

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098322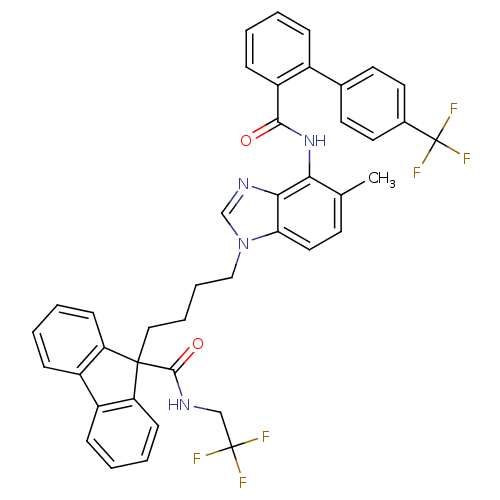 (9-(4-{5-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098321 (9-(4-{2,5-Dimethyl-4-[(4'-trifluoromethyl-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098326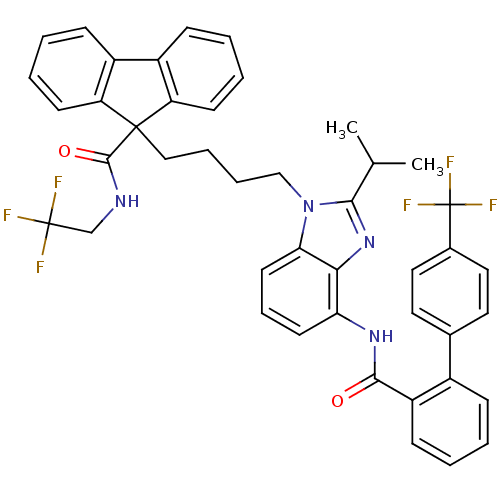 (9-(4-{2-Isopropyl-4-[(4'-trifluoromethyl-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098324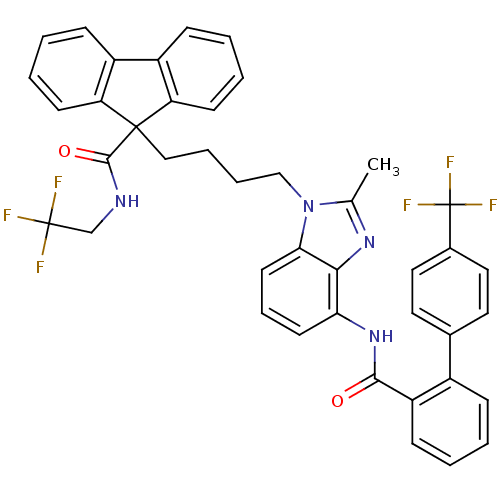 (9-(4-{2-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098325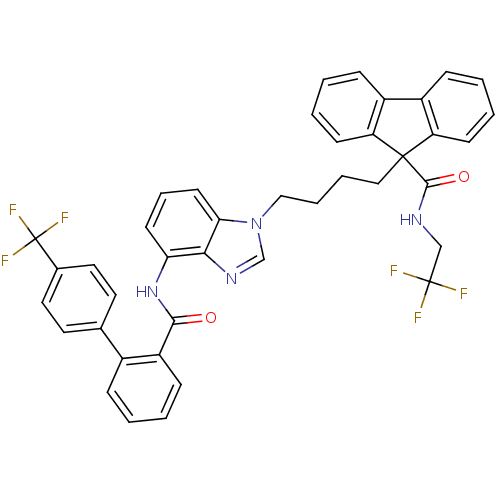 (9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098320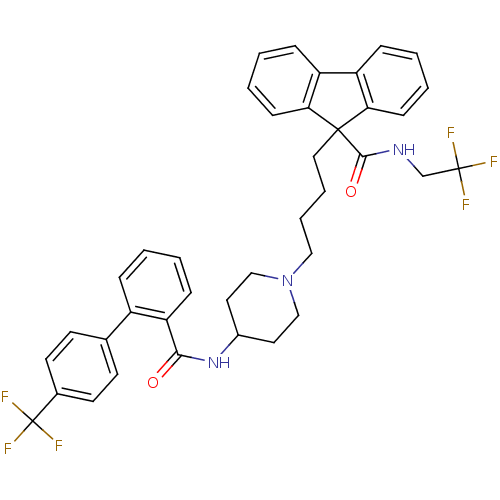 (9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098321 (9-(4-{2,5-Dimethyl-4-[(4'-trifluoromethyl-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098324 (9-(4-{2-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098322 (9-(4-{5-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098323 (9-(4-{2-Propyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098326 (9-(4-{2-Isopropyl-4-[(4'-trifluoromethyl-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098325 (9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098320 (9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein using triglyceride transfer assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098327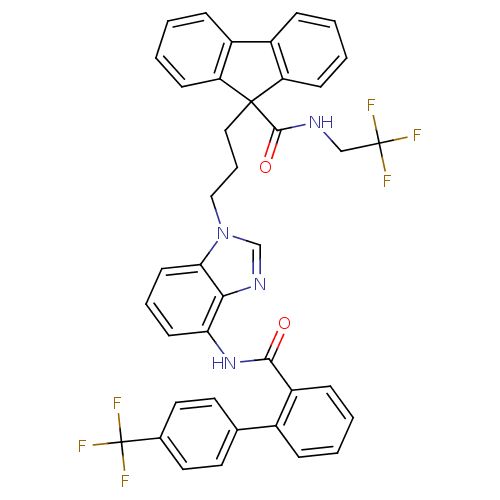 (9-(3-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209010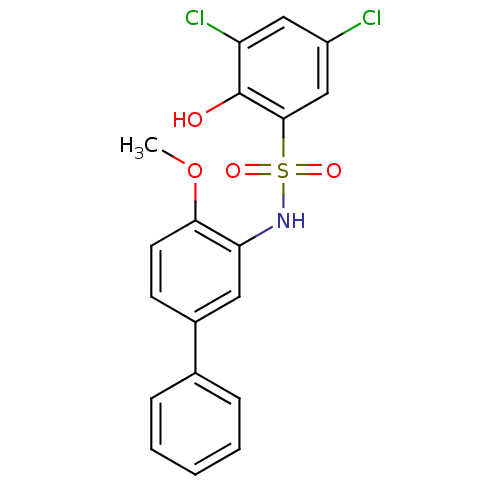 (3,5-dichloro-2-hydroxy-N-(4-methoxy-biphenyl-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50036210 ((2S,3S)-3-Carboxy-2,3-dihydroxy-pentanedioic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209015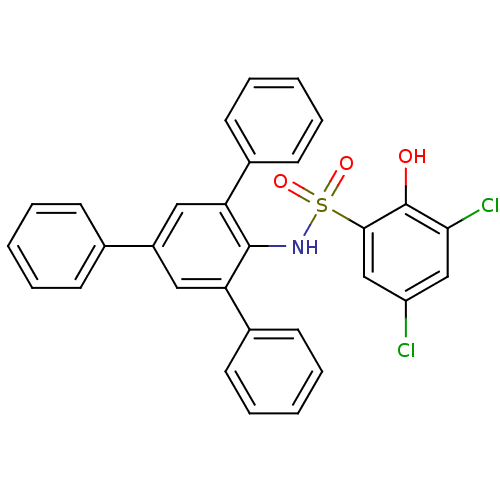 (3,5-dichloro-2-hydroxy-N-(2,4,6-triphenylphenyl)be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209014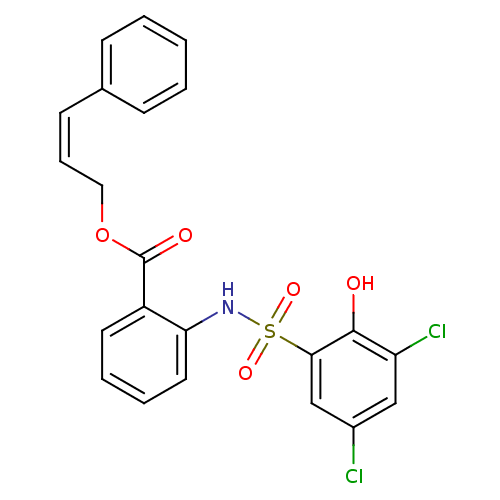 ((Z)-3-phenylallyl 2-(3,5-dichloro-2-hydroxyphenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209009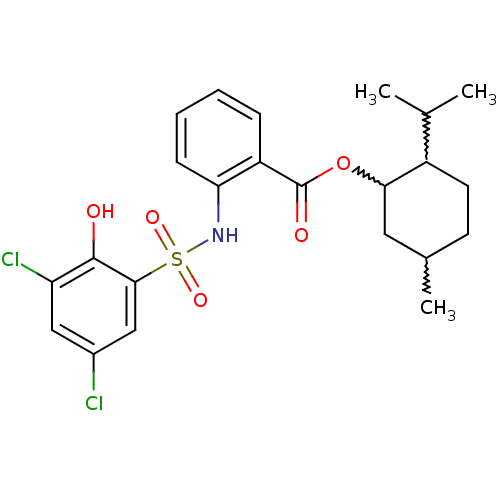 (2-isopropyl-5-methylcyclohexyl 2-(3,5-dichloro-2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209018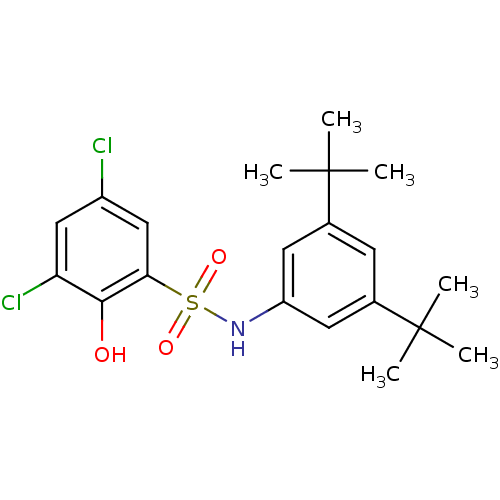 (3,5-dichloro-N-(3,5-di-tert-butylphenyl)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50066683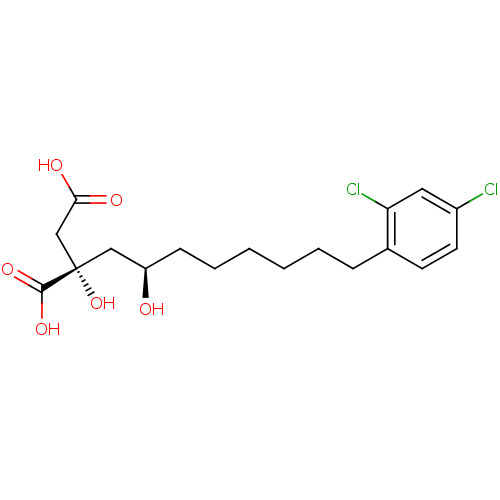 ((S)-2-((R)-8-(2,4-dichlorophenyl)-2-hydroxyoctyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209013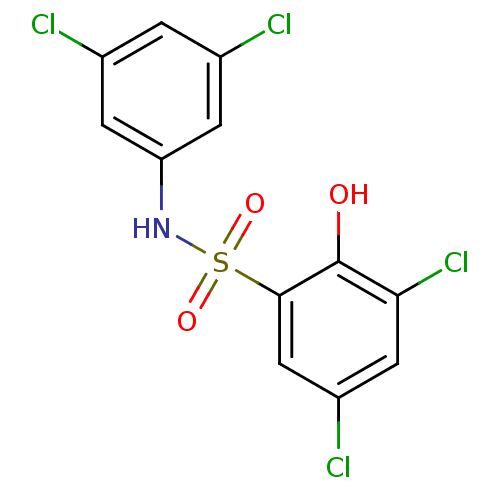 (3,5-dichloro-N-(3,5-dichlorophenyl)-2-hydroxybenze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209008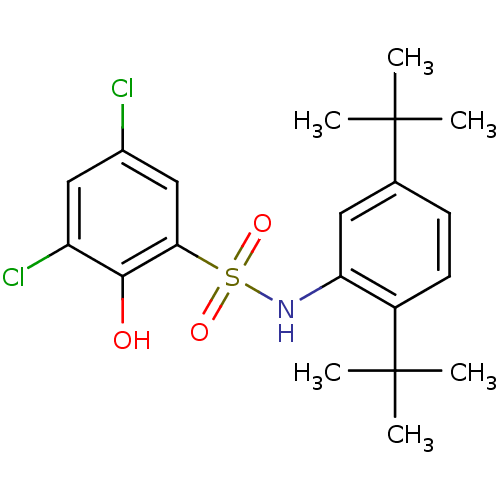 (3,5-dichloro-N-(2,5-di-tert-butylphenyl)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50209010 (3,5-dichloro-2-hydroxy-N-(4-methoxy-biphenyl-3-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human ACC1 | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209012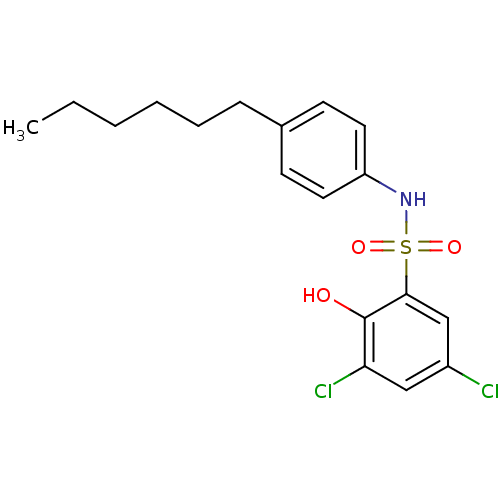 (3,5-dichloro-N-(4-hexylphenyl)-2-hydroxybenzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209007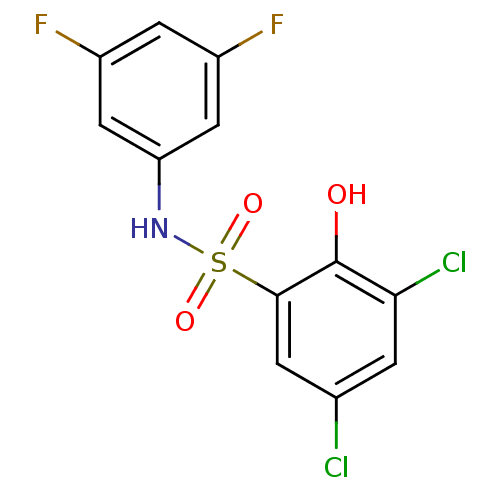 (3,5-dichloro-N-(3,5-difluorophenyl)-2-hydroxybenze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209017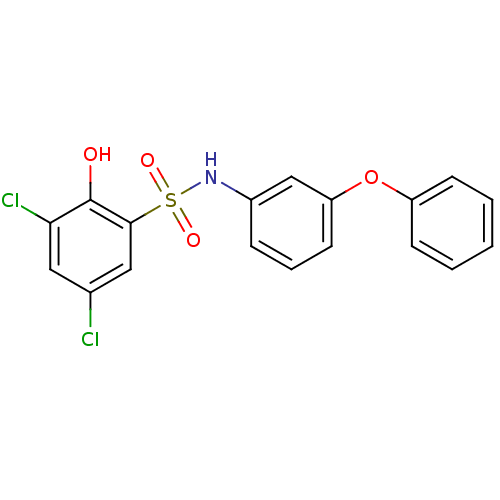 (3,5-dichloro-2-hydroxy-N-(3-phenoxyphenyl)benzenes...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Homo sapiens (Human)) | BDBM50209011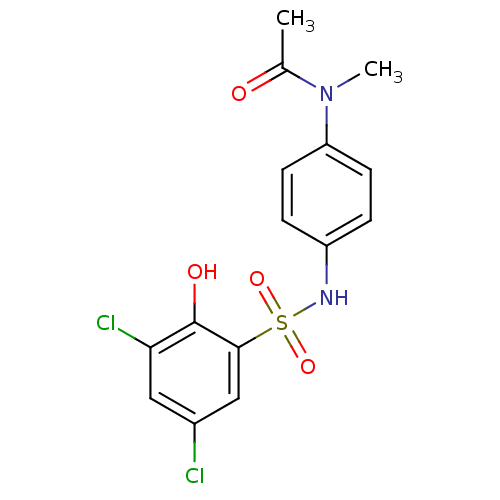 (CHEMBL250744 | N-(4-(3,5-dichloro-2-hydroxyphenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ATP-citrate lyase | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50209010 (3,5-dichloro-2-hydroxy-N-(4-methoxy-biphenyl-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human ACC2 | Bioorg Med Chem Lett 17: 3208-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.017 BindingDB Entry DOI: 10.7270/Q27P8Z26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||