 Found 49 hits with Last Name = 'jaramillo' and Initial = 'j'
Found 49 hits with Last Name = 'jaramillo' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50228710
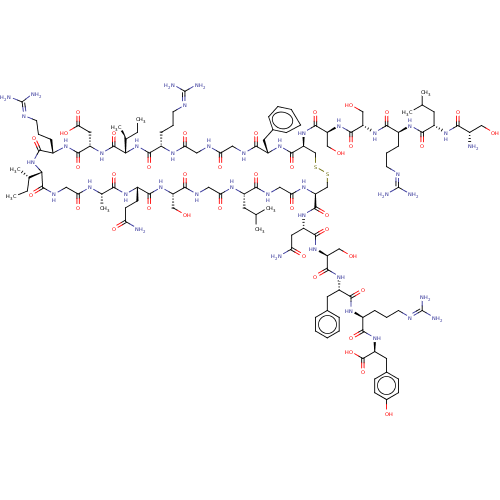
(CHEMBL3349899)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C122H193N41O38S2/c1-10-61(7)95-116(198)142-49-90(173)143-63(9)97(179)147-73(34-35-87(124)170)104(186)157-81(53-165)101(183)141-50-92(175)145-74(40-59(3)4)99(181)140-51-93(176)146-85(114(196)154-78(45-88(125)171)109(191)159-82(54-166)111(193)153-77(43-65-24-16-13-17-25-65)108(190)149-70(27-19-37-135-120(128)129)102(184)156-80(118(200)201)44-66-30-32-67(169)33-31-66)57-202-203-58-86(161-113(195)84(56-168)160-112(194)83(55-167)158-103(185)71(28-20-38-136-121(130)131)148-107(189)75(41-60(5)6)151-98(180)68(123)52-164)115(197)152-76(42-64-22-14-12-15-23-64)100(182)139-47-89(172)138-48-91(174)144-69(26-18-36-134-119(126)127)105(187)163-96(62(8)11-2)117(199)155-79(46-94(177)178)110(192)150-72(106(188)162-95)29-21-39-137-122(132)133/h12-17,22-25,30-33,59-63,68-86,95-96,164-169H,10-11,18-21,26-29,34-58,123H2,1-9H3,(H2,124,170)(H2,125,171)(H,138,172)(H,139,182)(H,140,181)(H,141,183)(H,142,198)(H,143,173)(H,144,174)(H,145,175)(H,146,176)(H,147,179)(H,148,189)(H,149,190)(H,150,192)(H,151,180)(H,152,197)(H,153,193)(H,154,196)(H,155,199)(H,156,184)(H,157,186)(H,158,185)(H,159,191)(H,160,194)(H,161,195)(H,162,188)(H,163,187)(H,177,178)(H,200,201)(H4,126,127,134)(H4,128,129,135)(H4,130,131,136)(H4,132,133,137)/t61-,62-,63-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,95-,96-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228710

(CHEMBL3349899)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C122H193N41O38S2/c1-10-61(7)95-116(198)142-49-90(173)143-63(9)97(179)147-73(34-35-87(124)170)104(186)157-81(53-165)101(183)141-50-92(175)145-74(40-59(3)4)99(181)140-51-93(176)146-85(114(196)154-78(45-88(125)171)109(191)159-82(54-166)111(193)153-77(43-65-24-16-13-17-25-65)108(190)149-70(27-19-37-135-120(128)129)102(184)156-80(118(200)201)44-66-30-32-67(169)33-31-66)57-202-203-58-86(161-113(195)84(56-168)160-112(194)83(55-167)158-103(185)71(28-20-38-136-121(130)131)148-107(189)75(41-60(5)6)151-98(180)68(123)52-164)115(197)152-76(42-64-22-14-12-15-23-64)100(182)139-47-89(172)138-48-91(174)144-69(26-18-36-134-119(126)127)105(187)163-96(62(8)11-2)117(199)155-79(46-94(177)178)110(192)150-72(106(188)162-95)29-21-39-137-122(132)133/h12-17,22-25,30-33,59-63,68-86,95-96,164-169H,10-11,18-21,26-29,34-58,123H2,1-9H3,(H2,124,170)(H2,125,171)(H,138,172)(H,139,182)(H,140,181)(H,141,183)(H,142,198)(H,143,173)(H,144,174)(H,145,175)(H,146,176)(H,147,179)(H,148,189)(H,149,190)(H,150,192)(H,151,180)(H,152,197)(H,153,193)(H,154,196)(H,155,199)(H,156,184)(H,157,186)(H,158,185)(H,159,191)(H,160,194)(H,161,195)(H,162,188)(H,163,187)(H,177,178)(H,200,201)(H4,126,127,134)(H4,128,129,135)(H4,130,131,136)(H4,132,133,137)/t61-,62-,63-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,95-,96-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013340
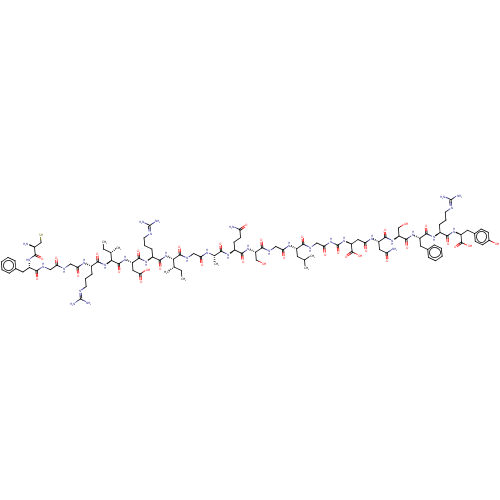
(CHEMBL3349626)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16])-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H158N34O33S/c1-8-51(5)81(136-91(159)61(25-18-34-115-102(111)112)127-94(162)67(41-80(149)150)130-97(165)82(52(6)9-2)137-90(158)59(23-16-32-113-100(107)108)122-77(146)43-116-75(144)42-117-86(154)64(128-84(152)58(104)49-171)36-54-19-12-10-13-20-54)96(164)120-44-76(145)121-53(7)83(151)125-62(30-31-72(105)141)89(157)132-70(47-138)87(155)119-45-78(147)124-63(35-50(3)4)85(153)118-46-79(148)135-103(170)134-69(99(168)169)40-74(143)123-66(39-73(106)142)93(161)133-71(48-139)95(163)129-65(37-55-21-14-11-15-22-55)92(160)126-60(24-17-33-114-101(109)110)88(156)131-68(98(166)167)38-56-26-28-57(140)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,138-140,171H,8-9,16-18,23-25,30-49,104H2,1-7H3,(H2,105,141)(H2,106,142)(H,116,144)(H,117,154)(H,118,153)(H,119,155)(H,120,164)(H,121,145)(H,122,146)(H,123,143)(H,124,147)(H,125,151)(H,126,160)(H,127,162)(H,128,152)(H,129,163)(H,130,165)(H,131,156)(H,132,157)(H,133,161)(H,136,159)(H,137,158)(H,149,150)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,148,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013343
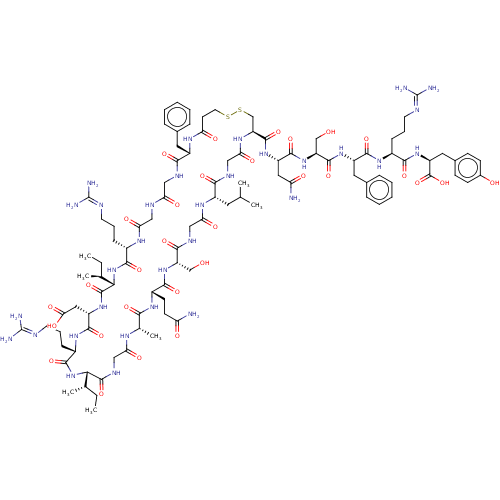
(CHEMBL3349621 | deamino [Mpr105,Cys121] r-ANF (99-...)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H154N32O30S2/c1-8-52(5)81-96(160)117-45-76(141)118-54(7)83(147)123-62(30-31-72(102)137)88(152)130-69(48-134)86(150)116-46-78(143)121-63(37-51(3)4)84(148)115-47-79(144)122-71(95(159)127-66(41-73(103)138)92(156)131-70(49-135)94(158)126-65(39-56-21-14-11-15-22-56)91(155)124-60(24-17-34-111-100(106)107)87(151)129-68(98(162)163)40-57-26-28-58(136)29-27-57)50-165-164-36-32-74(139)120-64(38-55-19-12-10-13-20-55)85(149)114-43-75(140)113-44-77(142)119-59(23-16-33-110-99(104)105)89(153)133-82(53(6)9-2)97(161)128-67(42-80(145)146)93(157)125-61(90(154)132-81)25-18-35-112-101(108)109/h10-15,19-22,26-29,51-54,59-71,81-82,134-136H,8-9,16-18,23-25,30-50H2,1-7H3,(H2,102,137)(H2,103,138)(H,113,140)(H,114,149)(H,115,148)(H,116,150)(H,117,160)(H,118,141)(H,119,142)(H,120,139)(H,121,143)(H,122,144)(H,123,147)(H,124,155)(H,125,157)(H,126,158)(H,127,159)(H,128,161)(H,129,151)(H,130,152)(H,131,156)(H,132,154)(H,133,153)(H,145,146)(H,162,163)(H4,104,105,110)(H4,106,107,111)(H4,108,109,112)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013341
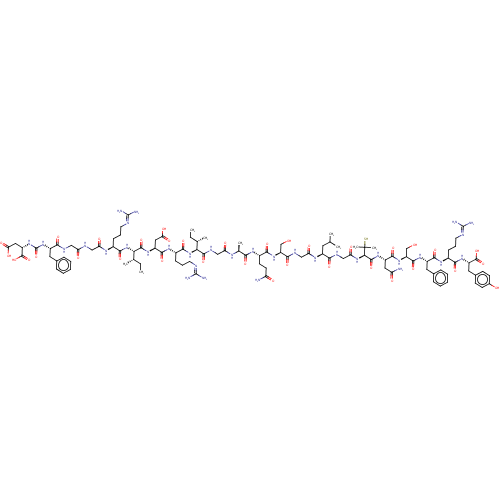
(CHEMBL3349629)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)C([#6])([#6])[#16] |r| Show InChI InChI=1S/C105H161N33O34S/c1-10-52(5)81(137-91(160)61(27-20-36-116-103(112)113)127-94(163)67(42-79(149)150)129-97(166)82(53(6)11-2)138-90(159)59(25-18-34-114-101(108)109)123-76(146)45-117-74(144)44-118-86(155)64(38-55-21-14-12-15-22-55)134-104(172)135-69(100(170)171)43-80(151)152)96(165)121-46-75(145)122-54(7)84(153)125-62(32-33-72(106)142)89(158)132-70(49-139)87(156)120-47-77(147)124-63(37-51(3)4)85(154)119-48-78(148)136-83(105(8,9)173)98(167)130-66(41-73(107)143)93(162)133-71(50-140)95(164)128-65(39-56-23-16-13-17-24-56)92(161)126-60(26-19-35-115-102(110)111)88(157)131-68(99(168)169)40-57-28-30-58(141)31-29-57/h12-17,21-24,28-31,51-54,59-71,81-83,139-141,173H,10-11,18-20,25-27,32-50H2,1-9H3,(H2,106,142)(H2,107,143)(H,117,144)(H,118,155)(H,119,154)(H,120,156)(H,121,165)(H,122,145)(H,123,146)(H,124,147)(H,125,153)(H,126,161)(H,127,163)(H,128,164)(H,129,166)(H,130,167)(H,131,157)(H,132,158)(H,133,162)(H,136,148)(H,137,160)(H,138,159)(H,149,150)(H,151,152)(H,168,169)(H,170,171)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,135,172)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-,83+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013338
(CHEMBL413659 | r-ANF (103-126)(Atrial Natriuretic ...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO)[C@@H](C)CC Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71+,72-,73-,74-,75-,84-,85-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013342

(CHEMBL3349625)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#8]-[#6])-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C104H159N33O34S/c1-9-52(5)82(136-91(158)61(26-19-35-115-103(111)112)127-94(161)67(41-80(148)149)130-98(165)83(53(6)10-2)137-90(157)59(24-17-33-113-101(107)108)122-77(145)44-116-75(143)43-117-86(153)64(37-55-20-13-11-14-21-55)134-104(170)135-69(100(168)169)42-81(150)171-8)97(164)120-45-76(144)121-54(7)84(151)125-62(31-32-73(105)141)89(156)132-70(48-138)87(154)119-46-78(146)123-63(36-51(3)4)85(152)118-47-79(147)124-72(50-172)96(163)129-66(40-74(106)142)93(160)133-71(49-139)95(162)128-65(38-56-22-15-12-16-23-56)92(159)126-60(25-18-34-114-102(109)110)88(155)131-68(99(166)167)39-57-27-29-58(140)30-28-57/h11-16,20-23,27-30,51-54,59-72,82-83,138-140,172H,9-10,17-19,24-26,31-50H2,1-8H3,(H2,105,141)(H2,106,142)(H,116,143)(H,117,153)(H,118,152)(H,119,154)(H,120,164)(H,121,144)(H,122,145)(H,123,146)(H,124,147)(H,125,151)(H,126,159)(H,127,161)(H,128,162)(H,129,163)(H,130,165)(H,131,155)(H,132,156)(H,133,160)(H,136,158)(H,137,157)(H,148,149)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,170)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,72-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228711

(CHEMBL3349900)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H155N33O30S2/c1-8-51(5)80-96(161)118-43-75(141)119-53(7)82(147)123-62(30-31-72(103)138)88(153)131-69(46-135)86(151)117-44-77(143)121-63(35-50(3)4)84(149)116-45-78(144)122-71(95(160)128-66(39-73(104)139)92(157)132-70(47-136)94(159)127-65(37-55-21-14-11-15-22-55)91(156)124-60(24-17-33-112-100(107)108)87(152)130-68(98(163)164)38-56-26-28-57(137)29-27-56)49-166-165-48-58(102)83(148)126-64(36-54-19-12-10-13-20-54)85(150)115-41-74(140)114-42-76(142)120-59(23-16-32-111-99(105)106)89(154)134-81(52(6)9-2)97(162)129-67(40-79(145)146)93(158)125-61(90(155)133-80)25-18-34-113-101(109)110/h10-15,19-22,26-29,50-53,58-71,80-81,135-137H,8-9,16-18,23-25,30-49,102H2,1-7H3,(H2,103,138)(H2,104,139)(H,114,140)(H,115,150)(H,116,149)(H,117,151)(H,118,161)(H,119,141)(H,120,142)(H,121,143)(H,122,144)(H,123,147)(H,124,156)(H,125,158)(H,126,148)(H,127,159)(H,128,160)(H,129,162)(H,130,152)(H,131,153)(H,132,157)(H,133,155)(H,134,154)(H,145,146)(H,163,164)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,80-,81-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013344
(CHEMBL3349628)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H157N33O34S/c1-8-51(5)81(135-90(158)60(25-18-34-114-102(110)111)126-93(161)66(40-79(147)148)129-97(165)82(52(6)9-2)136-89(157)58(23-16-32-112-100(106)107)121-76(144)43-115-74(142)42-116-85(153)63(36-54-19-12-10-13-20-54)133-103(170)134-68(99(168)169)41-80(149)150)96(164)119-44-75(143)120-53(7)83(151)124-61(30-31-72(104)140)88(156)131-69(47-137)86(154)118-45-77(145)122-62(35-50(3)4)84(152)117-46-78(146)123-71(49-171)95(163)128-65(39-73(105)141)92(160)132-70(48-138)94(162)127-64(37-55-21-14-11-15-22-55)91(159)125-59(24-17-33-113-101(108)109)87(155)130-67(98(166)167)38-56-26-28-57(139)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,137-139,171H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,104,140)(H2,105,141)(H,115,142)(H,116,153)(H,117,152)(H,118,154)(H,119,164)(H,120,143)(H,121,144)(H,122,145)(H,123,146)(H,124,151)(H,125,159)(H,126,161)(H,127,162)(H,128,163)(H,129,165)(H,130,155)(H,131,156)(H,132,160)(H,135,158)(H,136,157)(H,147,148)(H,149,150)(H,166,167)(H,168,169)(H4,106,107,112)(H4,108,109,113)(H4,110,111,114)(H2,133,134,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053967
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C31H54N6O9/c1-9-18(10-2)32-29(46)36-24(31(6,7)8)27(43)34-19(15-22(38)37-13-11-12-14-37)25(41)33-20(16-23(39)40)26(42)35-21(28(44)45)17-30(3,4)5/h18-21,24H,9-17H2,1-8H3,(H,33,41)(H,34,43)(H,35,42)(H,39,40)(H,44,45)(H2,32,36,46)/t19-,20-,21-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228712
(CHEMBL3350078)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H154N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,40,42,50-53,58-67,70-71,82-83,140-142H,8-9,16-18,23-25,30-39,41,43-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/b68-40+,69-42+/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,70-,71-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013339

(CHEMBL3349627)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H158N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,50-53,58-71,82-83,140-142H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013343

(CHEMBL3349621 | deamino [Mpr105,Cys121] r-ANF (99-...)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H154N32O30S2/c1-8-52(5)81-96(160)117-45-76(141)118-54(7)83(147)123-62(30-31-72(102)137)88(152)130-69(48-134)86(150)116-46-78(143)121-63(37-51(3)4)84(148)115-47-79(144)122-71(95(159)127-66(41-73(103)138)92(156)131-70(49-135)94(158)126-65(39-56-21-14-11-15-22-56)91(155)124-60(24-17-34-111-100(106)107)87(151)129-68(98(162)163)40-57-26-28-58(136)29-27-57)50-165-164-36-32-74(139)120-64(38-55-19-12-10-13-20-55)85(149)114-43-75(140)113-44-77(142)119-59(23-16-33-110-99(104)105)89(153)133-82(53(6)9-2)97(161)128-67(42-80(145)146)93(157)125-61(90(154)132-81)25-18-35-112-101(108)109/h10-15,19-22,26-29,51-54,59-71,81-82,134-136H,8-9,16-18,23-25,30-50H2,1-7H3,(H2,102,137)(H2,103,138)(H,113,140)(H,114,149)(H,115,148)(H,116,150)(H,117,160)(H,118,141)(H,119,142)(H,120,139)(H,121,143)(H,122,144)(H,123,147)(H,124,155)(H,125,157)(H,126,158)(H,127,159)(H,128,161)(H,129,151)(H,130,152)(H,131,156)(H,132,154)(H,133,153)(H,145,146)(H,162,163)(H4,104,105,110)(H4,106,107,111)(H4,108,109,112)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013338

(CHEMBL413659 | r-ANF (103-126)(Atrial Natriuretic ...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO)[C@@H](C)CC Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71+,72-,73-,74-,75-,84-,85-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50369164

(CHEMBL1169533)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@H](NC(=O)NC1[C@@H](C)CCC[C@H]1C)C(C)C)C(O)=O |r| Show InChI InChI=1S/C32H54N6O9/c1-17(2)14-23(31(45)46)35-29(43)22(16-25(40)41)33-28(42)21(15-24(39)38-12-7-8-13-38)34-30(44)26(18(3)4)36-32(47)37-27-19(5)10-9-11-20(27)6/h17-23,26-27H,7-16H2,1-6H3,(H,33,42)(H,34,44)(H,35,43)(H,40,41)(H,45,46)(H2,36,37,47)/t19-,20+,21-,22+,23+,26+,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50369164

(CHEMBL1169533)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@H](NC(=O)NC1[C@@H](C)CCC[C@H]1C)C(C)C)C(O)=O |r| Show InChI InChI=1S/C32H54N6O9/c1-17(2)14-23(31(45)46)35-29(43)22(16-25(40)41)33-28(42)21(15-24(39)38-12-7-8-13-38)34-30(44)26(18(3)4)36-32(47)37-27-19(5)10-9-11-20(27)6/h17-23,26-27H,7-16H2,1-6H3,(H,33,42)(H,34,44)(H,35,43)(H,40,41)(H,45,46)(H2,36,37,47)/t19-,20+,21-,22+,23+,26+,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053968
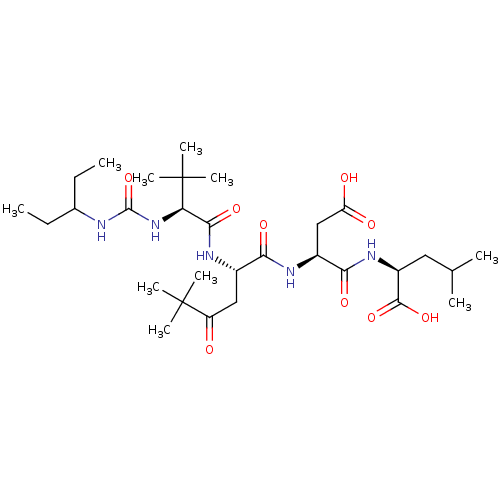
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)C(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C30H53N5O9/c1-11-17(12-2)31-28(44)35-23(30(8,9)10)26(41)33-18(14-21(36)29(5,6)7)24(39)32-19(15-22(37)38)25(40)34-20(27(42)43)13-16(3)4/h16-20,23H,11-15H2,1-10H3,(H,32,39)(H,33,41)(H,34,40)(H,37,38)(H,42,43)(H2,31,35,44)/t18-,19-,20-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50050831
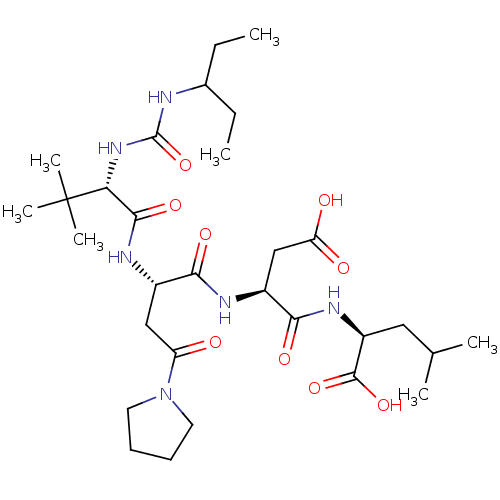
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C30H52N6O9/c1-8-18(9-2)31-29(45)35-24(30(5,6)7)27(42)33-19(15-22(37)36-12-10-11-13-36)25(40)32-20(16-23(38)39)26(41)34-21(28(43)44)14-17(3)4/h17-21,24H,8-16H2,1-7H3,(H,32,40)(H,33,42)(H,34,41)(H,38,39)(H,43,44)(H2,31,35,45)/t19-,20-,21-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50228711

(CHEMBL3349900)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H155N33O30S2/c1-8-51(5)80-96(161)118-43-75(141)119-53(7)82(147)123-62(30-31-72(103)138)88(153)131-69(46-135)86(151)117-44-77(143)121-63(35-50(3)4)84(149)116-45-78(144)122-71(95(160)128-66(39-73(104)139)92(157)132-70(47-136)94(159)127-65(37-55-21-14-11-15-22-55)91(156)124-60(24-17-33-112-100(107)108)87(152)130-68(98(163)164)38-56-26-28-57(137)29-27-56)49-166-165-48-58(102)83(148)126-64(36-54-19-12-10-13-20-54)85(150)115-41-74(140)114-42-76(142)120-59(23-16-32-111-99(105)106)89(154)134-81(52(6)9-2)97(162)129-67(40-79(145)146)93(158)125-61(90(155)133-80)25-18-34-113-101(109)110/h10-15,19-22,26-29,50-53,58-71,80-81,135-137H,8-9,16-18,23-25,30-49,102H2,1-7H3,(H2,103,138)(H2,104,139)(H,114,140)(H,115,150)(H,116,149)(H,117,151)(H,118,161)(H,119,141)(H,120,142)(H,121,143)(H,122,144)(H,123,147)(H,124,156)(H,125,158)(H,126,148)(H,127,159)(H,128,160)(H,129,162)(H,130,152)(H,131,153)(H,132,157)(H,133,155)(H,134,154)(H,145,146)(H,163,164)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,80-,81-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053981

((S)-2-((S)-3-Carboxy-2-{(2R,5S)-2-(3,3-dimethyl-2-...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)C[C@@H](CC(=O)C(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C31H54N4O9/c1-11-19(12-2)32-29(44)35-25(31(8,9)10)22(36)14-18(15-23(37)30(5,6)7)26(40)33-20(16-24(38)39)27(41)34-21(28(42)43)13-17(3)4/h17-21,25H,11-16H2,1-10H3,(H,33,40)(H,34,41)(H,38,39)(H,42,43)(H2,32,35,44)/t18-,20-,21-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053973

((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](CO)CC(C)(C)C)C(C)(C)C Show InChI InChI=1S/C31H56N6O8/c1-9-19(10-2)33-29(45)36-25(31(6,7)8)28(44)35-21(15-23(39)37-13-11-12-14-37)27(43)34-22(16-24(40)41)26(42)32-20(18-38)17-30(3,4)5/h19-22,25,38H,9-18H2,1-8H3,(H,32,42)(H,34,43)(H,35,44)(H,40,41)(H2,33,36,45)/t20-,21-,22-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013344

(CHEMBL3349628)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H157N33O34S/c1-8-51(5)81(135-90(158)60(25-18-34-114-102(110)111)126-93(161)66(40-79(147)148)129-97(165)82(52(6)9-2)136-89(157)58(23-16-32-112-100(106)107)121-76(144)43-115-74(142)42-116-85(153)63(36-54-19-12-10-13-20-54)133-103(170)134-68(99(168)169)41-80(149)150)96(164)119-44-75(143)120-53(7)83(151)124-61(30-31-72(104)140)88(156)131-69(47-137)86(154)118-45-77(145)122-62(35-50(3)4)84(152)117-46-78(146)123-71(49-171)95(163)128-65(39-73(105)141)92(160)132-70(48-138)94(162)127-64(37-55-21-14-11-15-22-55)91(159)125-59(24-17-33-113-101(108)109)87(155)130-67(98(166)167)38-56-26-28-57(139)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,137-139,171H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,104,140)(H2,105,141)(H,115,142)(H,116,153)(H,117,152)(H,118,154)(H,119,164)(H,120,143)(H,121,144)(H,122,145)(H,123,146)(H,124,151)(H,125,159)(H,126,161)(H,127,162)(H,128,163)(H,129,165)(H,130,155)(H,131,156)(H,132,160)(H,135,158)(H,136,157)(H,147,148)(H,149,150)(H,166,167)(H,168,169)(H4,106,107,112)(H4,108,109,113)(H4,110,111,114)(H2,133,134,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50050828
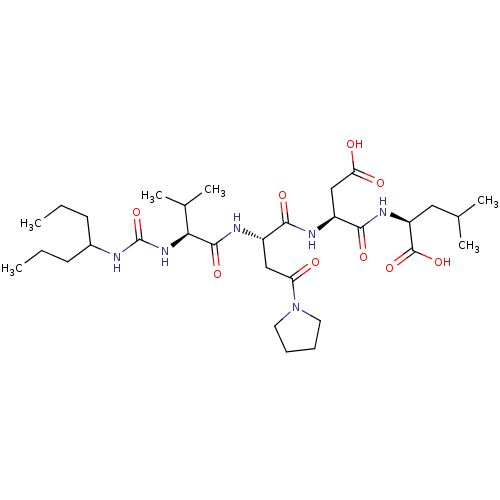
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[3-(...)Show SMILES CCCC(CCC)NC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C31H54N6O9/c1-7-11-20(12-8-2)32-31(46)36-26(19(5)6)29(43)34-21(16-24(38)37-13-9-10-14-37)27(41)33-22(17-25(39)40)28(42)35-23(30(44)45)15-18(3)4/h18-23,26H,7-17H2,1-6H3,(H,33,41)(H,34,43)(H,35,42)(H,39,40)(H,44,45)(H2,32,36,46)/t21-,22-,23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053984
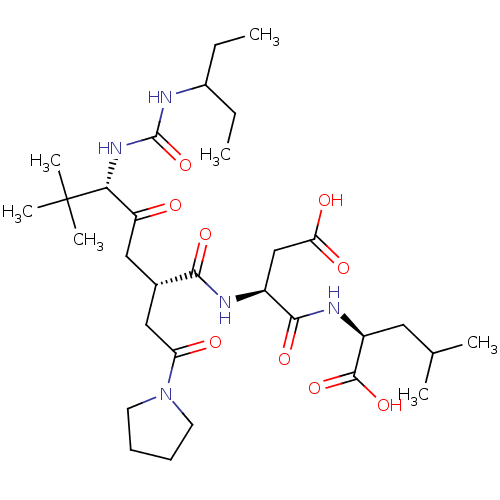
((S)-2-{(S)-3-Carboxy-2-[(2S,5S)-5-[3-(1-ethyl-prop...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)C[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C31H53N5O9/c1-8-20(9-2)32-30(45)35-26(31(5,6)7)23(37)15-19(16-24(38)36-12-10-11-13-36)27(41)33-21(17-25(39)40)28(42)34-22(29(43)44)14-18(3)4/h18-22,26H,8-17H2,1-7H3,(H,33,41)(H,34,42)(H,39,40)(H,43,44)(H2,32,35,45)/t19-,21-,22-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013340

(CHEMBL3349626)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16])-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H158N34O33S/c1-8-51(5)81(136-91(159)61(25-18-34-115-102(111)112)127-94(162)67(41-80(149)150)130-97(165)82(52(6)9-2)137-90(158)59(23-16-32-113-100(107)108)122-77(146)43-116-75(144)42-117-86(154)64(128-84(152)58(104)49-171)36-54-19-12-10-13-20-54)96(164)120-44-76(145)121-53(7)83(151)125-62(30-31-72(105)141)89(157)132-70(47-138)87(155)119-45-78(147)124-63(35-50(3)4)85(153)118-46-79(148)135-103(170)134-69(99(168)169)40-74(143)123-66(39-73(106)142)93(161)133-71(48-139)95(163)129-65(37-55-21-14-11-15-22-55)92(160)126-60(24-17-33-114-101(109)110)88(156)131-68(98(166)167)38-56-26-28-57(140)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,138-140,171H,8-9,16-18,23-25,30-49,104H2,1-7H3,(H2,105,141)(H2,106,142)(H,116,144)(H,117,154)(H,118,153)(H,119,155)(H,120,164)(H,121,145)(H,122,146)(H,123,143)(H,124,147)(H,125,151)(H,126,160)(H,127,162)(H,128,152)(H,129,163)(H,130,165)(H,131,156)(H,132,157)(H,133,161)(H,136,159)(H,137,158)(H,149,150)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,148,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013341

(CHEMBL3349629)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)C([#6])([#6])[#16] |r| Show InChI InChI=1S/C105H161N33O34S/c1-10-52(5)81(137-91(160)61(27-20-36-116-103(112)113)127-94(163)67(42-79(149)150)129-97(166)82(53(6)11-2)138-90(159)59(25-18-34-114-101(108)109)123-76(146)45-117-74(144)44-118-86(155)64(38-55-21-14-12-15-22-55)134-104(172)135-69(100(170)171)43-80(151)152)96(165)121-46-75(145)122-54(7)84(153)125-62(32-33-72(106)142)89(158)132-70(49-139)87(156)120-47-77(147)124-63(37-51(3)4)85(154)119-48-78(148)136-83(105(8,9)173)98(167)130-66(41-73(107)143)93(162)133-71(50-140)95(164)128-65(39-56-23-16-13-17-24-56)92(161)126-60(26-19-35-115-102(110)111)88(157)131-68(99(168)169)40-57-28-30-58(141)31-29-57/h12-17,21-24,28-31,51-54,59-71,81-83,139-141,173H,10-11,18-20,25-27,32-50H2,1-9H3,(H2,106,142)(H2,107,143)(H,117,144)(H,118,155)(H,119,154)(H,120,156)(H,121,165)(H,122,145)(H,123,146)(H,124,147)(H,125,153)(H,126,161)(H,127,163)(H,128,164)(H,129,166)(H,130,167)(H,131,157)(H,132,158)(H,133,162)(H,136,148)(H,137,160)(H,138,159)(H,149,150)(H,151,152)(H,168,169)(H,170,171)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,135,172)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-,83+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013342

(CHEMBL3349625)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#8]-[#6])-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C104H159N33O34S/c1-9-52(5)82(136-91(158)61(26-19-35-115-103(111)112)127-94(161)67(41-80(148)149)130-98(165)83(53(6)10-2)137-90(157)59(24-17-33-113-101(107)108)122-77(145)44-116-75(143)43-117-86(153)64(37-55-20-13-11-14-21-55)134-104(170)135-69(100(168)169)42-81(150)171-8)97(164)120-45-76(144)121-54(7)84(151)125-62(31-32-73(105)141)89(156)132-70(48-138)87(154)119-46-78(146)123-63(36-51(3)4)85(152)118-47-79(147)124-72(50-172)96(163)129-66(40-74(106)142)93(160)133-71(49-139)95(162)128-65(38-56-22-15-12-16-23-56)92(159)126-60(25-18-34-114-102(109)110)88(155)131-68(99(166)167)39-57-27-29-58(140)30-28-57/h11-16,20-23,27-30,51-54,59-72,82-83,138-140,172H,9-10,17-19,24-26,31-50H2,1-8H3,(H2,105,141)(H2,106,142)(H,116,143)(H,117,153)(H,118,152)(H,119,154)(H,120,164)(H,121,144)(H,122,145)(H,123,146)(H,124,147)(H,125,151)(H,126,159)(H,127,161)(H,128,162)(H,129,163)(H,130,165)(H,131,155)(H,132,156)(H,133,160)(H,136,158)(H,137,157)(H,148,149)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,170)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,72-,82-,83-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50050826
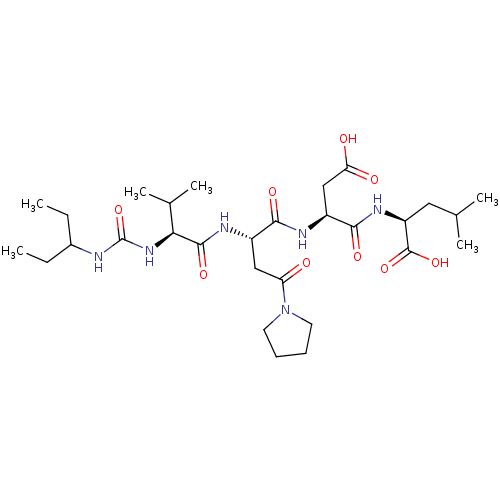
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...)Show SMILES CCC(CC)NC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C29H50N6O9/c1-7-18(8-2)30-29(44)34-24(17(5)6)27(41)32-19(14-22(36)35-11-9-10-12-35)25(39)31-20(15-23(37)38)26(40)33-21(28(42)43)13-16(3)4/h16-21,24H,7-15H2,1-6H3,(H,31,39)(H,32,41)(H,33,40)(H,37,38)(H,42,43)(H2,30,34,44)/t19-,20-,21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053962

((S)-N-((R)-1-Ethyl-2,2-dimethyl-propyl)-3-((S)-2-{...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](CC)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C31H56N6O7/c1-10-19(11-2)32-29(44)36-25(31(7,8)9)28(43)34-20(17-23(38)37-15-13-14-16-37)26(41)33-21(18-24(39)40)27(42)35-22(12-3)30(4,5)6/h19-22,25H,10-18H2,1-9H3,(H,33,41)(H,34,43)(H,35,42)(H,39,40)(H2,32,36,44)/t20-,21-,22+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50369165

(CHEMBL1169532)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@H](NC(=O)NC1[C@@H](C)CCC[C@@H]1C)C(C)C)C(O)=O |r| Show InChI InChI=1S/C32H54N6O9/c1-17(2)14-23(31(45)46)35-29(43)22(16-25(40)41)33-28(42)21(15-24(39)38-12-7-8-13-38)34-30(44)26(18(3)4)36-32(47)37-27-19(5)10-9-11-20(27)6/h17-23,26-27H,7-16H2,1-6H3,(H,33,42)(H,34,44)(H,35,43)(H,40,41)(H,45,46)(H2,36,37,47)/t19-,20-,21-,22+,23+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033462
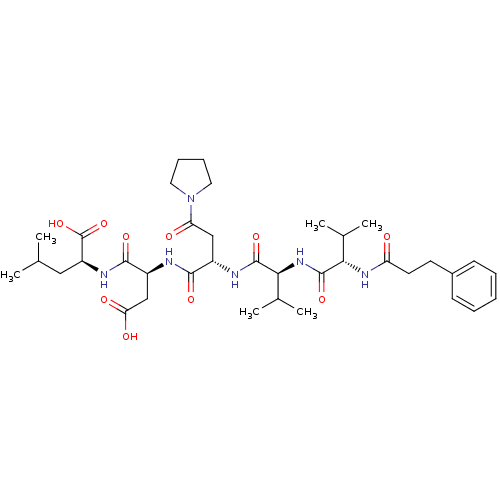
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCc1ccccc1)C(C)C)C(C)C)C(O)=O Show InChI InChI=1S/C37H56N6O10/c1-21(2)18-27(37(52)53)40-34(49)26(20-30(46)47)38-33(48)25(19-29(45)43-16-10-11-17-43)39-35(50)32(23(5)6)42-36(51)31(22(3)4)41-28(44)15-14-24-12-8-7-9-13-24/h7-9,12-13,21-23,25-27,31-32H,10-11,14-20H2,1-6H3,(H,38,48)(H,39,50)(H,40,49)(H,41,44)(H,42,51)(H,46,47)(H,52,53)/t25-,26-,27-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
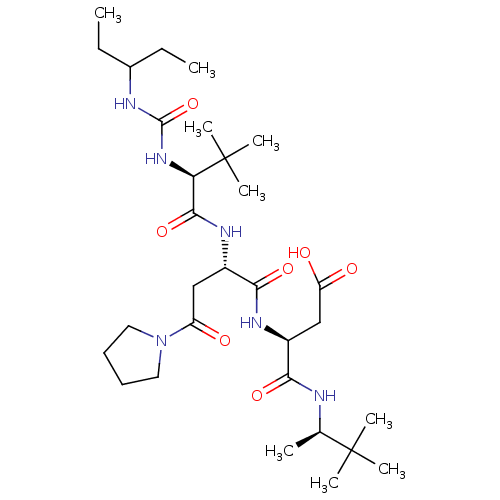
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053963
((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C30H54N6O7/c1-10-19(11-2)32-28(43)35-24(30(7,8)9)27(42)34-20(16-22(37)36-14-12-13-15-36)26(41)33-21(17-23(38)39)25(40)31-18(3)29(4,5)6/h18-21,24H,10-17H2,1-9H3,(H,31,40)(H,33,41)(H,34,42)(H,38,39)(H2,32,35,43)/t18-,20+,21+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013339

(CHEMBL3349627)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H158N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,50-53,58-71,82-83,140-142H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,82-,83-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053987
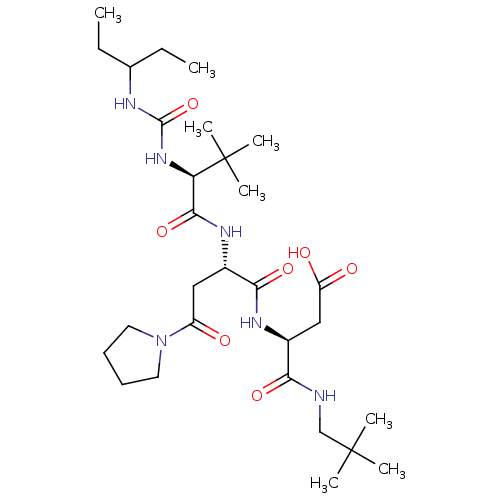
((S)-N-(2,2-Dimethyl-propyl)-3-((S)-2-{(S)-2-[3-(1-...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(C)(C)C)C(C)(C)C Show InChI InChI=1S/C29H52N6O7/c1-9-18(10-2)31-27(42)34-23(29(6,7)8)26(41)33-19(15-21(36)35-13-11-12-14-35)25(40)32-20(16-22(37)38)24(39)30-17-28(3,4)5/h18-20,23H,9-17H2,1-8H3,(H,30,39)(H,32,40)(H,33,41)(H,37,38)(H2,31,34,42)/t19-,20-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50228712

(CHEMBL3350078)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H154N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,40,42,50-53,58-67,70-71,82-83,140-142H,8-9,16-18,23-25,30-39,41,43-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/b68-40+,69-42+/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,70-,71-,82-,83-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053970
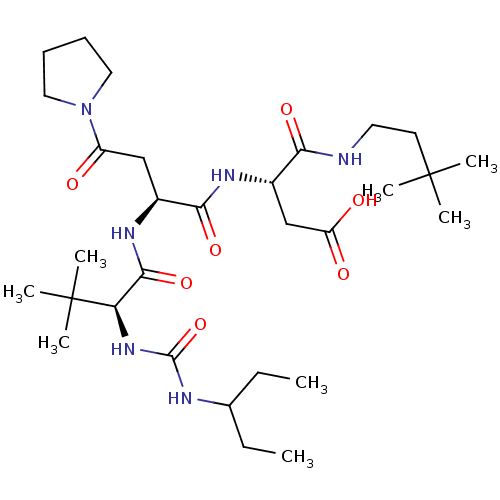
((S)-N-(3,3-Dimethyl-butyl)-3-((S)-2-{(S)-2-[3-(1-e...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)NCCC(C)(C)C)C(C)(C)C Show InChI InChI=1S/C30H54N6O7/c1-9-19(10-2)32-28(43)35-24(30(6,7)8)27(42)34-20(17-22(37)36-15-11-12-16-36)26(41)33-21(18-23(38)39)25(40)31-14-13-29(3,4)5/h19-21,24H,9-18H2,1-8H3,(H,31,40)(H,33,41)(H,34,42)(H,38,39)(H2,32,35,43)/t20-,21-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053985

((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)NCCC(C)C)C(C)(C)C Show InChI InChI=1S/C29H52N6O7/c1-8-19(9-2)31-28(42)34-24(29(5,6)7)27(41)33-20(16-22(36)35-14-10-11-15-35)26(40)32-21(17-23(37)38)25(39)30-13-12-18(3)4/h18-21,24H,8-17H2,1-7H3,(H,30,39)(H,32,40)(H,33,41)(H,37,38)(H2,31,34,42)/t20-,21-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50050829
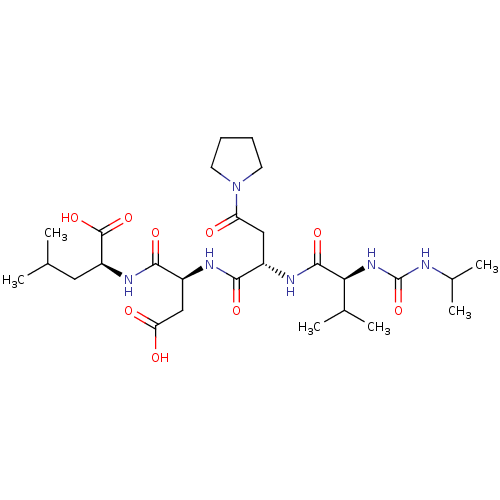
((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(3-isopropyl-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)NC(C)C)C(C)C)C(O)=O Show InChI InChI=1S/C27H46N6O9/c1-14(2)11-19(26(40)41)31-24(38)18(13-21(35)36)29-23(37)17(12-20(34)33-9-7-8-10-33)30-25(39)22(15(3)4)32-27(42)28-16(5)6/h14-19,22H,7-13H2,1-6H3,(H,29,37)(H,30,39)(H,31,38)(H,35,36)(H,40,41)(H2,28,32,42)/t17-,18-,19-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053972
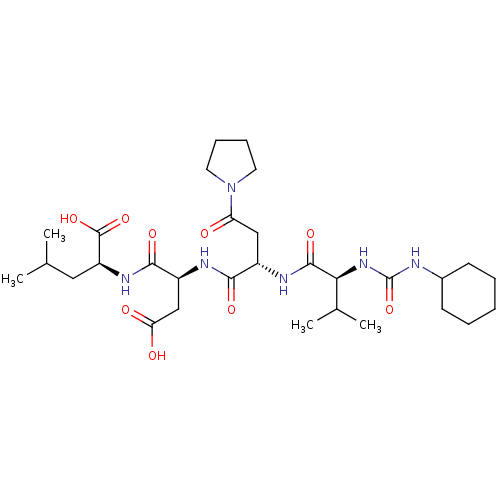
((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(3-cyclohexyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)NC1CCCCC1)C(C)C)C(O)=O Show InChI InChI=1S/C30H50N6O9/c1-17(2)14-22(29(43)44)34-27(41)21(16-24(38)39)32-26(40)20(15-23(37)36-12-8-9-13-36)33-28(42)25(18(3)4)35-30(45)31-19-10-6-5-7-11-19/h17-22,25H,5-16H2,1-4H3,(H,32,40)(H,33,42)(H,34,41)(H,38,39)(H,43,44)(H2,31,35,45)/t20-,21-,22-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033460
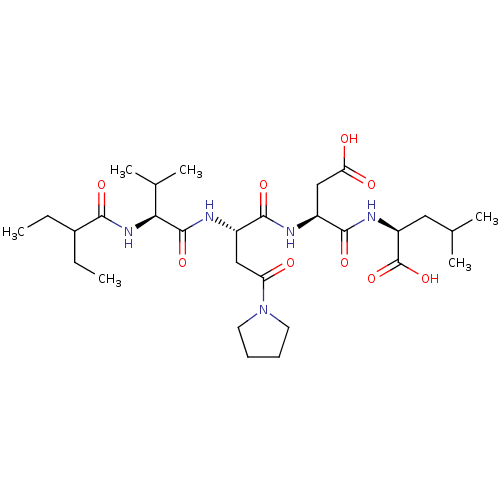
((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(2-ethyl-buty...)Show SMILES CCC(CC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C29H49N5O9/c1-7-18(8-2)25(38)33-24(17(5)6)28(41)31-19(14-22(35)34-11-9-10-12-34)26(39)30-20(15-23(36)37)27(40)32-21(29(42)43)13-16(3)4/h16-21,24H,7-15H2,1-6H3,(H,30,39)(H,31,41)(H,32,40)(H,33,38)(H,36,37)(H,42,43)/t19-,20-,21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053980
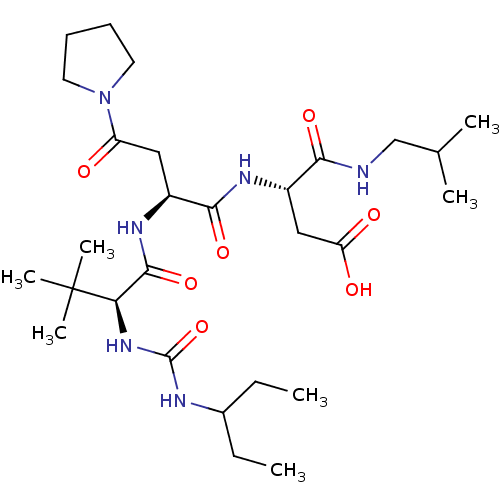
((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(C)C)C(C)(C)C Show InChI InChI=1S/C28H50N6O7/c1-8-18(9-2)30-27(41)33-23(28(5,6)7)26(40)32-19(14-21(35)34-12-10-11-13-34)25(39)31-20(15-22(36)37)24(38)29-16-17(3)4/h17-20,23H,8-16H2,1-7H3,(H,29,38)(H,31,39)(H,32,40)(H,36,37)(H2,30,33,41)/t19-,20-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053964
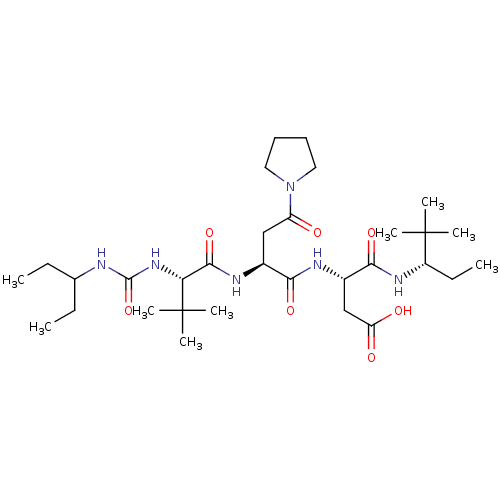
((S)-N-((S)-1-Ethyl-2,2-dimethyl-propyl)-3-((S)-2-{...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C31H56N6O7/c1-10-19(11-2)32-29(44)36-25(31(7,8)9)28(43)34-20(17-23(38)37-15-13-14-16-37)26(41)33-21(18-24(39)40)27(42)35-22(12-3)30(4,5)6/h19-22,25H,10-18H2,1-9H3,(H,33,41)(H,34,43)(H,35,42)(H,39,40)(H2,32,36,44)/t20-,21-,22-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50053986
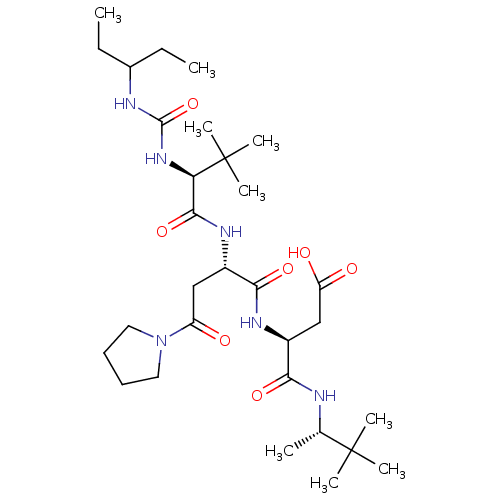
((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...)Show SMILES CCC(CC)NC(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C30H54N6O7/c1-10-19(11-2)32-28(43)35-24(30(7,8)9)27(42)34-20(16-22(37)36-14-12-13-15-36)26(41)33-21(17-23(38)39)25(40)31-18(3)29(4,5)6/h18-21,24H,10-17H2,1-9H3,(H,31,40)(H,33,41)(H,34,42)(H,38,39)(H2,32,35,43)/t18-,20-,21-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |
CviR transcriptional regulator
(Chromobacterium violaceum) | BDBM50136221
(CHEMBL3754734)Show InChI InChI=1S/C17H17N3O2/c21-16(20-14-4-2-1-3-5-14)12-22-15-8-6-13(7-9-15)17-18-10-11-19-17/h1-9H,10-12H2,(H,18,19)(H,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Polit£cnico Nacional
Curated by ChEMBL
| Assay Description
Inhibition of CviR in Chromobacterium violaceum CV026 assessed as reduction of violacein production after 15 hrs |
Bioorg Med Chem 23: 7565-77 (2015)
Article DOI: 10.1016/j.bmc.2015.10.046
BindingDB Entry DOI: 10.7270/Q2RF5WVS |
More data for this
Ligand-Target Pair | |
CviR transcriptional regulator
(Chromobacterium violaceum) | BDBM50136219
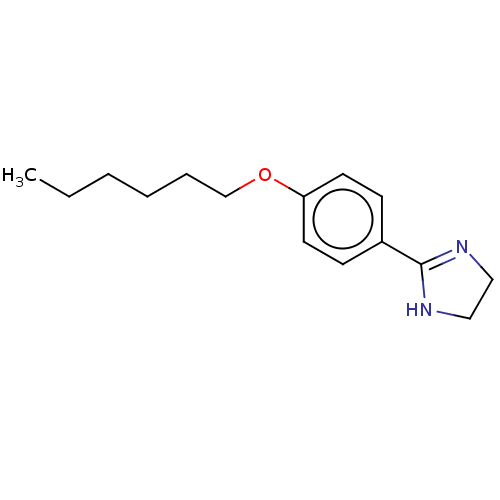
(CHEMBL3753564)Show InChI InChI=1S/C15H22N2O/c1-2-3-4-5-12-18-14-8-6-13(7-9-14)15-16-10-11-17-15/h6-9H,2-5,10-12H2,1H3,(H,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Polit£cnico Nacional
Curated by ChEMBL
| Assay Description
Inhibition of CviR in Chromobacterium violaceum CV026 assessed as reduction of violacein production after 15 hrs |
Bioorg Med Chem 23: 7565-77 (2015)
Article DOI: 10.1016/j.bmc.2015.10.046
BindingDB Entry DOI: 10.7270/Q2RF5WVS |
More data for this
Ligand-Target Pair | |
CviR transcriptional regulator
(Chromobacterium violaceum) | BDBM50136296

(CHEMBL3754531)Show InChI InChI=1S/C15H21NO2/c1-2-3-4-5-11-17-14-8-6-13(7-9-14)15-16-10-12-18-15/h6-9H,2-5,10-12H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Polit£cnico Nacional
Curated by ChEMBL
| Assay Description
Inhibition of CviR in Chromobacterium violaceum CV026 assessed as reduction of violacein production after 15 hrs |
Bioorg Med Chem 23: 7565-77 (2015)
Article DOI: 10.1016/j.bmc.2015.10.046
BindingDB Entry DOI: 10.7270/Q2RF5WVS |
More data for this
Ligand-Target Pair | |
CviR transcriptional regulator
(Chromobacterium violaceum) | BDBM50136220
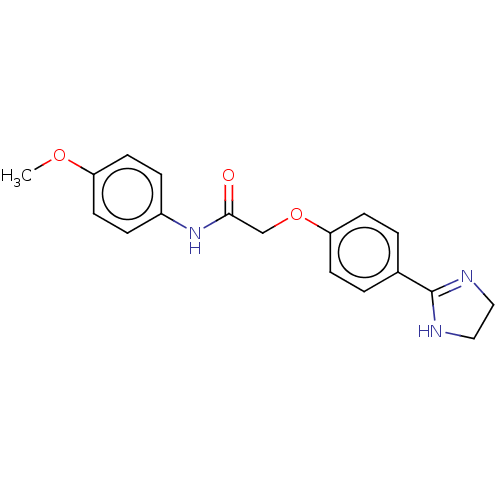
(CHEMBL3752577)Show SMILES COc1ccc(NC(=O)COc2ccc(cc2)C2=NCCN2)cc1 |t:18| Show InChI InChI=1S/C18H19N3O3/c1-23-15-8-4-14(5-9-15)21-17(22)12-24-16-6-2-13(3-7-16)18-19-10-11-20-18/h2-9H,10-12H2,1H3,(H,19,20)(H,21,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Polit£cnico Nacional
Curated by ChEMBL
| Assay Description
Inhibition of CviR in Chromobacterium violaceum CV026 assessed as reduction of violacein production after 15 hrs |
Bioorg Med Chem 23: 7565-77 (2015)
Article DOI: 10.1016/j.bmc.2015.10.046
BindingDB Entry DOI: 10.7270/Q2RF5WVS |
More data for this
Ligand-Target Pair | |
CviR transcriptional regulator
(Chromobacterium violaceum) | BDBM50136222

(CHEMBL3754036)Show InChI InChI=1S/C11H12N2OS/c14-10(13-11-12-6-7-15-11)8-9-4-2-1-3-5-9/h1-5H,6-8H2,(H,12,13,14) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Polit£cnico Nacional
Curated by ChEMBL
| Assay Description
Inhibition of CviR in Chromobacterium violaceum CV026 assessed as reduction of violacein production after 15 hrs |
Bioorg Med Chem 23: 7565-77 (2015)
Article DOI: 10.1016/j.bmc.2015.10.046
BindingDB Entry DOI: 10.7270/Q2RF5WVS |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50013358
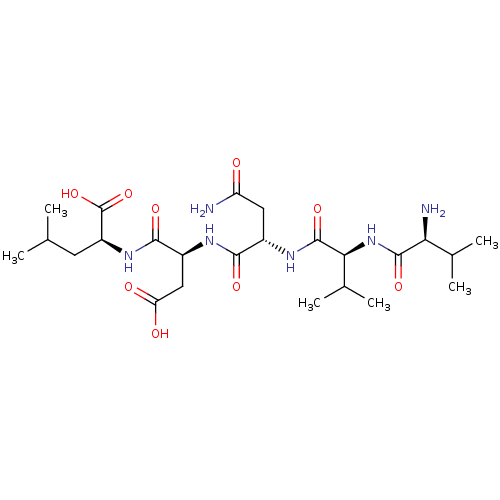
(CHEMBL102379 | H-Val-Val-Asn-Asp-Leu-OH | Val-Val-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)C(O)=O Show InChI InChI=1S/C24H42N6O9/c1-10(2)7-15(24(38)39)29-21(35)14(9-17(32)33)27-20(34)13(8-16(25)31)28-23(37)19(12(5)6)30-22(36)18(26)11(3)4/h10-15,18-19H,7-9,26H2,1-6H3,(H2,25,31)(H,27,34)(H,28,37)(H,29,35)(H,30,36)(H,32,33)(H,38,39)/t13-,14-,15-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. |
J Med Chem 39: 4173-80 (1996)
Article DOI: 10.1021/jm960324r
BindingDB Entry DOI: 10.7270/Q28W3DZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
















































