1-19-11-5-6-14(19)16(17(20)21-2)12(9-11)10-4-7-15(22-3)13(18)8-10/h4,7-8,11-12,14,16H,5-6,9H2,1-3H3/t11?,12-,14?,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
Bioorg Med Chem 17: 5126-32 (2009)
Article DOI: 10.1016/j.bmc.2009.05.052
BindingDB Entry DOI: 10.7270/Q2611186 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data