 Found 1184 hits with Last Name = 'janc' and Initial = 'j'
Found 1184 hits with Last Name = 'janc' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM17284
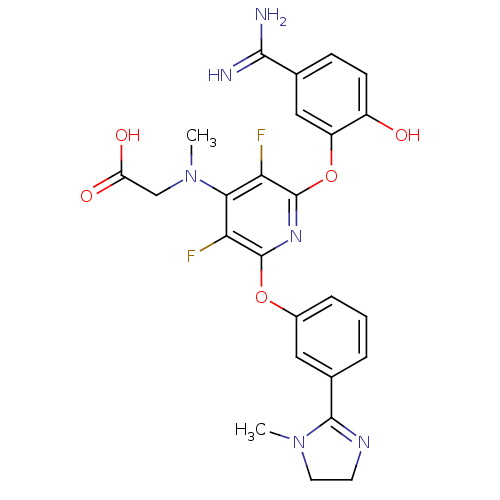
(2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C25H24F2N6O5/c1-32-9-8-30-23(32)14-4-3-5-15(10-14)37-24-19(26)21(33(2)12-18(35)36)20(27)25(31-24)38-17-11-13(22(28)29)6-7-16(17)34/h3-7,10-11,34H,8-9,12H2,1-2H3,(H3,28,29)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Biochemistry 39: 12534-42 (2000)
Article DOI: 10.1021/bi001477q
BindingDB Entry DOI: 10.7270/Q2C827JM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17280

(1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(N3CCCC(C3)C(O)=O)c2F)c1 |c:4| Show InChI InChI=1S/C28H28F2N6O5/c1-35-11-9-33-25(35)16-4-2-6-18(12-16)40-26-21(29)23(36-10-3-5-17(14-36)28(38)39)22(30)27(34-26)41-20-13-15(24(31)32)7-8-19(20)37/h2,4,6-8,12-13,17,37H,3,5,9-11,14H2,1H3,(H3,31,32)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Biochemistry 39: 12534-42 (2000)
Article DOI: 10.1021/bi001477q
BindingDB Entry DOI: 10.7270/Q2C827JM |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50410979
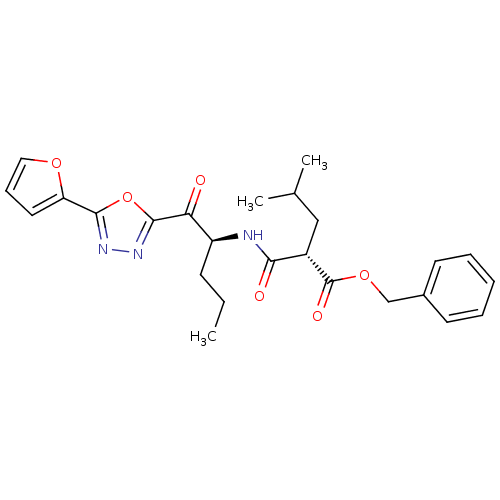
(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50410979

(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50410971
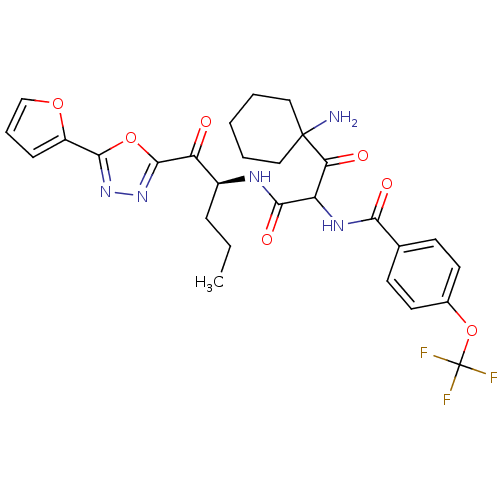
(CHEMBL378899)Show SMILES CCC[C@H](NC(=O)C(NC(=O)c1ccc(OC(F)(F)F)cc1)C(=O)C1(N)CCCCC1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C28H30F3N5O7/c1-2-7-18(21(37)26-36-35-25(42-26)19-8-6-15-41-19)33-24(40)20(22(38)27(32)13-4-3-5-14-27)34-23(39)16-9-11-17(12-10-16)43-28(29,30)31/h6,8-12,15,18,20H,2-5,7,13-14,32H2,1H3,(H,33,40)(H,34,39)/t18-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410611
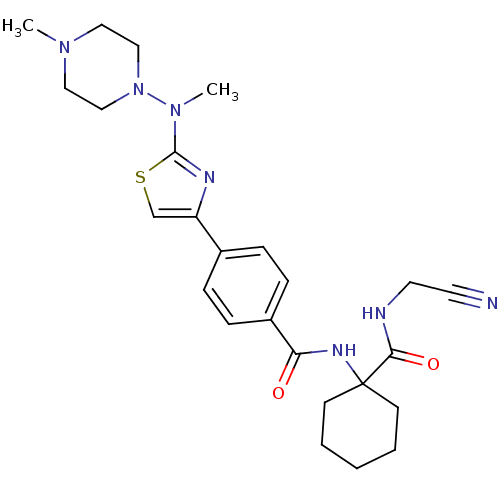
(CHEMBL414669)Show SMILES CN(N1CCN(C)CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H33N7O2S/c1-30-14-16-32(17-15-30)31(2)24-28-21(18-35-24)19-6-8-20(9-7-19)22(33)29-25(10-4-3-5-11-25)23(34)27-13-12-26/h6-9,18H,3-5,10-11,13-17H2,1-2H3,(H,27,34)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410588
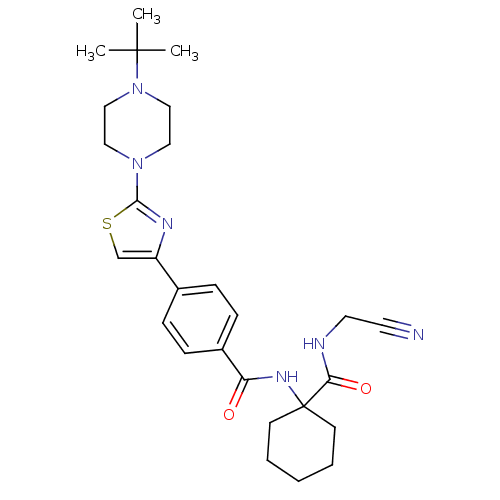
(CHEMBL200708)Show SMILES CC(C)(C)N1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-26(2,3)33-17-15-32(16-18-33)25-30-22(19-36-25)20-7-9-21(10-8-20)23(34)31-27(11-5-4-6-12-27)24(35)29-14-13-28/h7-10,19H,4-6,11-12,14-18H2,1-3H3,(H,29,35)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410609
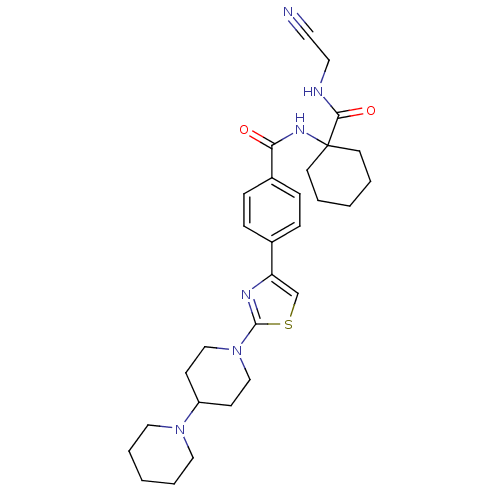
(CHEMBL198798)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C29H38N6O2S/c30-15-16-31-27(37)29(13-3-1-4-14-29)33-26(36)23-9-7-22(8-10-23)25-21-38-28(32-25)35-19-11-24(12-20-35)34-17-5-2-6-18-34/h7-10,21,24H,1-6,11-14,16-20H2,(H,31,37)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410590
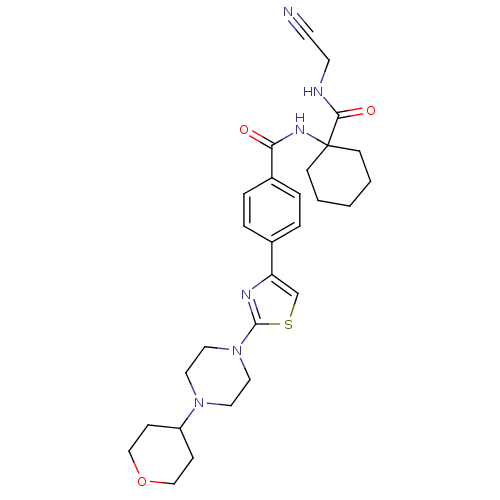
(CHEMBL200543)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCN(CC1)C1CCOCC1 Show InChI InChI=1S/C28H36N6O3S/c29-12-13-30-26(36)28(10-2-1-3-11-28)32-25(35)22-6-4-21(5-7-22)24-20-38-27(31-24)34-16-14-33(15-17-34)23-8-18-37-19-9-23/h4-7,20,23H,1-3,8-11,13-19H2,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410587
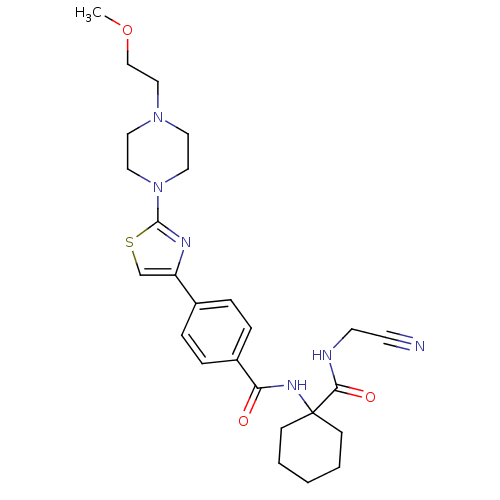
(CHEMBL200602)Show SMILES COCCN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H34N6O3S/c1-35-18-17-31-13-15-32(16-14-31)25-29-22(19-36-25)20-5-7-21(8-6-20)23(33)30-26(9-3-2-4-10-26)24(34)28-12-11-27/h5-8,19H,2-4,9-10,12-18H2,1H3,(H,28,34)(H,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410903
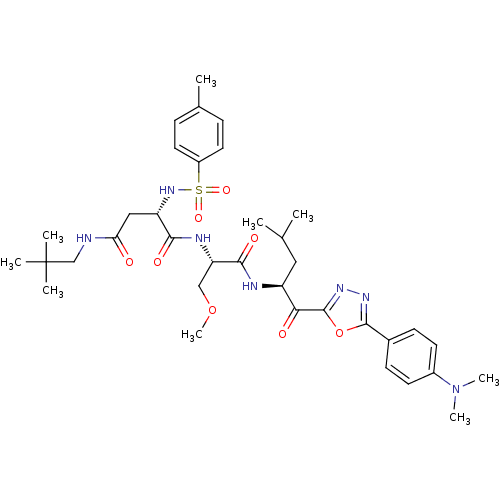
(CHEMBL207598)Show SMILES COC[C@H](NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)N[C@@H](CC(C)C)C(=O)c1nnc(o1)-c1ccc(cc1)N(C)C Show InChI InChI=1S/C36H51N7O8S/c1-22(2)18-27(31(45)35-41-40-34(51-35)24-12-14-25(15-13-24)43(7)8)38-33(47)29(20-50-9)39-32(46)28(19-30(44)37-21-36(4,5)6)42-52(48,49)26-16-10-23(3)11-17-26/h10-17,22,27-29,42H,18-21H2,1-9H3,(H,37,44)(H,38,47)(H,39,46)/t27-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410898
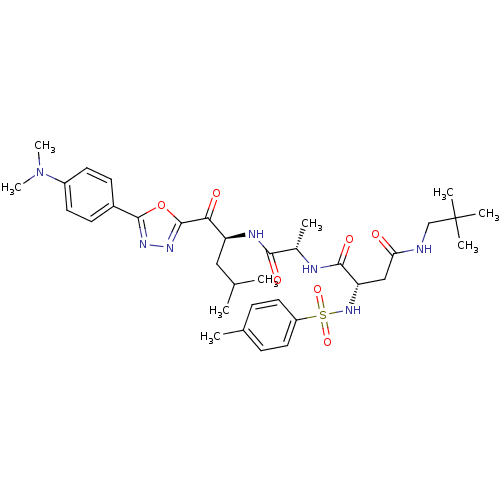
(CHEMBL205757)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)c1nnc(o1)-c1ccc(cc1)N(C)C Show InChI InChI=1S/C35H49N7O7S/c1-21(2)18-27(30(44)34-40-39-33(49-34)24-12-14-25(15-13-24)42(8)9)38-31(45)23(4)37-32(46)28(19-29(43)36-20-35(5,6)7)41-50(47,48)26-16-10-22(3)11-17-26/h10-17,21,23,27-28,41H,18-20H2,1-9H3,(H,36,43)(H,37,46)(H,38,45)/t23-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410607
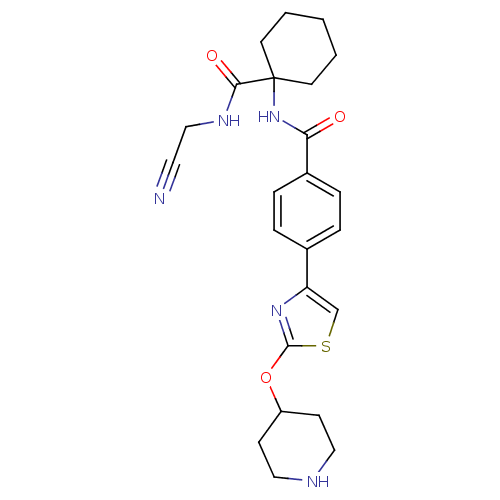
(CHEMBL200744)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(OC2CCNCC2)n1 Show InChI InChI=1S/C24H29N5O3S/c25-12-15-27-22(31)24(10-2-1-3-11-24)29-21(30)18-6-4-17(5-7-18)20-16-33-23(28-20)32-19-8-13-26-14-9-19/h4-7,16,19,26H,1-3,8-11,13-15H2,(H,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410571
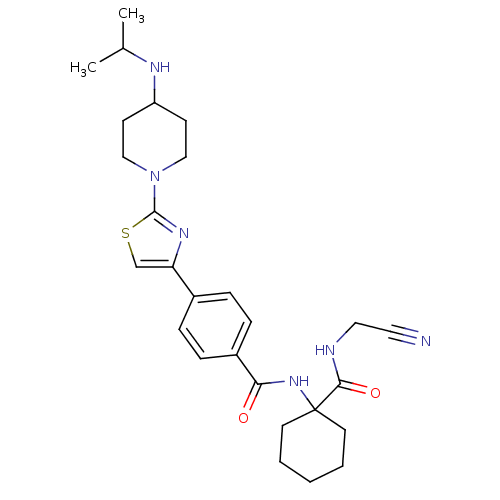
(CHEMBL200287)Show SMILES CC(C)NC1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-19(2)30-22-10-16-33(17-11-22)26-31-23(18-36-26)20-6-8-21(9-7-20)24(34)32-27(12-4-3-5-13-27)25(35)29-15-14-28/h6-9,18-19,22,30H,3-5,10-13,15-17H2,1-2H3,(H,29,35)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410901
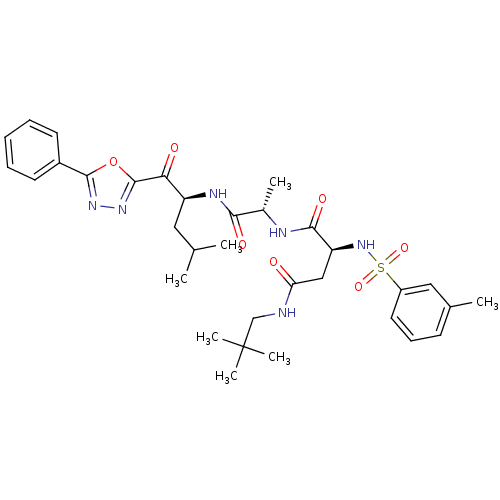
(CHEMBL206413)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1cccc(C)c1)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C33H44N6O7S/c1-20(2)16-25(28(41)32-38-37-31(46-32)23-13-9-8-10-14-23)36-29(42)22(4)35-30(43)26(18-27(40)34-19-33(5,6)7)39-47(44,45)24-15-11-12-21(3)17-24/h8-15,17,20,22,25-26,39H,16,18-19H2,1-7H3,(H,34,40)(H,35,43)(H,36,42)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410580
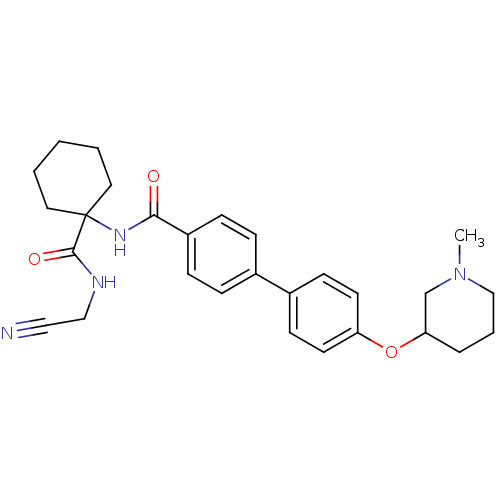
(CHEMBL435913)Show SMILES CN1CCCC(C1)Oc1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H34N4O3/c1-32-19-5-6-25(20-32)35-24-13-11-22(12-14-24)21-7-9-23(10-8-21)26(33)31-28(15-3-2-4-16-28)27(34)30-18-17-29/h7-14,25H,2-6,15-16,18-20H2,1H3,(H,30,34)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410904
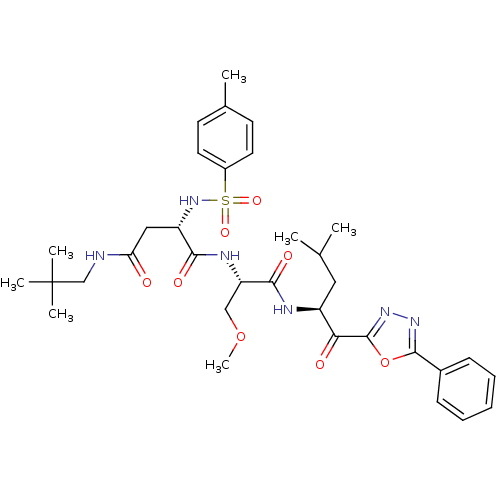
(CHEMBL377532)Show SMILES COC[C@H](NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)N[C@@H](CC(C)C)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H46N6O8S/c1-21(2)17-25(29(42)33-39-38-32(48-33)23-11-9-8-10-12-23)36-31(44)27(19-47-7)37-30(43)26(18-28(41)35-20-34(4,5)6)40-49(45,46)24-15-13-22(3)14-16-24/h8-16,21,25-27,40H,17-20H2,1-7H3,(H,35,41)(H,36,44)(H,37,43)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410899

(CHEMBL383674)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C33H44N6O7S/c1-20(2)17-25(28(41)32-38-37-31(46-32)23-11-9-8-10-12-23)36-29(42)22(4)35-30(43)26(18-27(40)34-19-33(5,6)7)39-47(44,45)24-15-13-21(3)14-16-24/h8-16,20,22,25-26,39H,17-19H2,1-7H3,(H,34,40)(H,35,43)(H,36,42)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410575
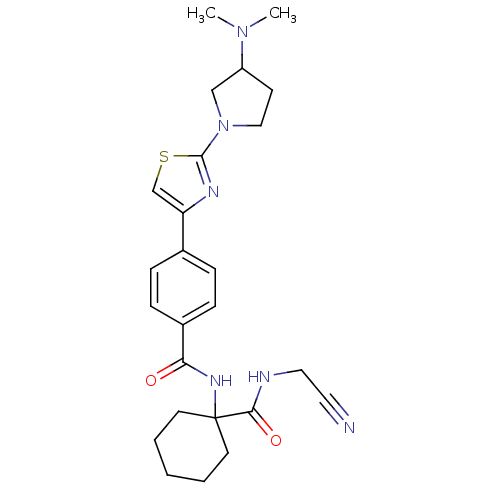
(CHEMBL199470)Show SMILES CN(C)C1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H32N6O2S/c1-30(2)20-10-15-31(16-20)24-28-21(17-34-24)18-6-8-19(9-7-18)22(32)29-25(11-4-3-5-12-25)23(33)27-14-13-26/h6-9,17,20H,3-5,10-12,14-16H2,1-2H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410591

(CHEMBL200506)Show SMILES CN1CCN(Cc2nc(cs2)-c2ccc(cc2)C(=O)NC2(CCCCC2)C(=O)NCC#N)CC1 Show InChI InChI=1S/C25H32N6O2S/c1-30-13-15-31(16-14-30)17-22-28-21(18-34-22)19-5-7-20(8-6-19)23(32)29-25(9-3-2-4-10-25)24(33)27-12-11-26/h5-8,18H,2-4,9-10,12-17H2,1H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50374399

(CHEMBL209293)Show SMILES CCC[C@H](NC(=O)C1(CCCCC1)NC(=O)c1ccc(cc1)N(C)C)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C27H33N5O5/c1-4-9-20(22(33)25-31-30-24(37-25)21-10-8-17-36-21)28-26(35)27(15-6-5-7-16-27)29-23(34)18-11-13-19(14-12-18)32(2)3/h8,10-14,17,20H,4-7,9,15-16H2,1-3H3,(H,28,35)(H,29,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410612
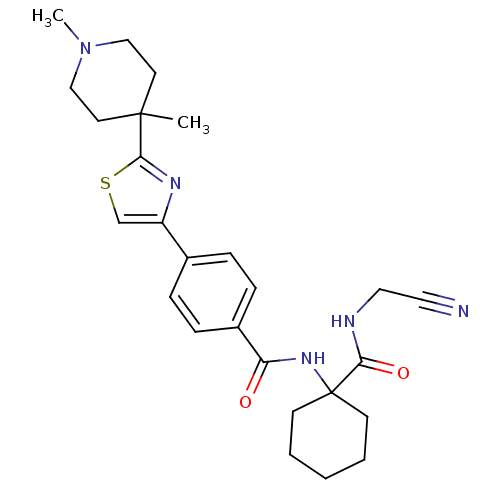
(CHEMBL200166)Show SMILES CN1CCC(C)(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H33N5O2S/c1-25(12-16-31(2)17-13-25)24-29-21(18-34-24)19-6-8-20(9-7-19)22(32)30-26(10-4-3-5-11-26)23(33)28-15-14-27/h6-9,18H,3-5,10-13,15-17H2,1-2H3,(H,28,33)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176251

(2-((6-chloro-1H-benzo[d]imidazol-2-yl)methyl)-1H-b...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(Cl)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H13ClN6/c17-9-2-4-11-13(6-9)23-15(21-11)7-14-20-10-3-1-8(16(18)19)5-12(10)22-14/h1-6H,7H2,(H3,18,19)(H,20,22)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM16303
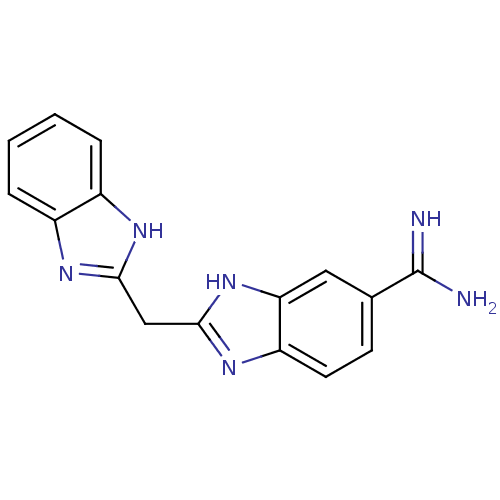
(2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...)Show InChI InChI=1S/C16H14N6/c17-16(18)9-5-6-12-13(7-9)22-15(21-12)8-14-19-10-3-1-2-4-11(10)20-14/h1-7H,8H2,(H3,17,18)(H,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM16303

(2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...)Show InChI InChI=1S/C16H14N6/c17-16(18)9-5-6-12-13(7-9)22-15(21-12)8-14-19-10-3-1-2-4-11(10)20-14/h1-7H,8H2,(H3,17,18)(H,19,20)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50181921
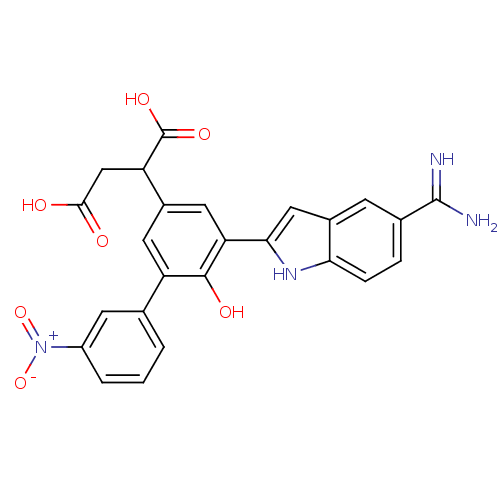
(2-[5-(5-carbamimidoyl-1H-indol-2-yl)-6-hydroxy-3'-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1cccc(c1)[N+]([O-])=O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H20N4O7/c26-24(27)13-4-5-20-15(6-13)10-21(28-20)19-9-14(18(25(33)34)11-22(30)31)8-17(23(19)32)12-2-1-3-16(7-12)29(35)36/h1-10,18,28,32H,11H2,(H3,26,27)(H,30,31)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to factor7a/TF complex |
Bioorg Med Chem Lett 16: 2243-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.037
BindingDB Entry DOI: 10.7270/Q2GB23NX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176252
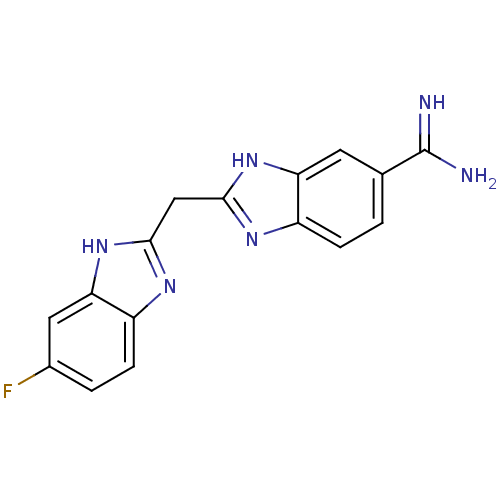
(2-((6-fluoro-1H-benzo[d]imidazol-2-yl)methyl)-1H-b...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(F)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H13FN6/c17-9-2-4-11-13(6-9)23-15(21-11)7-14-20-10-3-1-8(16(18)19)5-12(10)22-14/h1-6H,7H2,(H3,18,19)(H,20,22)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50410979

(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14338
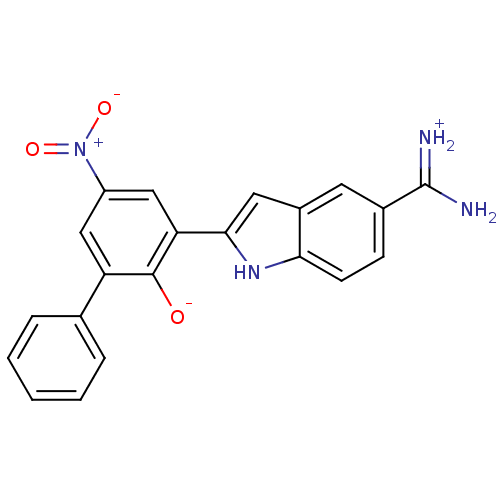
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(cc(-c2ccccc2)c1[O-])[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19855
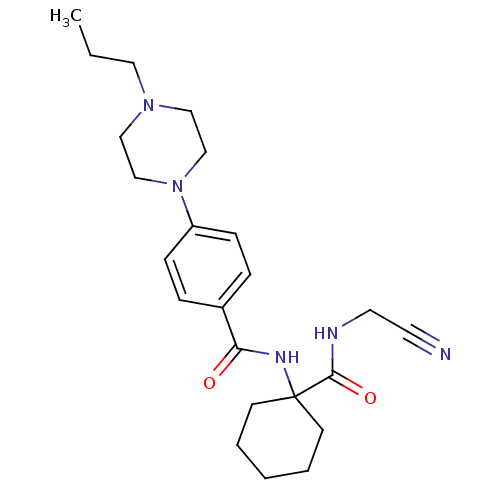
(Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...)Show SMILES CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H33N5O2/c1-2-14-27-15-17-28(18-16-27)20-8-6-19(7-9-20)21(29)26-23(10-4-3-5-11-23)22(30)25-13-12-24/h6-9H,2-5,10-11,13-18H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410595
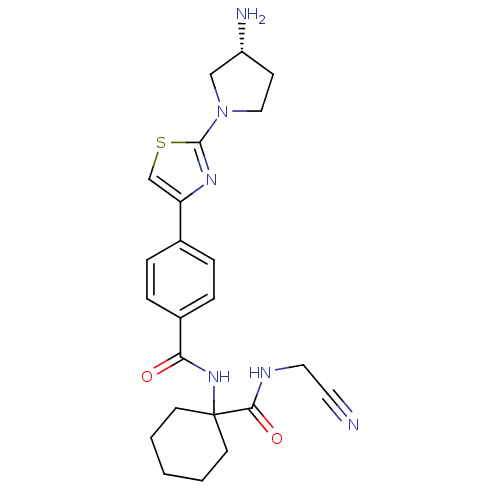
(CHEMBL200507)Show SMILES N[C@@H]1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H28N6O2S/c24-11-12-26-21(31)23(9-2-1-3-10-23)28-20(30)17-6-4-16(5-7-17)19-15-32-22(27-19)29-13-8-18(25)14-29/h4-7,15,18H,1-3,8-10,12-14,25H2,(H,26,31)(H,28,30)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410572
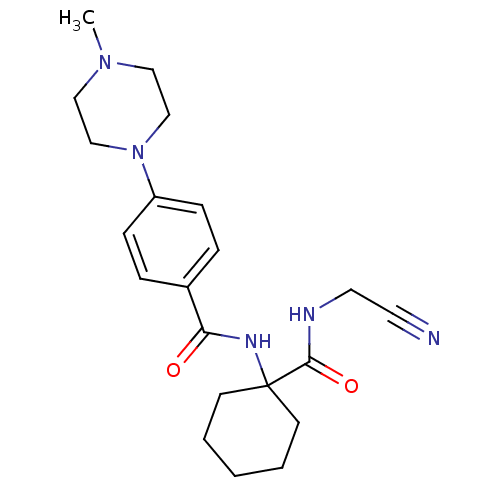
(CHEMBL440035)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C21H29N5O2/c1-25-13-15-26(16-14-25)18-7-5-17(6-8-18)19(27)24-21(9-3-2-4-10-21)20(28)23-12-11-22/h5-8H,2-4,9-10,12-16H2,1H3,(H,23,28)(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187167
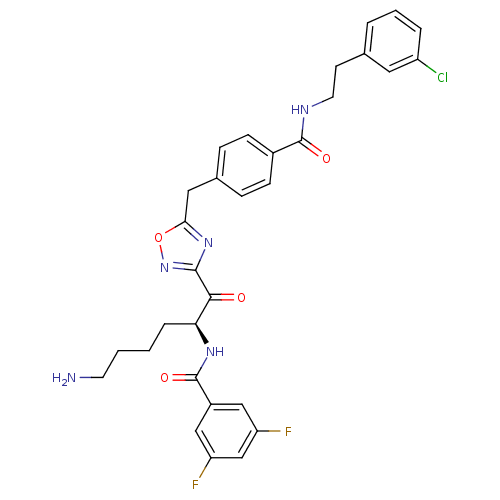
(CHEMBL211357 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1cc(F)cc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(14-23)11-13-36-30(41)21-9-7-20(8-10-21)15-27-38-29(39-43-27)28(40)26(6-1-2-12-35)37-31(42)22-16-24(33)18-25(34)17-22/h3-5,7-10,14,16-18,26H,1-2,6,11-13,15,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410594
(CHEMBL200596)Show SMILES CC(C)N(C)C1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H38N6O2S/c1-20(2)33(3)23-11-17-34(18-12-23)27-31-24(19-37-27)21-7-9-22(10-8-21)25(35)32-28(13-5-4-6-14-28)26(36)30-16-15-29/h7-10,19-20,23H,4-6,11-14,16-18H2,1-3H3,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
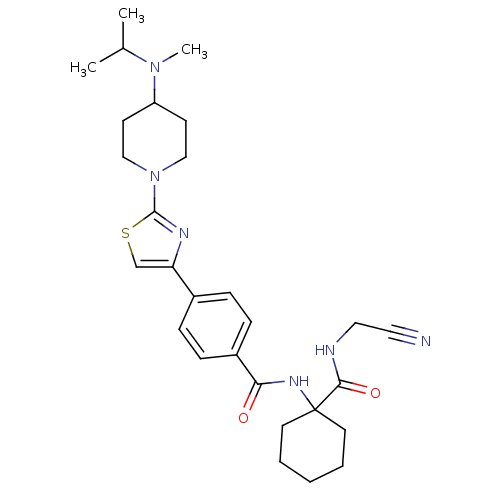
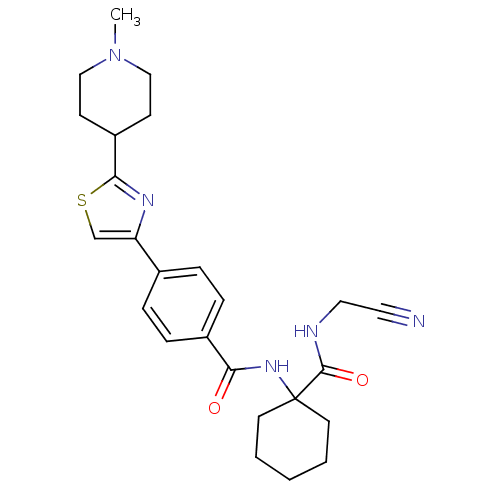
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410592
(CHEMBL200455)Show SMILES CN1CCC(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H31N5O2S/c1-30-15-9-20(10-16-30)23-28-21(17-33-23)18-5-7-19(8-6-18)22(31)29-25(11-3-2-4-12-25)24(32)27-14-13-26/h5-8,17,20H,2-4,9-12,14-16H2,1H3,(H,27,32)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187166
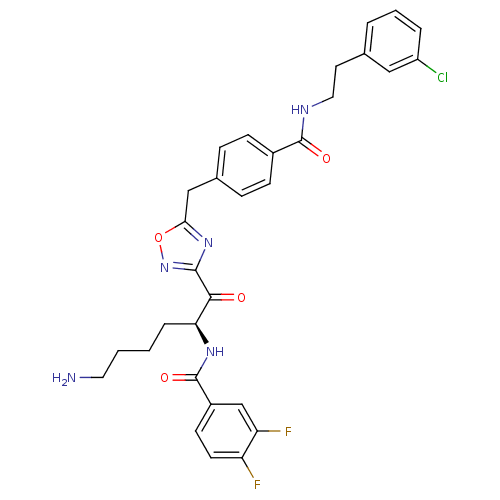
(CHEMBL380293 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(16-23)13-15-36-30(41)21-9-7-20(8-10-21)17-27-38-29(39-43-27)28(40)26(6-1-2-14-35)37-31(42)22-11-12-24(33)25(34)18-22/h3-5,7-12,16,18,26H,1-2,6,13-15,17,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50374365

(CHEMBL209215)Show SMILES CCC[C@H](NC(=O)C1(CCCCC1)NC(=O)c1ccc(OC(F)(F)F)cc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C26H27F3N4O6/c1-2-7-18(20(34)23-33-32-22(38-23)19-8-6-15-37-19)30-24(36)25(13-4-3-5-14-25)31-21(35)16-9-11-17(12-10-16)39-26(27,28)29/h6,8-12,15,18H,2-5,7,13-14H2,1H3,(H,30,36)(H,31,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187177
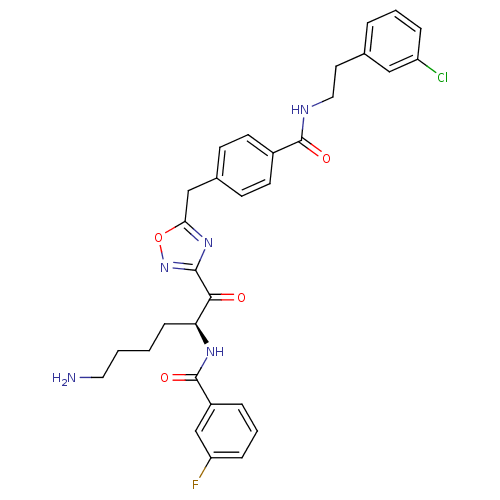
(CHEMBL212774 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1cccc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-7-3-5-20(17-24)14-16-35-30(40)22-12-10-21(11-13-22)18-27-37-29(38-42-27)28(39)26(9-1-2-15-34)36-31(41)23-6-4-8-25(33)19-23/h3-8,10-13,17,19,26H,1-2,9,14-16,18,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187160

(CHEMBL213928 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-5-3-4-20(18-24)15-17-35-30(40)22-9-7-21(8-10-22)19-27-37-29(38-42-27)28(39)26(6-1-2-16-34)36-31(41)23-11-13-25(33)14-12-23/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187163
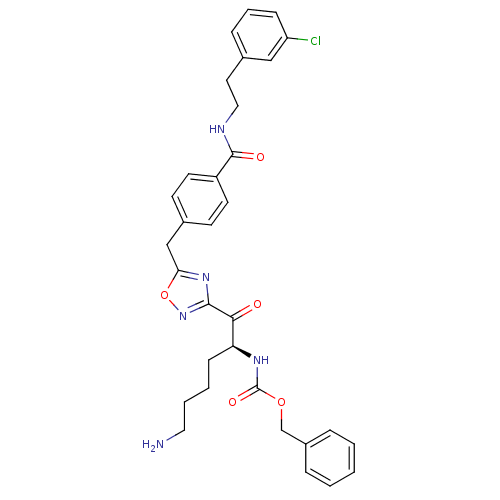
((S)-benzyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)b...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H34ClN5O5/c33-26-10-6-9-22(19-26)16-18-35-31(40)25-14-12-23(13-15-25)20-28-37-30(38-43-28)29(39)27(11-4-5-17-34)36-32(41)42-21-24-7-2-1-3-8-24/h1-3,6-10,12-15,19,27H,4-5,11,16-18,20-21,34H2,(H,35,40)(H,36,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187168
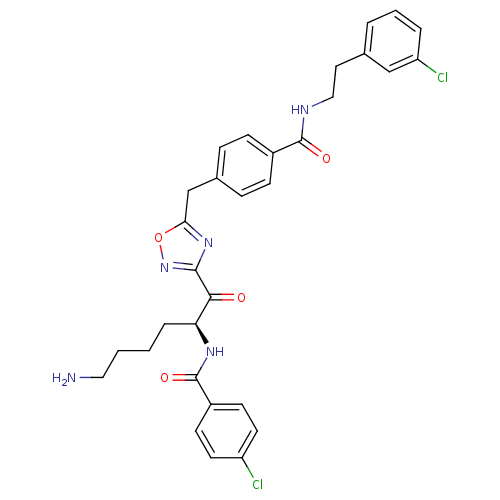
(CHEMBL377656 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(Cl)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31Cl2N5O4/c32-24-13-11-23(12-14-24)31(41)36-26(6-1-2-16-34)28(39)29-37-27(42-38-29)19-21-7-9-22(10-8-21)30(40)35-17-15-20-4-3-5-25(33)18-20/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187176
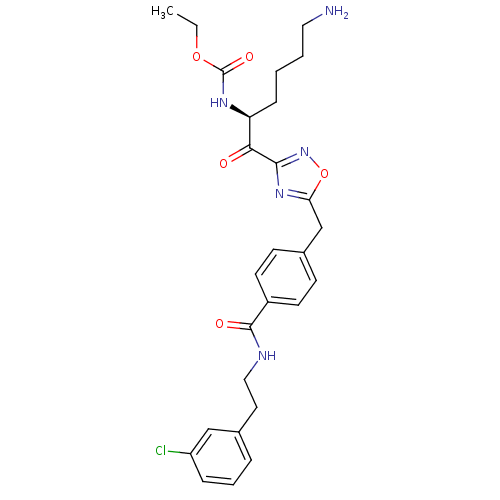
((S)-ethyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)be...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C27H32ClN5O5/c1-2-37-27(36)31-22(8-3-4-14-29)24(34)25-32-23(38-33-25)17-19-9-11-20(12-10-19)26(35)30-15-13-18-6-5-7-21(28)16-18/h5-7,9-12,16,22H,2-4,8,13-15,17,29H2,1H3,(H,30,35)(H,31,36)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50410979

(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180400
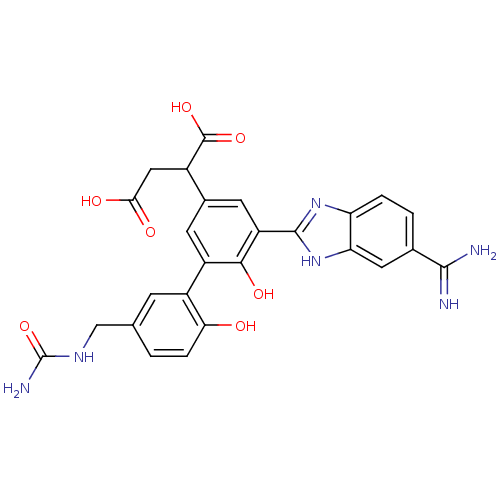
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-6,2'-...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H24N6O7/c27-23(28)12-2-3-18-19(8-12)32-24(31-18)17-7-13(14(25(37)38)9-21(34)35)6-16(22(17)36)15-5-11(1-4-20(15)33)10-30-26(29)39/h1-8,14,33,36H,9-10H2,(H3,27,28)(H,31,32)(H,34,35)(H,37,38)(H3,29,30,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410606
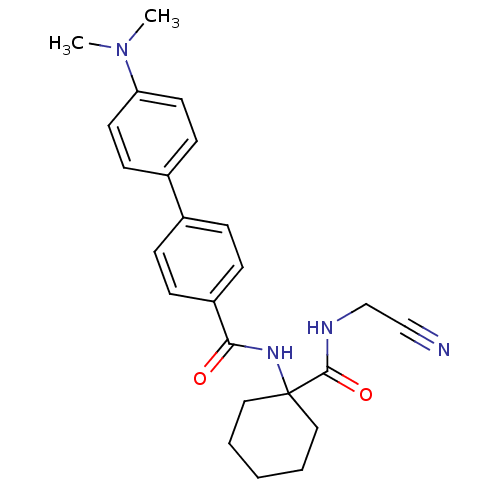
(CHEMBL383186)Show SMILES CN(C)c1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H28N4O2/c1-28(2)21-12-10-19(11-13-21)18-6-8-20(9-7-18)22(29)27-24(14-4-3-5-15-24)23(30)26-17-16-25/h6-13H,3-5,14-15,17H2,1-2H3,(H,26,30)(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50181916
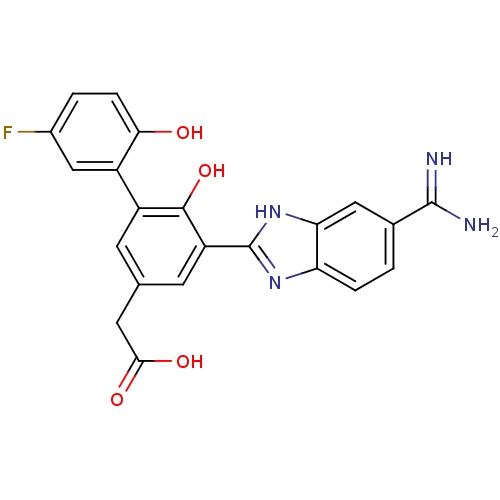
(CHEMBL206168 | [5-(5-carbamimidoyl-1H-benzoimidazo...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(CC(O)=O)cc(c1O)-c1cc(F)ccc1O Show InChI InChI=1S/C22H17FN4O4/c23-12-2-4-18(28)13(9-12)14-5-10(7-19(29)30)6-15(20(14)31)22-26-16-3-1-11(21(24)25)8-17(16)27-22/h1-6,8-9,28,31H,7H2,(H3,24,25)(H,26,27)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to factor7a/TF complex |
Bioorg Med Chem Lett 16: 2243-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.037
BindingDB Entry DOI: 10.7270/Q2GB23NX |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187162
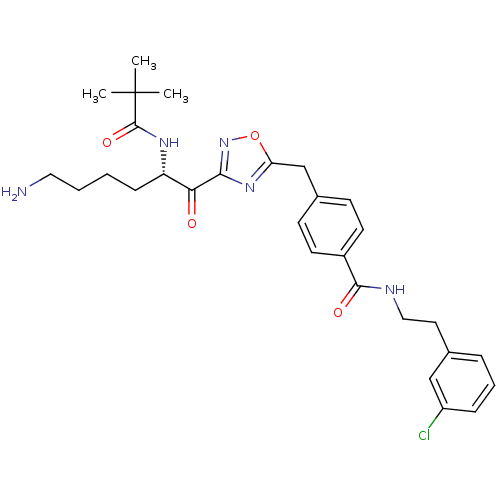
((S)-N-(3-chlorophenethyl)-4-((3-(6-amino-2-pivalam...)Show SMILES CC(C)(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C29H36ClN5O4/c1-29(2,3)28(38)33-23(9-4-5-15-31)25(36)26-34-24(39-35-26)18-20-10-12-21(13-11-20)27(37)32-16-14-19-7-6-8-22(30)17-19/h6-8,10-13,17,23H,4-5,9,14-16,18,31H2,1-3H3,(H,32,37)(H,33,38)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176257
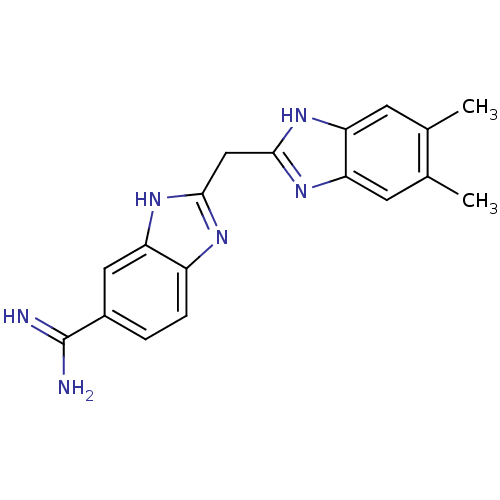
(2-((5,6-dimethyl-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES Cc1cc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2cc1C Show InChI InChI=1S/C18H18N6/c1-9-5-13-14(6-10(9)2)23-17(22-13)8-16-21-12-4-3-11(18(19)20)7-15(12)24-16/h3-7H,8H2,1-2H3,(H3,19,20)(H,21,24)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data