 Found 648 hits with Last Name = 'jacobs' and Initial = 'md'
Found 648 hits with Last Name = 'jacobs' and Initial = 'md' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463499
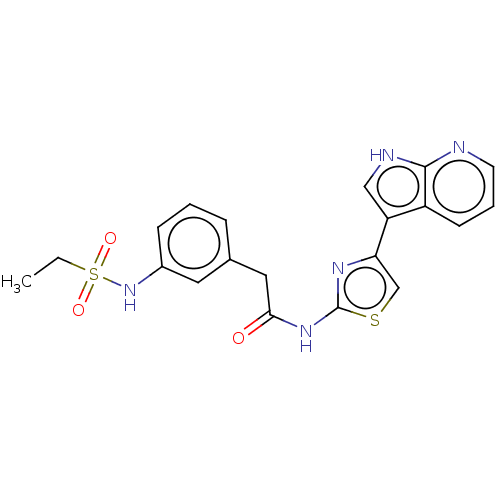
(CHEMBL4250547)Show SMILES CCS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C20H19N5O3S2/c1-2-30(27,28)25-14-6-3-5-13(9-14)10-18(26)24-20-23-17(12-29-20)16-11-22-19-15(16)7-4-8-21-19/h3-9,11-12,25H,2,10H2,1H3,(H,21,22)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463484
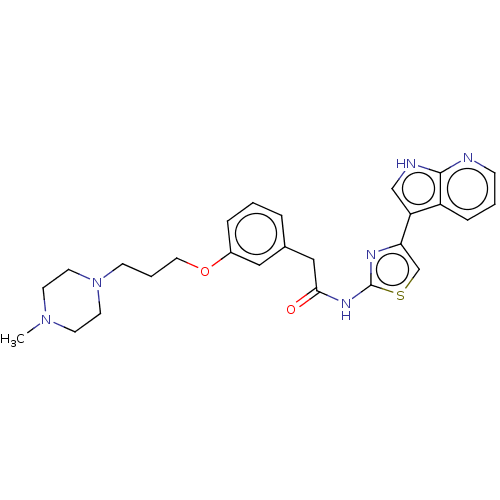
(CHEMBL4248525)Show SMILES CN1CCN(CCCOc2cccc(CC(=O)Nc3nc(cs3)-c3c[nH]c4ncccc34)c2)CC1 Show InChI InChI=1S/C26H30N6O2S/c1-31-10-12-32(13-11-31)9-4-14-34-20-6-2-5-19(15-20)16-24(33)30-26-29-23(18-35-26)22-17-28-25-21(22)7-3-8-27-25/h2-3,5-8,15,17-18H,4,9-14,16H2,1H3,(H,27,28)(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463498

(CHEMBL4241959)Show SMILES COCCNS(=O)(=O)c1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C21H21N5O4S2/c1-30-9-8-24-32(28,29)15-5-2-4-14(10-15)11-19(27)26-21-25-18(13-31-21)17-12-23-20-16(17)6-3-7-22-20/h2-7,10,12-13,24H,8-9,11H2,1H3,(H,22,23)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463492

(CHEMBL4241305)Show SMILES CCCS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C21H21N5O3S2/c1-2-9-31(28,29)26-15-6-3-5-14(10-15)11-19(27)25-21-24-18(13-30-21)17-12-23-20-16(17)7-4-8-22-20/h3-8,10,12-13,26H,2,9,11H2,1H3,(H,22,23)(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044287
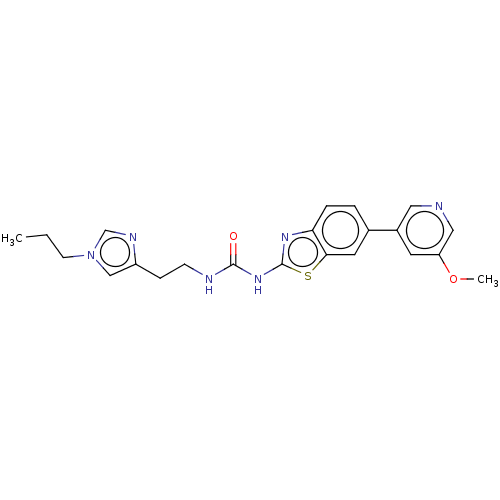
(CHEMBL3356900)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3ccc(cc3s2)-c2cncc(OC)c2)c1 Show InChI InChI=1S/C22H24N6O2S/c1-3-8-28-13-17(25-14-28)6-7-24-21(29)27-22-26-19-5-4-15(10-20(19)31-22)16-9-18(30-2)12-23-11-16/h4-5,9-14H,3,6-8H2,1-2H3,(H2,24,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463481

(CHEMBL4244717)Show SMILES CC(C)S(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C21H21N5O3S2/c1-13(2)31(28,29)26-15-6-3-5-14(9-15)10-19(27)25-21-24-18(12-30-21)17-11-23-20-16(17)7-4-8-22-20/h3-9,11-13,26H,10H2,1-2H3,(H,22,23)(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463500
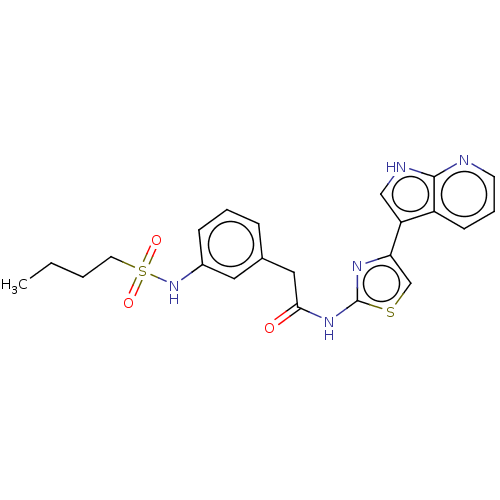
(CHEMBL4243658)Show SMILES CCCCS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C22H23N5O3S2/c1-2-3-10-32(29,30)27-16-7-4-6-15(11-16)12-20(28)26-22-25-19(14-31-22)18-13-24-21-17(18)8-5-9-23-21/h4-9,11,13-14,27H,2-3,10,12H2,1H3,(H,23,24)(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463495

(CHEMBL4244623)Show SMILES COc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H16N4O2S/c1-25-13-5-2-4-12(8-13)9-17(24)23-19-22-16(11-26-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11H,9H2,1H3,(H,20,21)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274571
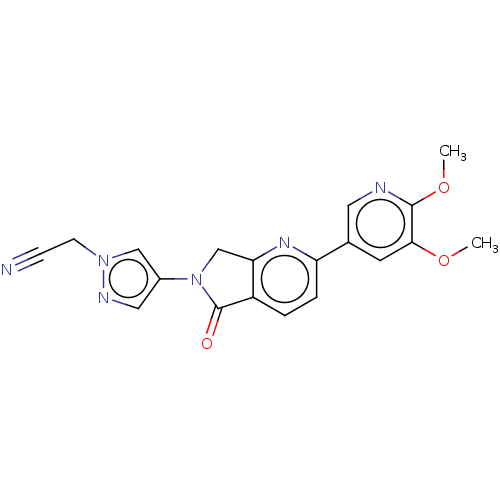
(CHEMBL4127784)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC#N)c1 Show InChI InChI=1S/C19H16N6O3/c1-27-17-7-12(8-21-18(17)28-2)15-4-3-14-16(23-15)11-25(19(14)26)13-9-22-24(10-13)6-5-20/h3-4,7-10H,6,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463489

(CHEMBL4245089)Show SMILES Oc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C18H14N4O2S/c23-12-4-1-3-11(7-12)8-16(24)22-18-21-15(10-25-18)14-9-20-17-13(14)5-2-6-19-17/h1-7,9-10,23H,8H2,(H,19,20)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463491
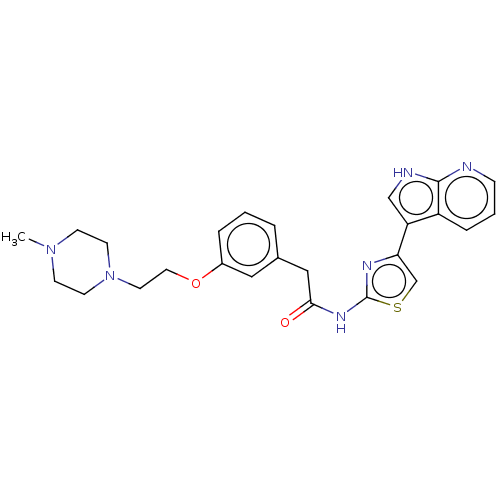
(CHEMBL4245197)Show SMILES CN1CCN(CCOc2cccc(CC(=O)Nc3nc(cs3)-c3c[nH]c4ncccc34)c2)CC1 Show InChI InChI=1S/C25H28N6O2S/c1-30-8-10-31(11-9-30)12-13-33-19-5-2-4-18(14-19)15-23(32)29-25-28-22(17-34-25)21-16-27-24-20(21)6-3-7-26-24/h2-7,14,16-17H,8-13,15H2,1H3,(H,26,27)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463490

(CHEMBL4240605)Show SMILES CCCNS(=O)(=O)c1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C21H21N5O3S2/c1-2-8-24-31(28,29)15-6-3-5-14(10-15)11-19(27)26-21-25-18(13-30-21)17-12-23-20-16(17)7-4-9-22-20/h3-7,9-10,12-13,24H,2,8,11H2,1H3,(H,22,23)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274538
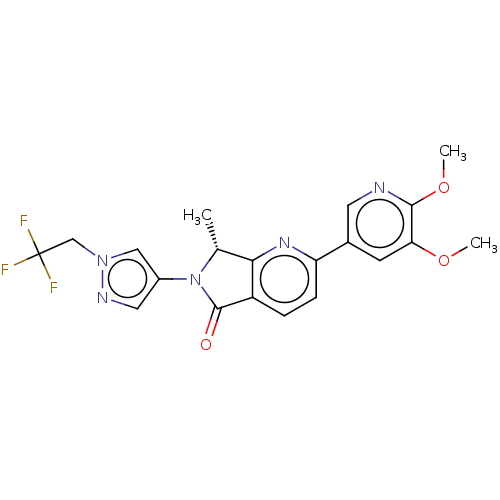
(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274537
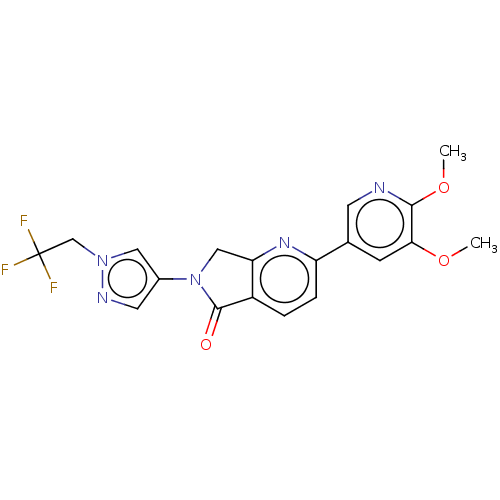
(CHEMBL4129974)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC(F)(F)F)c1 Show InChI InChI=1S/C19H16F3N5O3/c1-29-16-5-11(6-23-17(16)30-2)14-4-3-13-15(25-14)9-27(18(13)28)12-7-24-26(8-12)10-19(20,21)22/h3-8H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463483
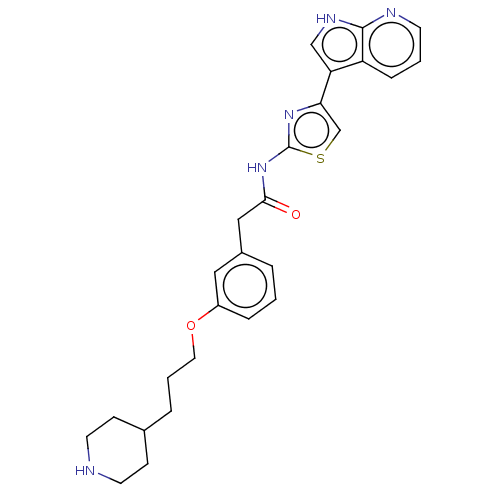
(CHEMBL4245242)Show SMILES O=C(Cc1cccc(OCCCC2CCNCC2)c1)Nc1nc(cs1)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C26H29N5O2S/c32-24(31-26-30-23(17-34-26)22-16-29-25-21(22)7-2-10-28-25)15-19-4-1-6-20(14-19)33-13-3-5-18-8-11-27-12-9-18/h1-2,4,6-7,10,14,16-18,27H,3,5,8-9,11-13,15H2,(H,28,29)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463494

(CHEMBL4240883)Show InChI InChI=1S/C18H14N4OS/c23-16(9-12-5-2-1-3-6-12)22-18-21-15(11-24-18)14-10-20-17-13(14)7-4-8-19-17/h1-8,10-11H,9H2,(H,19,20)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463487

(CHEMBL4248883)Show SMILES O=C(Cc1ccc2OCOc2c1)Nc1nc(cs1)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C19H14N4O3S/c24-17(7-11-3-4-15-16(6-11)26-10-25-15)23-19-22-14(9-27-19)13-8-21-18-12(13)2-1-5-20-18/h1-6,8-9H,7,10H2,(H,20,21)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274572
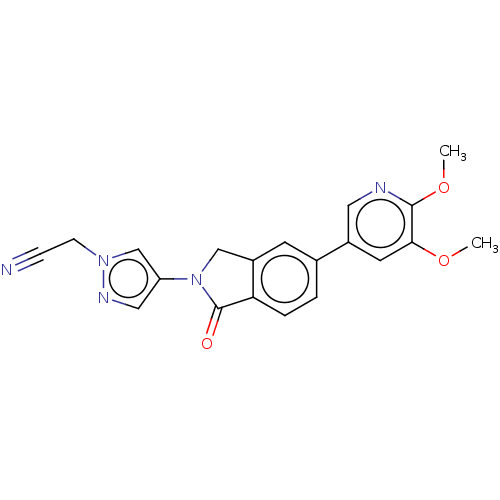
(CHEMBL4129180)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2c1)c1cnn(CC#N)c1 Show InChI InChI=1S/C20H17N5O3/c1-27-18-8-14(9-22-19(18)28-2)13-3-4-17-15(7-13)11-25(20(17)26)16-10-23-24(12-16)6-5-21/h3-4,7-10,12H,6,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463497

(CHEMBL4249070)Show SMILES CC(C)NS(=O)(=O)c1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C21H21N5O3S2/c1-13(2)26-31(28,29)15-6-3-5-14(9-15)10-19(27)25-21-24-18(12-30-21)17-11-23-20-16(17)7-4-8-22-20/h3-9,11-13,26H,10H2,1-2H3,(H,22,23)(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274541
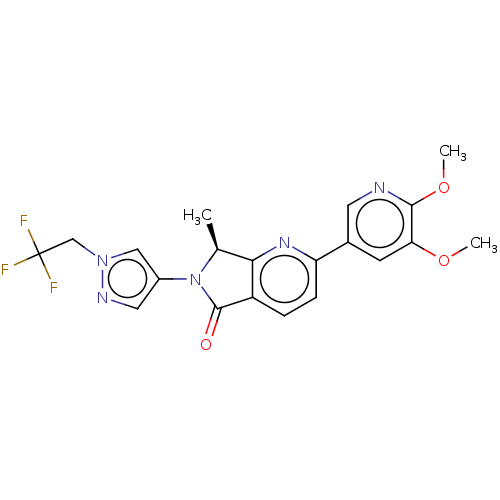
(CHEMBL4130036)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274559

(CHEMBL4126707)Show SMILES CCn1cc(cn1)N1Cc2nc(ccc2C1=O)-c1cnc(OC)c(OC)c1 Show InChI InChI=1S/C19H19N5O3/c1-4-23-10-13(9-21-23)24-11-16-14(19(24)25)5-6-15(22-16)12-7-17(26-2)18(27-3)20-8-12/h5-10H,4,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463482

(CHEMBL4238696)Show SMILES O=C(Cc1cccc(OCCN2CCNCC2)c1)Nc1nc(cs1)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C24H26N6O2S/c31-22(14-17-3-1-4-18(13-17)32-12-11-30-9-7-25-8-10-30)29-24-28-21(16-33-24)20-15-27-23-19(20)5-2-6-26-23/h1-6,13,15-16,25H,7-12,14H2,(H,26,27)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274542

(CHEMBL4127853)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC#C)c1 Show InChI InChI=1S/C20H17N5O3/c1-4-7-24-11-14(10-22-24)25-12-17-15(20(25)26)5-6-16(23-17)13-8-18(27-2)19(28-3)21-9-13/h1,5-6,8-11H,7,12H2,2-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274543

(CHEMBL4129251)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC(F)F)c1 Show InChI InChI=1S/C19H17F2N5O3/c1-28-16-5-11(6-22-18(16)29-2)14-4-3-13-15(24-14)9-26(19(13)27)12-7-23-25(8-12)10-17(20)21/h3-8,17H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463486

(CHEMBL4242191)Show SMILES O=C(Cc1cccc(OCCCN2CCNCC2)c1)Nc1nc(cs1)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C25H28N6O2S/c32-23(30-25-29-22(17-34-25)21-16-28-24-20(21)6-2-7-27-24)15-18-4-1-5-19(14-18)33-13-3-10-31-11-8-26-9-12-31/h1-2,4-7,14,16-17,26H,3,8-13,15H2,(H,27,28)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50462718

(CHEMBL4237479)Show SMILES CN1CCC(CCCOc2cccc(CC(=O)Nc3nc(cs3)-c3ccncc3)c2)CC1 Show InChI InChI=1S/C25H30N4O2S/c1-29-13-9-19(10-14-29)5-3-15-31-22-6-2-4-20(16-22)17-24(30)28-25-27-23(18-32-25)21-7-11-26-12-8-21/h2,4,6-8,11-12,16,18-19H,3,5,9-10,13-15,17H2,1H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate by pyruvate kinase-lactate dehydrogen... |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM26668
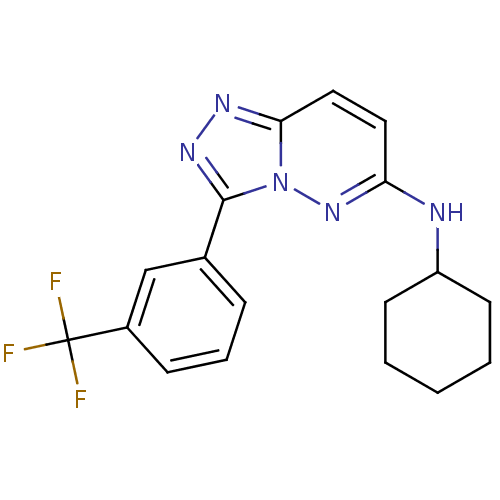
(CHEMBL494360 | N-cyclohexyl-3-[3-(trifluoromethyl)...)Show SMILES FC(F)(F)c1cccc(c1)-c1nnc2ccc(NC3CCCCC3)nn12 Show InChI InChI=1S/C18H18F3N5/c19-18(20,21)13-6-4-5-12(11-13)17-24-23-16-10-9-15(25-26(16)17)22-14-7-2-1-3-8-14/h4-6,9-11,14H,1-3,7-8H2,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) assessed as [32P] incorporation preincubated for 15 mins before ATP substrate addition by coupled spectrophotomet... |
Bioorg Med Chem Lett 19: 3019-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.061
BindingDB Entry DOI: 10.7270/Q21J99PJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463485

(CHEMBL4240458)Show SMILES CN(C)S(=O)(=O)c1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C20H19N5O3S2/c1-25(2)30(27,28)14-6-3-5-13(9-14)10-18(26)24-20-23-17(12-29-20)16-11-22-19-15(16)7-4-8-21-19/h3-9,11-12H,10H2,1-2H3,(H,21,22)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274560
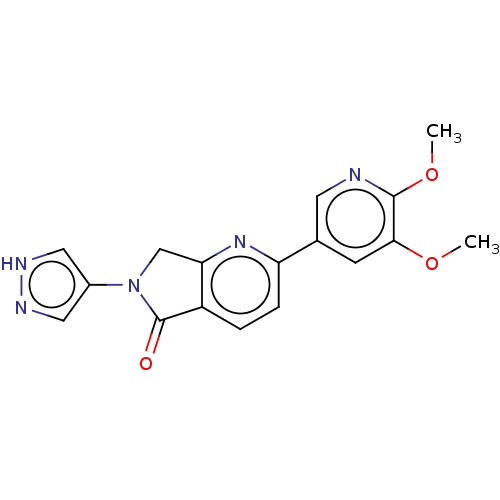
(CHEMBL4125738)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cn[nH]c1 Show InChI InChI=1S/C17H15N5O3/c1-24-15-5-10(6-18-16(15)25-2)13-4-3-12-14(21-13)9-22(17(12)23)11-7-19-20-8-11/h3-8H,9H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274540

(CHEMBL4128822)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(c3cnn(CC(F)(F)F)c3)C(C)(C)c2n1 Show InChI InChI=1S/C21H20F3N5O3/c1-20(2)17-14(19(30)29(20)13-9-26-28(10-13)11-21(22,23)24)5-6-15(27-17)12-7-16(31-3)18(32-4)25-8-12/h5-10H,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50462710
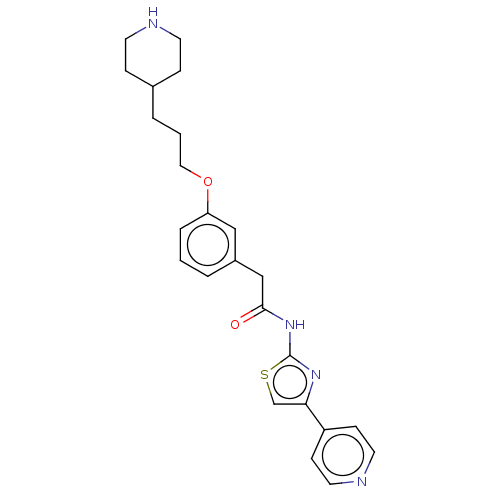
(CHEMBL4251324)Show SMILES O=C(Cc1cccc(OCCCC2CCNCC2)c1)Nc1nc(cs1)-c1ccncc1 Show InChI InChI=1S/C24H28N4O2S/c29-23(28-24-27-22(17-31-24)20-8-12-26-13-9-20)16-19-3-1-5-21(15-19)30-14-2-4-18-6-10-25-11-7-18/h1,3,5,8-9,12-13,15,17-18,25H,2,4,6-7,10-11,14,16H2,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate by pyruvate kinase-lactate dehydrogen... |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463501

(CHEMBL4239032)Show SMILES O=C(Cc1cccc(c1)C#N)Nc1nc(cs1)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C19H13N5OS/c20-9-13-4-1-3-12(7-13)8-17(25)24-19-23-16(11-26-19)15-10-22-18-14(15)5-2-6-21-18/h1-7,10-11H,8H2,(H,21,22)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463488
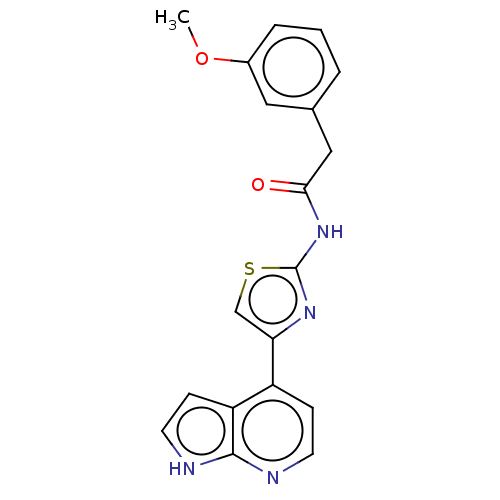
(CHEMBL4247780)Show SMILES COc1cccc(CC(=O)Nc2nc(cs2)-c2ccnc3[nH]ccc23)c1 Show InChI InChI=1S/C19H16N4O2S/c1-25-13-4-2-3-12(9-13)10-17(24)23-19-22-16(11-26-19)14-5-7-20-18-15(14)6-8-21-18/h2-9,11H,10H2,1H3,(H,20,21)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50258853
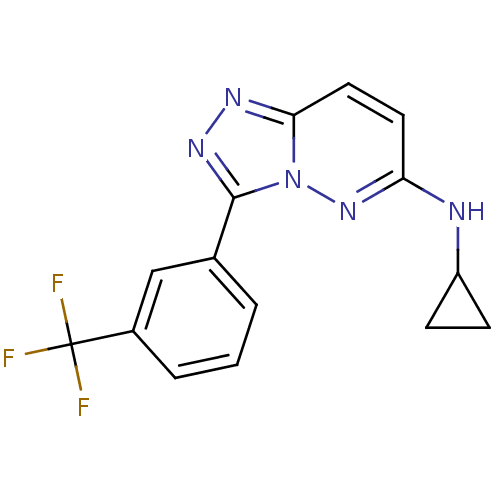
(CHEMBL468940 | N-cyclopropyl-3-(3-(trifluoromethyl...)Show InChI InChI=1S/C15H12F3N5/c16-15(17,18)10-3-1-2-9(8-10)14-21-20-13-7-6-12(22-23(13)14)19-11-4-5-11/h1-3,6-8,11H,4-5H2,(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) assessed as [32P] incorporation preincubated for 15 mins before ATP substrate addition by coupled spectrophotomet... |
Bioorg Med Chem Lett 19: 3019-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.061
BindingDB Entry DOI: 10.7270/Q21J99PJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463493
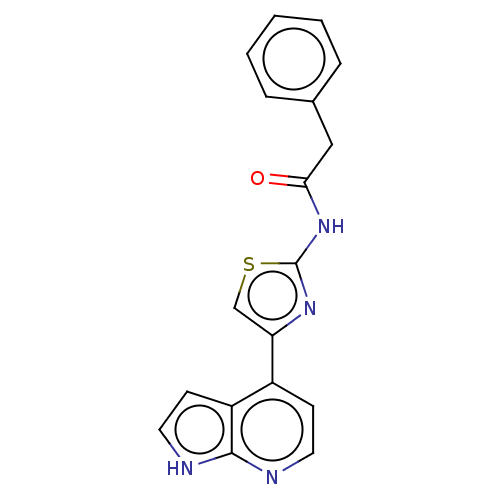
(CHEMBL4251448)Show InChI InChI=1S/C18H14N4OS/c23-16(10-12-4-2-1-3-5-12)22-18-21-15(11-24-18)13-6-8-19-17-14(13)7-9-20-17/h1-9,11H,10H2,(H,19,20)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463496

(CHEMBL4243123)Show SMILES CCNS(=O)(=O)c1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C20H19N5O3S2/c1-2-23-30(27,28)14-6-3-5-13(9-14)10-18(26)25-20-24-17(12-29-20)16-11-22-19-15(16)7-4-8-21-19/h3-9,11-12,23H,2,10H2,1H3,(H,21,22)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274580

(CHEMBL4128006)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CCC#N)c1 Show InChI InChI=1S/C20H18N6O3/c1-28-18-8-13(9-22-19(18)29-2)16-5-4-15-17(24-16)12-26(20(15)27)14-10-23-25(11-14)7-3-6-21/h4-5,8-11H,3,7,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50258287
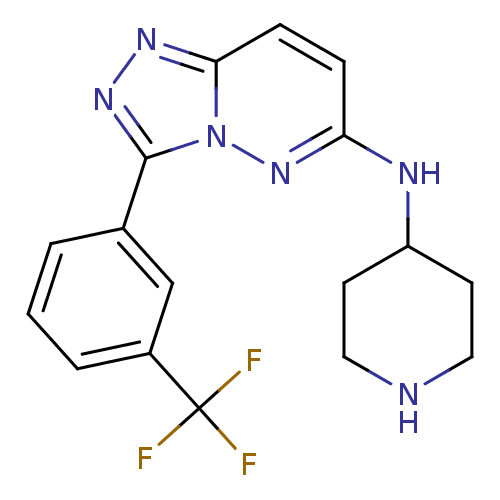
(CHEMBL493169 | N-(piperidin-4-yl)-3-(3-(trifluorom...)Show SMILES FC(F)(F)c1cccc(c1)-c1nnc2ccc(NC3CCNCC3)nn12 Show InChI InChI=1S/C17H17F3N6/c18-17(19,20)12-3-1-2-11(10-12)16-24-23-15-5-4-14(25-26(15)16)22-13-6-8-21-9-7-13/h1-5,10,13,21H,6-9H2,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) assessed as [32P] incorporation preincubated for 15 mins before ATP substrate addition by coupled spectrophotomet... |
Bioorg Med Chem Lett 19: 3019-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.061
BindingDB Entry DOI: 10.7270/Q21J99PJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM26697
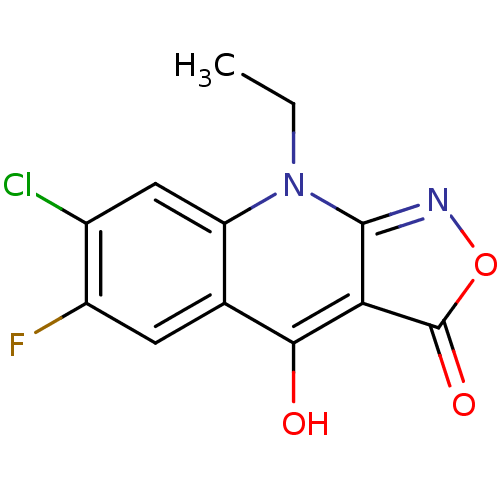
(7-chloro-9-ethyl-6-fluoro-1H,3H,4H,9H-quinolino[2,...)Show InChI InChI=1S/C12H8ClFN2O3/c1-2-16-8-4-6(13)7(14)3-5(8)10(17)9-11(16)15-19-12(9)18/h3-4,17H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) assessed as [32P] incorporation preincubated for 15 mins before ATP substrate addition by coupled spectrophotomet... |
Bioorg Med Chem Lett 19: 3019-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.061
BindingDB Entry DOI: 10.7270/Q21J99PJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274544

(CHEMBL4126620)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(c1)C(C)(C)C#N Show InChI InChI=1S/C21H20N6O3/c1-21(2,12-22)27-10-14(9-24-27)26-11-17-15(20(26)28)5-6-16(25-17)13-7-18(29-3)19(30-4)23-8-13/h5-10H,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274573
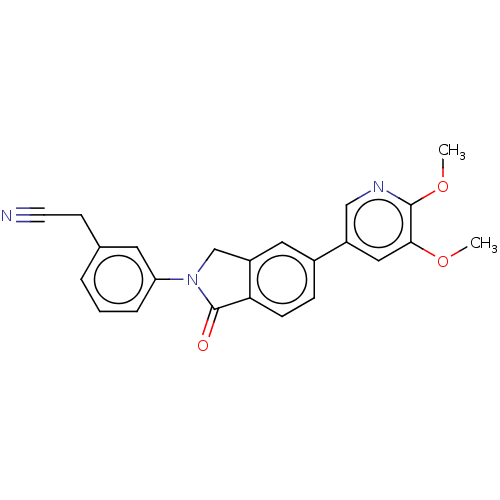
(CHEMBL4126829)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2c1)c1cccc(CC#N)c1 Show InChI InChI=1S/C23H19N3O3/c1-28-21-12-17(13-25-22(21)29-2)16-6-7-20-18(11-16)14-26(23(20)27)19-5-3-4-15(10-19)8-9-24/h3-7,10-13H,8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50463480
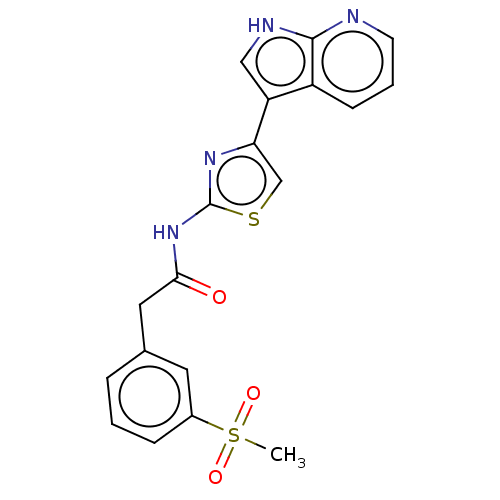
(CHEMBL4237255)Show SMILES CS(=O)(=O)c1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H16N4O3S2/c1-28(25,26)13-5-2-4-12(8-13)9-17(24)23-19-22-16(11-27-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11H,9H2,1H3,(H,20,21)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate in presence of ATP by spectrophotomet... |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50258285
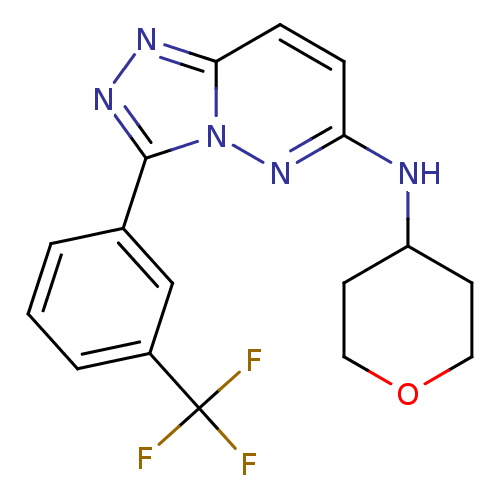
(CHEMBL493168 | N-(tetrahydro-2H-pyran-4-yl)-3-(3-(...)Show SMILES FC(F)(F)c1cccc(c1)-c1nnc2ccc(NC3CCOCC3)nn12 Show InChI InChI=1S/C17H16F3N5O/c18-17(19,20)12-3-1-2-11(10-12)16-23-22-15-5-4-14(24-25(15)16)21-13-6-8-26-9-7-13/h1-5,10,13H,6-9H2,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) assessed as [32P] incorporation preincubated for 15 mins before ATP substrate addition by coupled spectrophotomet... |
Bioorg Med Chem Lett 19: 3019-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.061
BindingDB Entry DOI: 10.7270/Q21J99PJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50462711

(CHEMBL4241204)Show SMILES CN1CCN(CCCOc2cccc(CC(=O)Nc3nc(cs3)-c3ccncc3)c2)CC1 Show InChI InChI=1S/C24H29N5O2S/c1-28-11-13-29(14-12-28)10-3-15-31-21-5-2-4-19(16-21)17-23(30)27-24-26-22(18-32-24)20-6-8-25-9-7-20/h2,4-9,16,18H,3,10-15,17H2,1H3,(H,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate by pyruvate kinase-lactate dehydrogen... |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50462716
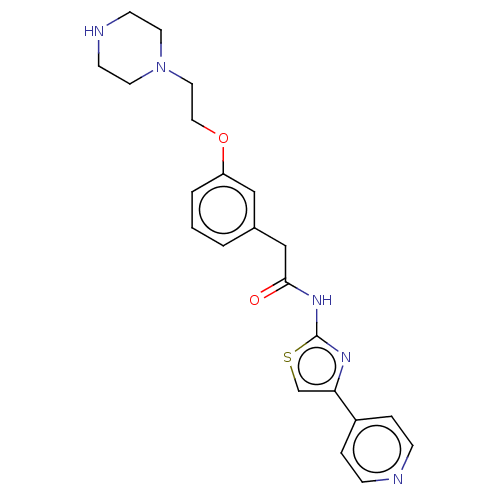
(CHEMBL4240949)Show SMILES O=C(Cc1cccc(OCCN2CCNCC2)c1)Nc1nc(cs1)-c1ccncc1 Show InChI InChI=1S/C22H25N5O2S/c28-21(26-22-25-20(16-30-22)18-4-6-23-7-5-18)15-17-2-1-3-19(14-17)29-13-12-27-10-8-24-9-11-27/h1-7,14,16,24H,8-13,15H2,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate by pyruvate kinase-lactate dehydrogen... |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50258425
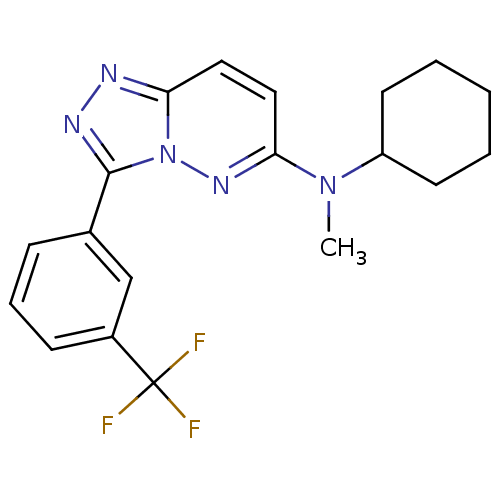
(CHEMBL494222 | N-cyclohexyl-N-methyl-3-(3-(trifluo...)Show SMILES CN(C1CCCCC1)c1ccc2nnc(-c3cccc(c3)C(F)(F)F)n2n1 Show InChI InChI=1S/C19H20F3N5/c1-26(15-8-3-2-4-9-15)17-11-10-16-23-24-18(27(16)25-17)13-6-5-7-14(12-13)19(20,21)22/h5-7,10-12,15H,2-4,8-9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) assessed as [32P] incorporation preincubated for 15 mins before ATP substrate addition by coupled spectrophotomet... |
Bioorg Med Chem Lett 19: 3019-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.061
BindingDB Entry DOI: 10.7270/Q21J99PJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50258858
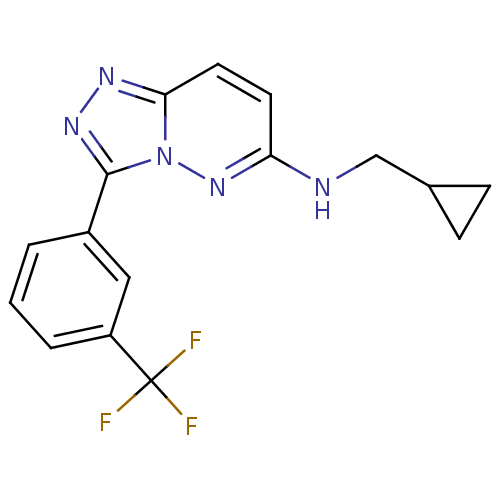
(CHEMBL450568 | N-(cyclopropylmethyl)-3-(3-(trifluo...)Show InChI InChI=1S/C16H14F3N5/c17-16(18,19)12-3-1-2-11(8-12)15-22-21-14-7-6-13(23-24(14)15)20-9-10-4-5-10/h1-3,6-8,10H,4-5,9H2,(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) assessed as [32P] incorporation preincubated for 15 mins before ATP substrate addition by coupled spectrophotomet... |
Bioorg Med Chem Lett 19: 3019-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.061
BindingDB Entry DOI: 10.7270/Q21J99PJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50462709
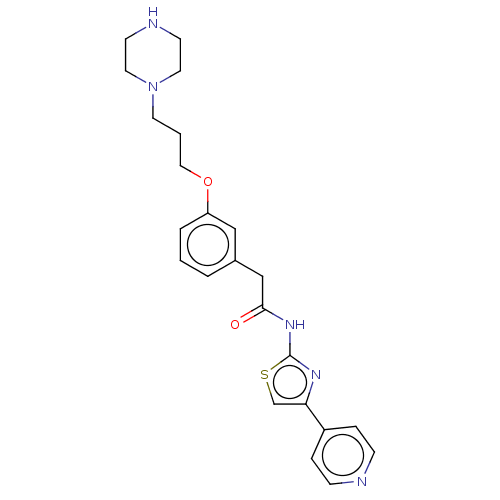
(CHEMBL4245507)Show SMILES O=C(Cc1cccc(OCCCN2CCNCC2)c1)Nc1nc(cs1)-c1ccncc1 Show InChI InChI=1S/C23H27N5O2S/c29-22(27-23-26-21(17-31-23)19-5-7-24-8-6-19)16-18-3-1-4-20(15-18)30-14-2-11-28-12-9-25-10-13-28/h1,3-8,15,17,25H,2,9-14,16H2,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (6 to 553 residues) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate by pyruvate kinase-lactate dehydrogen... |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data