 Found 444 hits with Last Name = 'jacobson' and Initial = 'ma'
Found 444 hits with Last Name = 'jacobson' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neurotensin receptor type 1
(MOUSE) | BDBM50240339
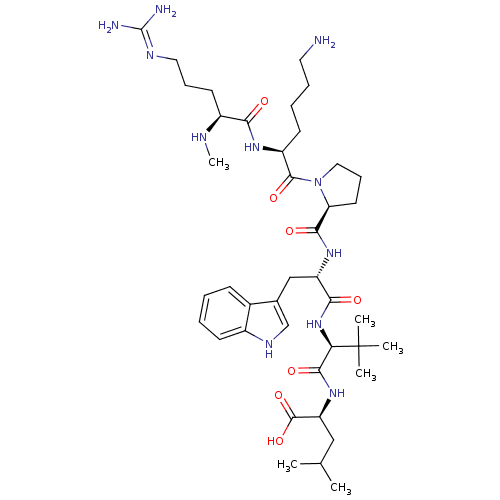
((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM82078
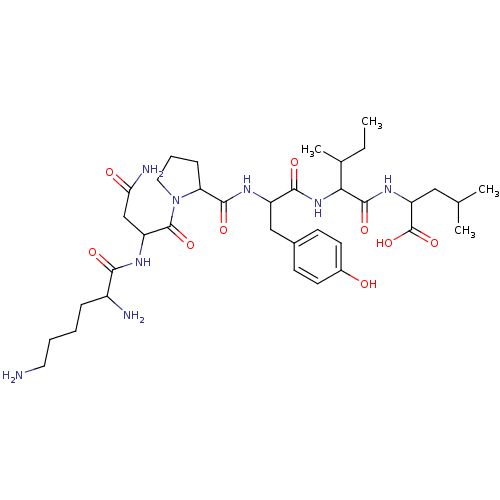
(CAS_55508-42-4 | NSC_128644 | Neurotensin)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(N)CCCCN)C(=O)NC(CC(C)C)C(O)=O Show InChI InChI=1S/C36H58N8O9/c1-5-21(4)30(34(50)42-27(36(52)53)17-20(2)3)43-32(48)25(18-22-11-13-23(45)14-12-22)40-33(49)28-10-8-16-44(28)35(51)26(19-29(39)46)41-31(47)24(38)9-6-7-15-37/h11-14,20-21,24-28,30,45H,5-10,15-19,37-38H2,1-4H3,(H2,39,46)(H,40,49)(H,41,47)(H,42,50)(H,43,48)(H,52,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077035

(1-(1-{2-[4-Morpholin-4-yl-2-(2,2,2-trifluoro-ethox...)Show SMILES FC(F)(F)COc1cc(ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12)N1CCOCC1 Show InChI InChI=1S/C27H30F3N3O5/c28-27(29,30)18-38-24-16-22(31-11-13-36-14-12-31)6-5-19(24)15-25(34)32-9-7-21(8-10-32)33-23-4-2-1-3-20(23)17-37-26(33)35/h1-6,16,21H,7-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077032
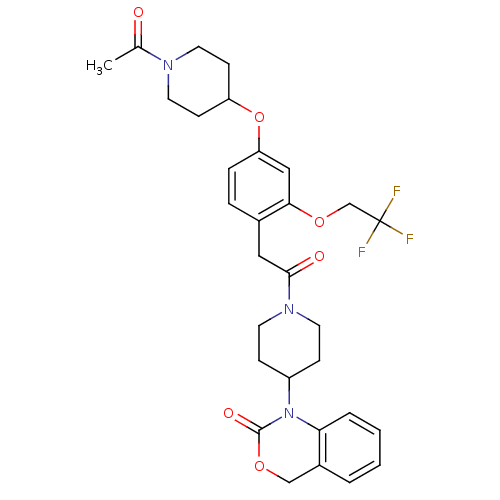
(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OCC(F)(F)F)c1 Show InChI InChI=1S/C30H34F3N3O6/c1-20(37)34-14-10-24(11-15-34)42-25-7-6-21(27(17-25)41-19-30(31,32)33)16-28(38)35-12-8-23(9-13-35)36-26-5-3-2-4-22(26)18-40-29(36)39/h2-7,17,23-24H,8-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077035

(1-(1-{2-[4-Morpholin-4-yl-2-(2,2,2-trifluoro-ethox...)Show SMILES FC(F)(F)COc1cc(ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12)N1CCOCC1 Show InChI InChI=1S/C27H30F3N3O5/c28-27(29,30)18-38-24-16-22(31-11-13-36-14-12-31)6-5-19(24)15-25(34)32-9-7-21(8-10-32)33-23-4-2-1-3-20(23)17-37-26(33)35/h1-6,16,21H,7-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077041

(1-(1-{2-[4-(1-Cyclopropylmethyl-piperidin-4-yloxy)...)Show SMILES FC(F)(F)COc1cc(OC2CCN(CC3CC3)CC2)ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C32H38F3N3O5/c33-32(34,35)21-42-29-18-27(43-26-11-13-36(14-12-26)19-22-5-6-22)8-7-23(29)17-30(39)37-15-9-25(10-16-37)38-28-4-2-1-3-24(28)20-41-31(38)40/h1-4,7-8,18,22,25-26H,5-6,9-17,19-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077041

(1-(1-{2-[4-(1-Cyclopropylmethyl-piperidin-4-yloxy)...)Show SMILES FC(F)(F)COc1cc(OC2CCN(CC3CC3)CC2)ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C32H38F3N3O5/c33-32(34,35)21-42-29-18-27(43-26-11-13-36(14-12-26)19-22-5-6-22)8-7-23(29)17-30(39)37-15-9-25(10-16-37)38-28-4-2-1-3-24(28)20-41-31(38)40/h1-4,7-8,18,22,25-26H,5-6,9-17,19-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077037
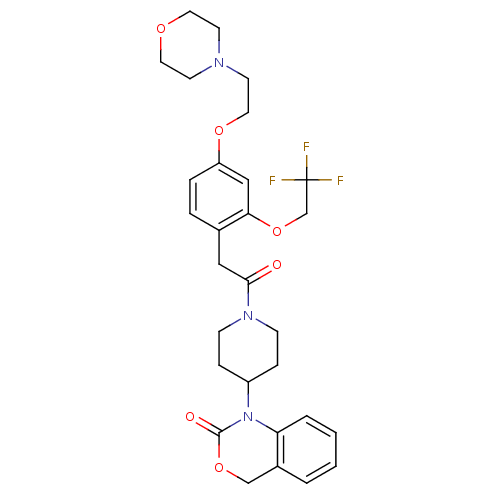
(1-(1-{2-[4-(2-Morpholin-4-yl-ethoxy)-2-(2,2,2-trif...)Show SMILES FC(F)(F)COc1cc(OCCN2CCOCC2)ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C29H34F3N3O6/c30-29(31,32)20-41-26-18-24(39-16-13-33-11-14-38-15-12-33)6-5-21(26)17-27(36)34-9-7-23(8-10-34)35-25-4-2-1-3-22(25)19-40-28(35)37/h1-6,18,23H,7-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077032

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OCC(F)(F)F)c1 Show InChI InChI=1S/C30H34F3N3O6/c1-20(37)34-14-10-24(11-15-34)42-25-7-6-21(27(17-25)41-19-30(31,32)33)16-28(38)35-12-8-23(9-13-35)36-26-5-3-2-4-22(26)18-40-29(36)39/h2-7,17,23-24H,8-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50077032

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OCC(F)(F)F)c1 Show InChI InChI=1S/C30H34F3N3O6/c1-20(37)34-14-10-24(11-15-34)42-25-7-6-21(27(17-25)41-19-30(31,32)33)16-28(38)35-12-8-23(9-13-35)36-26-5-3-2-4-22(26)18-40-29(36)39/h2-7,17,23-24H,8-16,18-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards rat liver Vasopressin V1a receptor by using functional assay |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077037

(1-(1-{2-[4-(2-Morpholin-4-yl-ethoxy)-2-(2,2,2-trif...)Show SMILES FC(F)(F)COc1cc(OCCN2CCOCC2)ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C29H34F3N3O6/c30-29(31,32)20-41-26-18-24(39-16-13-33-11-14-38-15-12-33)6-5-21(26)17-27(36)34-9-7-23(8-10-34)35-25-4-2-1-3-22(25)19-40-28(35)37/h1-6,18,23H,7-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077039
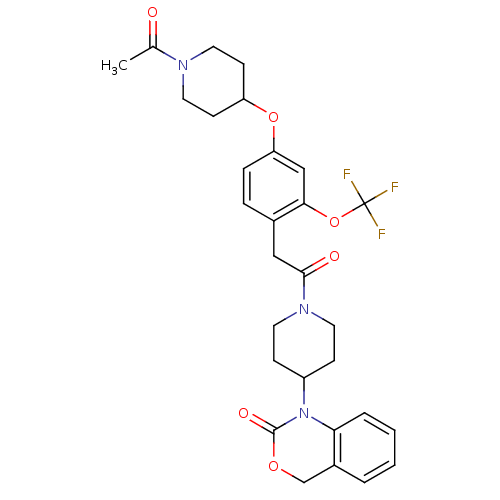
(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-trifluor...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OC(F)(F)F)c1 Show InChI InChI=1S/C29H32F3N3O6/c1-19(36)33-14-10-23(11-15-33)40-24-7-6-20(26(17-24)41-29(30,31)32)16-27(37)34-12-8-22(9-13-34)35-25-5-3-2-4-21(25)18-39-28(35)38/h2-7,17,22-23H,8-16,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077039

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-trifluor...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OC(F)(F)F)c1 Show InChI InChI=1S/C29H32F3N3O6/c1-19(36)33-14-10-23(11-15-33)40-24-7-6-20(26(17-24)41-29(30,31)32)16-27(37)34-12-8-22(9-13-34)35-25-5-3-2-4-21(25)18-39-28(35)38/h2-7,17,22-23H,8-16,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM50240339

((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077034

(1-(1-{2-[2-(2,2,2-Trifluoro-ethoxy)-phenyl]-acetyl...)Show SMILES FC(F)(F)COc1ccccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C23H23F3N2O4/c24-23(25,26)15-32-20-8-4-2-5-16(20)13-21(29)27-11-9-18(10-12-27)28-19-7-3-1-6-17(19)14-31-22(28)30/h1-8,18H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077034

(1-(1-{2-[2-(2,2,2-Trifluoro-ethoxy)-phenyl]-acetyl...)Show SMILES FC(F)(F)COc1ccccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C23H23F3N2O4/c24-23(25,26)15-32-20-8-4-2-5-16(20)13-21(29)27-11-9-18(10-12-27)28-19-7-3-1-6-17(19)14-31-22(28)30/h1-8,18H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM82078

(CAS_55508-42-4 | NSC_128644 | Neurotensin)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(N)CCCCN)C(=O)NC(CC(C)C)C(O)=O Show InChI InChI=1S/C36H58N8O9/c1-5-21(4)30(34(50)42-27(36(52)53)17-20(2)3)43-32(48)25(18-22-11-13-23(45)14-12-22)40-33(49)28-10-8-16-44(28)35(51)26(19-29(39)46)41-31(47)24(38)9-6-7-15-37/h11-14,20-21,24-28,30,45H,5-10,15-19,37-38H2,1-4H3,(H2,39,46)(H,40,49)(H,41,47)(H,42,50)(H,43,48)(H,52,53) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50029649
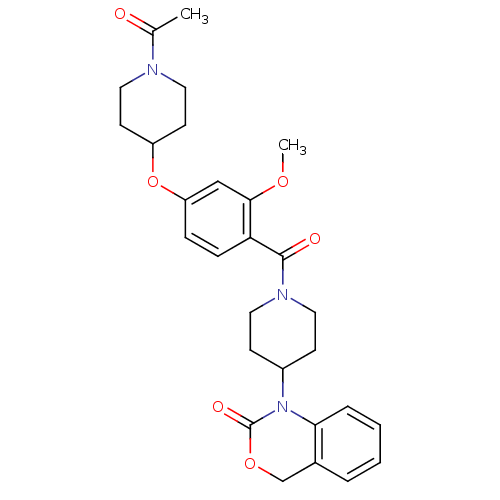
(1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...)Show SMILES COc1cc(OC2CCN(CC2)C(C)=O)ccc1C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C28H33N3O6/c1-19(32)29-15-11-22(12-16-29)37-23-7-8-24(26(17-23)35-2)27(33)30-13-9-21(10-14-30)31-25-6-4-3-5-20(25)18-36-28(31)34/h3-8,17,21-22H,9-16,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077040

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-methoxy-...)Show SMILES COc1cc(OC2CCN(CC2)C(C)=O)ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C29H35N3O6/c1-20(33)30-15-11-24(12-16-30)38-25-8-7-21(27(18-25)36-2)17-28(34)31-13-9-23(10-14-31)32-26-6-4-3-5-22(26)19-37-29(32)35/h3-8,18,23-24H,9-17,19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM82557
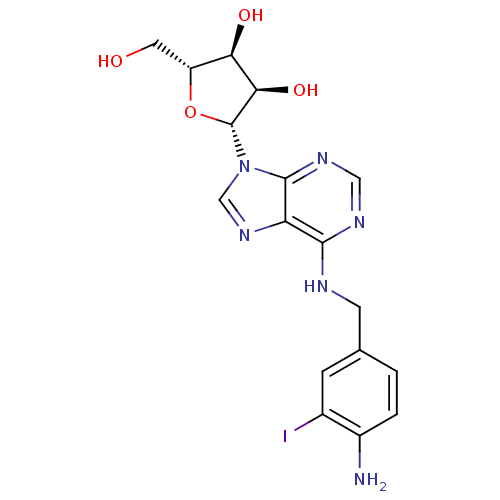
(I-ABA | IABA | N(6)-(4-Amino-3-iodobenzyl)adenosin...)Show SMILES Nc1ccc(CNc2ncnc3n(cnc23)[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)cc1I Show InChI InChI=1S/C17H19IN6O4/c18-9-3-8(1-2-10(9)19)4-20-15-12-16(22-6-21-15)24(7-23-12)17-14(27)13(26)11(5-25)28-17/h1-3,6-7,11,13-14,17,25-27H,4-5,19H2,(H,20,21,22)/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 10365-9 (1993)
Article DOI: 10.1073/pnas.90.21.10365
BindingDB Entry DOI: 10.7270/Q24Q7SH0 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM85844
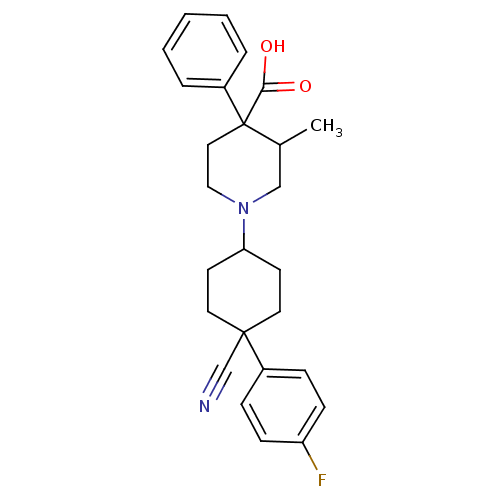
(CAS_54385 | Levocabastine | NSC_54385)Show SMILES CC1CN(CCC1(C(O)=O)c1ccccc1)C1CCC(CC1)(C#N)c1ccc(F)cc1 |(1.11,-6.21,;-.22,-6.98,;-.22,-8.52,;-1.56,-9.29,;-2.89,-8.52,;-2.89,-6.98,;-1.56,-6.21,;-.84,-4.85,;-.73,-3.31,;.7,-4.8,;-2.28,-4.85,;-3.82,-4.8,;-4.54,-3.44,;-3.73,-2.13,;-2.19,-2.18,;-1.47,-3.54,;-1.56,-10.83,;-2.89,-11.6,;-2.89,-13.14,;-1.56,-13.91,;-.22,-13.14,;-.22,-11.6,;-2.28,-15.27,;-3,-16.63,;-.84,-15.27,;.7,-15.32,;1.43,-16.68,;.61,-17.99,;1.33,-19.35,;-.93,-17.93,;-1.65,-16.57,)| Show InChI InChI=1S/C26H29FN2O2/c1-19-17-29(16-15-26(19,24(30)31)21-5-3-2-4-6-21)23-11-13-25(18-28,14-12-23)20-7-9-22(27)10-8-20/h2-10,19,23H,11-17H2,1H3,(H,30,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM82558
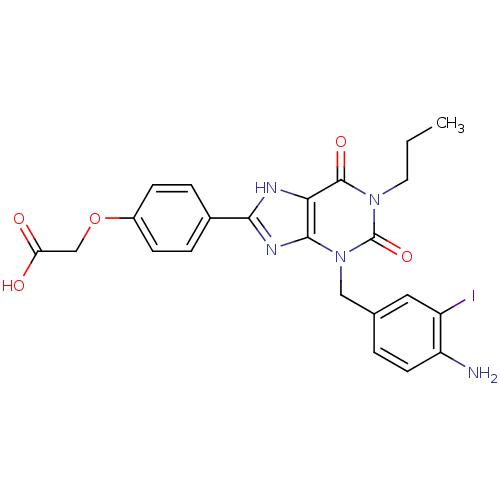
(CAS_163889 | CHEMBL355370 | I-ABOPX | NSC_163889)Show SMILES CCCn1c(=O)n(Cc2ccc(N)c(I)c2)c2nc([nH]c2c1=O)-c1ccc(OCC(O)=O)cc1 Show InChI InChI=1S/C23H22IN5O5/c1-2-9-28-22(32)19-21(29(23(28)33)11-13-3-8-17(25)16(24)10-13)27-20(26-19)14-4-6-15(7-5-14)34-12-18(30)31/h3-8,10H,2,9,11-12,25H2,1H3,(H,26,27)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 10365-9 (1993)
Article DOI: 10.1073/pnas.90.21.10365
BindingDB Entry DOI: 10.7270/Q24Q7SH0 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50029649

(1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...)Show SMILES COc1cc(OC2CCN(CC2)C(C)=O)ccc1C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C28H33N3O6/c1-19(32)29-15-11-22(12-16-29)37-23-7-8-24(26(17-23)35-2)27(33)30-13-9-21(10-14-30)31-25-6-4-3-5-20(25)18-36-28(31)34/h3-8,17,21-22H,9-16,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 25.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 10365-9 (1993)
Article DOI: 10.1073/pnas.90.21.10365
BindingDB Entry DOI: 10.7270/Q24Q7SH0 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077038
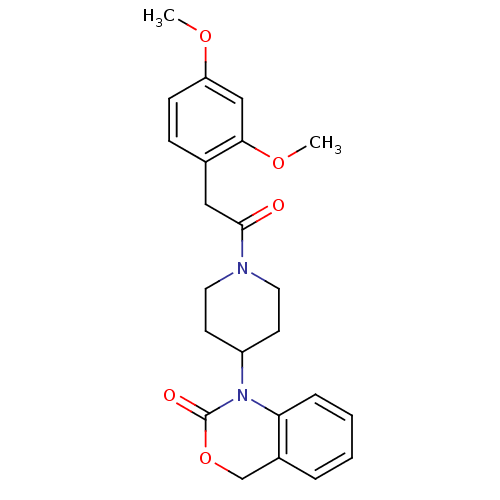
(1-{1-[2-(2,4-Dimethoxy-phenyl)-acetyl]-piperidin-4...)Show SMILES COc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OC)c1 Show InChI InChI=1S/C23H26N2O5/c1-28-19-8-7-16(21(14-19)29-2)13-22(26)24-11-9-18(10-12-24)25-20-6-4-3-5-17(20)15-30-23(25)27/h3-8,14,18H,9-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50006730
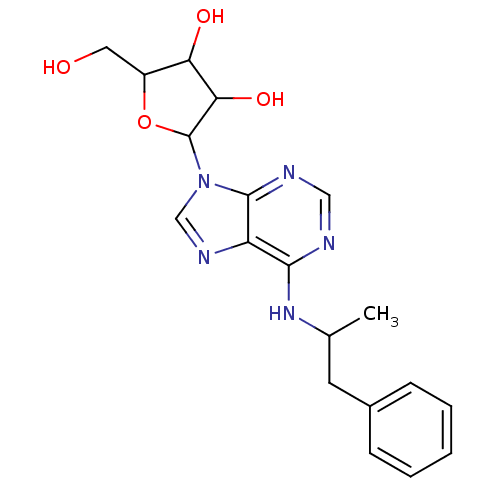
((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 10365-9 (1993)
Article DOI: 10.1073/pnas.90.21.10365
BindingDB Entry DOI: 10.7270/Q24Q7SH0 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077040

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-methoxy-...)Show SMILES COc1cc(OC2CCN(CC2)C(C)=O)ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C29H35N3O6/c1-20(33)30-15-11-24(12-16-30)38-25-8-7-21(27(18-25)36-2)17-28(34)31-13-9-23(10-14-31)32-26-6-4-3-5-22(26)19-37-29(32)35/h3-8,18,23-24H,9-17,19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077038

(1-{1-[2-(2,4-Dimethoxy-phenyl)-acetyl]-piperidin-4...)Show SMILES COc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OC)c1 Show InChI InChI=1S/C23H26N2O5/c1-28-19-8-7-16(21(14-19)29-2)13-22(26)24-11-9-18(10-12-24)25-20-6-4-3-5-17(20)15-30-23(25)27/h3-8,14,18H,9-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50207816
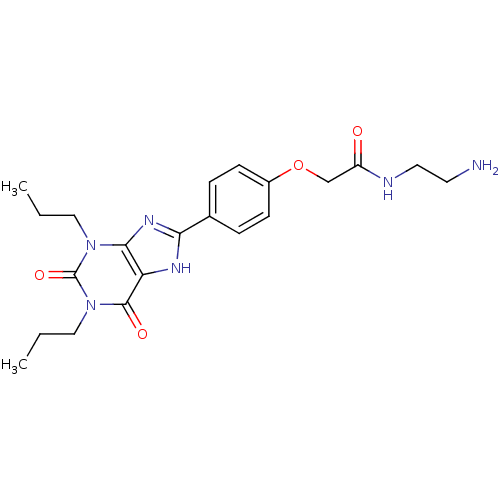
(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 70.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 10365-9 (1993)
Article DOI: 10.1073/pnas.90.21.10365
BindingDB Entry DOI: 10.7270/Q24Q7SH0 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50072368

(1-[1-(2,4-Dimethoxy-benzoyl)-piperidin-4-yl]-1,4-d...)Show SMILES COc1ccc(C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OC)c1 Show InChI InChI=1S/C22H24N2O5/c1-27-17-7-8-18(20(13-17)28-2)21(25)23-11-9-16(10-12-23)24-19-6-4-3-5-15(19)14-29-22(24)26/h3-8,13,16H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 89.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 10365-9 (1993)
Article DOI: 10.1073/pnas.90.21.10365
BindingDB Entry DOI: 10.7270/Q24Q7SH0 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50072368

(1-[1-(2,4-Dimethoxy-benzoyl)-piperidin-4-yl]-1,4-d...)Show SMILES COc1ccc(C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OC)c1 Show InChI InChI=1S/C22H24N2O5/c1-27-17-7-8-18(20(13-17)28-2)21(25)23-11-9-16(10-12-23)24-19-6-4-3-5-15(19)14-29-22(24)26/h3-8,13,16H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50077032

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OCC(F)(F)F)c1 Show InChI InChI=1S/C30H34F3N3O6/c1-20(37)34-14-10-24(11-15-34)42-25-7-6-21(27(17-25)41-19-30(31,32)33)16-28(38)35-12-8-23(9-13-35)36-26-5-3-2-4-22(26)18-40-29(36)39/h2-7,17,23-24H,8-16,18-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Vasopressin V2 receptor by using functional assay |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50077032

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OCC(F)(F)F)c1 Show InChI InChI=1S/C30H34F3N3O6/c1-20(37)34-14-10-24(11-15-34)42-25-7-6-21(27(17-25)41-19-30(31,32)33)16-28(38)35-12-8-23(9-13-35)36-26-5-3-2-4-22(26)18-40-29(36)39/h2-7,17,23-24H,8-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards rat kidney Vasopressin V2 receptor by using functional assay |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077036
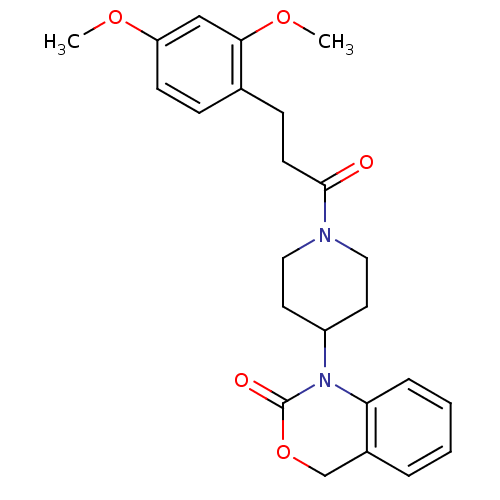
(1-{1-[3-(2,4-Dimethoxy-phenyl)-propionyl]-piperidi...)Show SMILES COc1ccc(CCC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OC)c1 Show InChI InChI=1S/C24H28N2O5/c1-29-20-9-7-17(22(15-20)30-2)8-10-23(27)25-13-11-19(12-14-25)26-21-6-4-3-5-18(21)16-31-24(26)28/h3-7,9,15,19H,8,10-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50006730

((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 10365-9 (1993)
Article DOI: 10.1073/pnas.90.21.10365
BindingDB Entry DOI: 10.7270/Q24Q7SH0 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077033
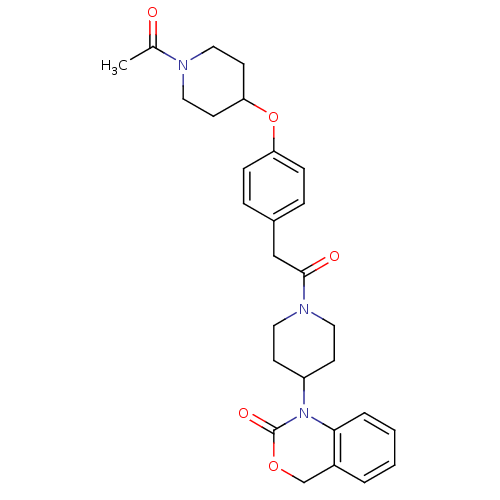
(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-phenyl]-ac...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)cc1 Show InChI InChI=1S/C28H33N3O5/c1-20(32)29-16-12-25(13-17-29)36-24-8-6-21(7-9-24)18-27(33)30-14-10-23(11-15-30)31-26-5-3-2-4-22(26)19-35-28(31)34/h2-9,23,25H,10-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077036

(1-{1-[3-(2,4-Dimethoxy-phenyl)-propionyl]-piperidi...)Show SMILES COc1ccc(CCC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OC)c1 Show InChI InChI=1S/C24H28N2O5/c1-29-20-9-7-17(22(15-20)30-2)8-10-23(27)25-13-11-19(12-14-25)26-21-6-4-3-5-18(21)16-31-24(26)28/h3-7,9,15,19H,8,10-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50169690
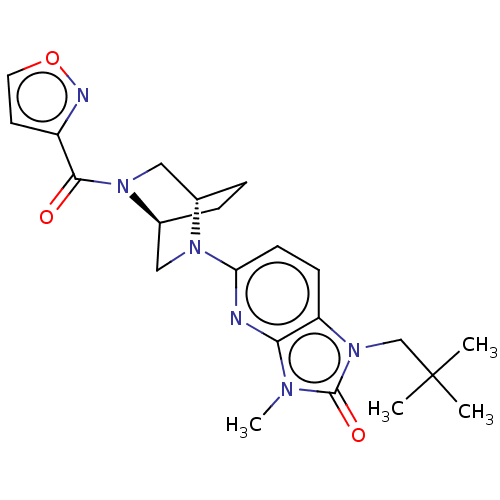
(CHEMBL3806137)Show SMILES [H][C@@]12CC[C@@]([H])(CN1C(=O)c1ccon1)N(C2)c1ccc2n(CC(C)(C)C)c(=O)n(C)c2n1 |r| Show InChI InChI=1S/C22H28N6O3/c1-22(2,3)13-28-17-7-8-18(23-19(17)25(4)21(28)30)26-11-15-6-5-14(26)12-27(15)20(29)16-9-10-31-24-16/h7-10,14-15H,5-6,11-13H2,1-4H3/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
ACS Med Chem Lett 7: 312-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00459
BindingDB Entry DOI: 10.7270/Q2W95C3K |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50077032

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(OCC(F)(F)F)c1 Show InChI InChI=1S/C30H34F3N3O6/c1-20(37)34-14-10-24(11-15-34)42-25-7-6-21(27(17-25)41-19-30(31,32)33)16-28(38)35-12-8-23(9-13-35)36-26-5-3-2-4-22(26)18-40-29(36)39/h2-7,17,23-24H,8-16,18-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human Vasopressin V1a receptor by using functional assay |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 10365-9 (1993)
Article DOI: 10.1073/pnas.90.21.10365
BindingDB Entry DOI: 10.7270/Q24Q7SH0 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077033

(1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-phenyl]-ac...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)cc1 Show InChI InChI=1S/C28H33N3O5/c1-20(32)29-16-12-25(13-17-29)36-24-8-6-21(7-9-24)18-27(33)30-14-10-23(11-15-30)31-26-5-3-2-4-22(26)19-35-28(31)34/h2-9,23,25H,10-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50145198
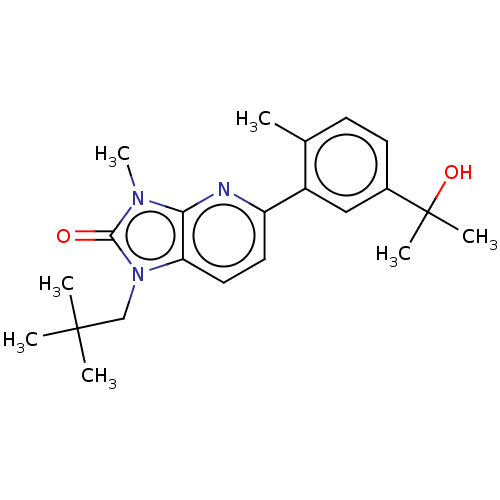
(CHEMBL3765778)Show SMILES Cc1ccc(cc1-c1ccc2n(CC(C)(C)C)c(=O)n(C)c2n1)C(C)(C)O Show InChI InChI=1S/C22H29N3O2/c1-14-8-9-15(22(5,6)27)12-16(14)17-10-11-18-19(23-17)24(7)20(26)25(18)13-21(2,3)4/h8-12,27H,13H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of 5-HT2B receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1260-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.021
BindingDB Entry DOI: 10.7270/Q2NV9M4S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50145198

(CHEMBL3765778)Show SMILES Cc1ccc(cc1-c1ccc2n(CC(C)(C)C)c(=O)n(C)c2n1)C(C)(C)O Show InChI InChI=1S/C22H29N3O2/c1-14-8-9-15(22(5,6)27)12-16(14)17-10-11-18-19(23-17)24(7)20(26)25(18)13-21(2,3)4/h8-12,27H,13H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of platelet-activating factor receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1260-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.021
BindingDB Entry DOI: 10.7270/Q2NV9M4S |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50029649

(1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...)Show SMILES COc1cc(OC2CCN(CC2)C(C)=O)ccc1C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C28H33N3O6/c1-19(32)29-15-11-22(12-16-29)37-23-7-8-24(26(17-23)35-2)27(33)30-13-9-21(10-14-30)31-25-6-4-3-5-20(25)18-36-28(31)34/h3-8,17,21-22H,9-16,18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human Vasopressin V1a receptor by using functional assay |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50029649

(1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...)Show SMILES COc1cc(OC2CCN(CC2)C(C)=O)ccc1C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C28H33N3O6/c1-19(32)29-15-11-22(12-16-29)37-23-7-8-24(26(17-23)35-2)27(33)30-13-9-21(10-14-30)31-25-6-4-3-5-20(25)18-36-28(31)34/h3-8,17,21-22H,9-16,18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human kidney Vasopressin V2 receptor by using functional assay |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent glycine transporter 1
(Homo sapiens (Human)) | BDBM50195169

(2,4-dichloro-N-((4-(morpholine-4-carbonyl)-1-(prop...)Show SMILES CCCS(=O)(=O)N1CCC(CNC(=O)c2ccc(Cl)cc2Cl)(CC1)C(=O)N1CCOCC1 Show InChI InChI=1S/C21H29Cl2N3O5S/c1-2-13-32(29,30)26-7-5-21(6-8-26,20(28)25-9-11-31-12-10-25)15-24-19(27)17-4-3-16(22)14-18(17)23/h3-4,14H,2,5-13,15H2,1H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human GlyT1 |
Bioorg Med Chem Lett 16: 5968-72 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.131
BindingDB Entry DOI: 10.7270/Q2C82B31 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent glycine transporter 1
(Homo sapiens (Human)) | BDBM50248651
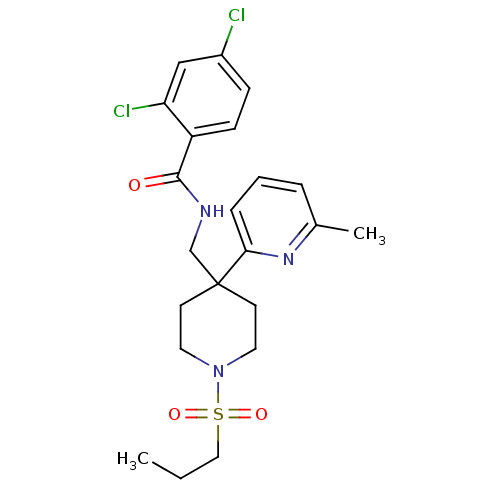
(2,4-dichloro-N-((4-(6-methylpyridin-2-yl)-1-(propy...)Show SMILES CCCS(=O)(=O)N1CCC(CNC(=O)c2ccc(Cl)cc2Cl)(CC1)c1cccc(C)n1 Show InChI InChI=1S/C22H27Cl2N3O3S/c1-3-13-31(29,30)27-11-9-22(10-12-27,20-6-4-5-16(2)26-20)15-25-21(28)18-8-7-17(23)14-19(18)24/h4-8,14H,3,9-13,15H2,1-2H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human glycine transporter 1 |
Bioorg Med Chem Lett 19: 1488-91 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.115
BindingDB Entry DOI: 10.7270/Q2V124P3 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent glycine transporter 1
(Homo sapiens (Human)) | BDBM50248641
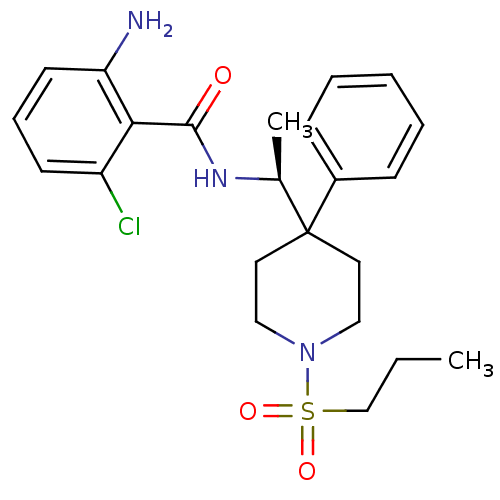
(2-amino-6-chloro-N-((4-phenyl-1-(propylsulfonyl)pi...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)([C@H](C)NC(=O)c1c(N)cccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C23H30ClN3O3S/c1-3-16-31(29,30)27-14-12-23(13-15-27,18-8-5-4-6-9-18)17(2)26-22(28)21-19(24)10-7-11-20(21)25/h4-11,17H,3,12-16,25H2,1-2H3,(H,26,28)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of glycine transporter 1 (unknown origin) by HTS assay |
Bioorg Med Chem Lett 19: 1488-91 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.115
BindingDB Entry DOI: 10.7270/Q2V124P3 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent glycine transporter 1
(Homo sapiens (Human)) | BDBM50248064
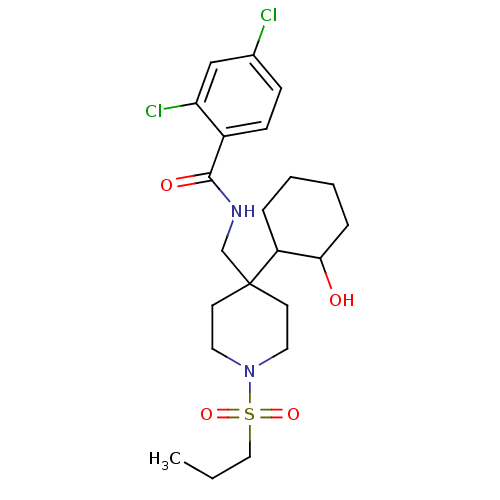
(2,4-dichloro-N-((4-(2-hydroxycyclohexyl)-1-(propyl...)Show SMILES CCCS(=O)(=O)N1CCC(CNC(=O)c2ccc(Cl)cc2Cl)(CC1)C1CCCCC1O Show InChI InChI=1S/C22H32Cl2N2O4S/c1-2-13-31(29,30)26-11-9-22(10-12-26,18-5-3-4-6-20(18)27)15-25-21(28)17-8-7-16(23)14-19(17)24/h7-8,14,18,20,27H,2-6,9-13,15H2,1H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of Glyt1 |
Bioorg Med Chem Lett 19: 1492-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.015
BindingDB Entry DOI: 10.7270/Q2B27V5N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data