

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50427810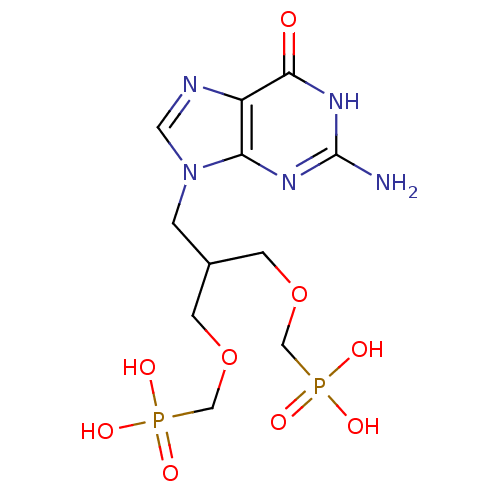 (CHEMBL2325752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human N-terminal hexahistidine-tagged HGPRT | J Med Chem 56: 2513-26 (2013) Article DOI: 10.1021/jm301893b BindingDB Entry DOI: 10.7270/Q2MW2JGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine phosphoribosyltransferase (Plasmodium vivax) | BDBM194499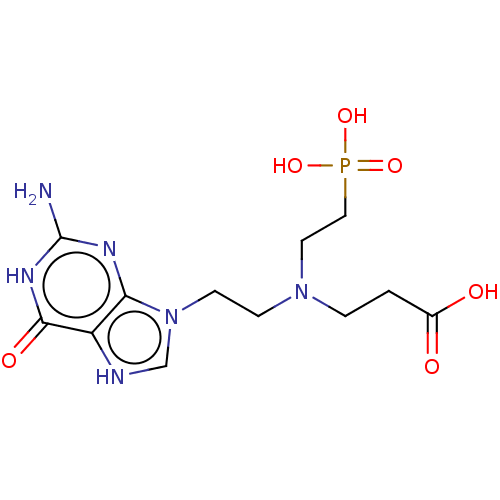 (US9200020, Table 3 compound 8) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 50 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392261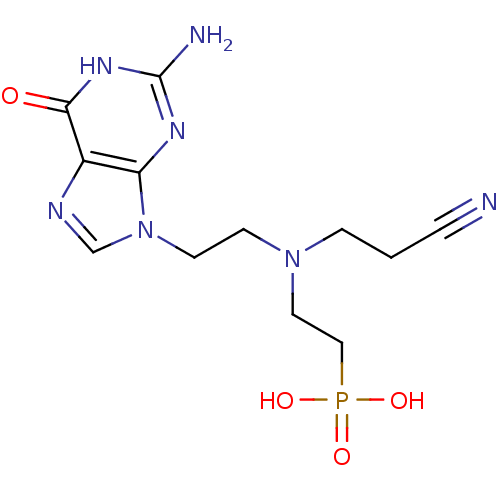 (CHEMBL2153480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194501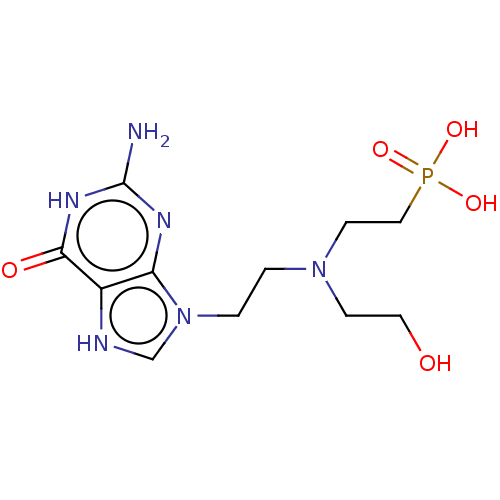 (US9200020, Table 3 compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194496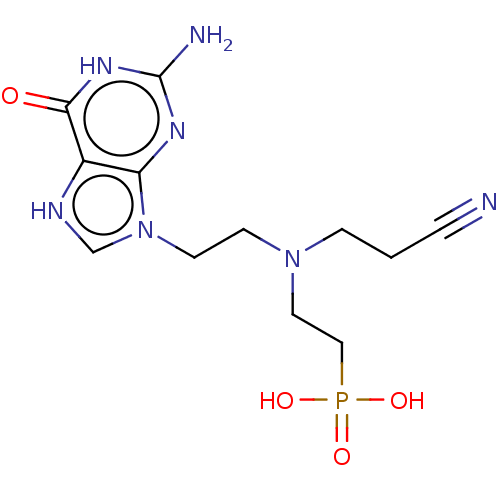 (US9200020, Table 3 compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392263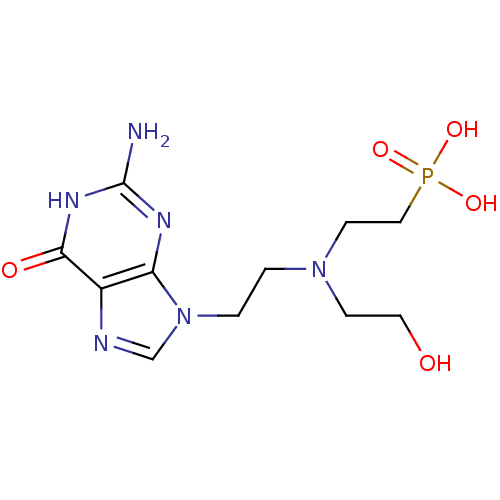 (CHEMBL2153497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194493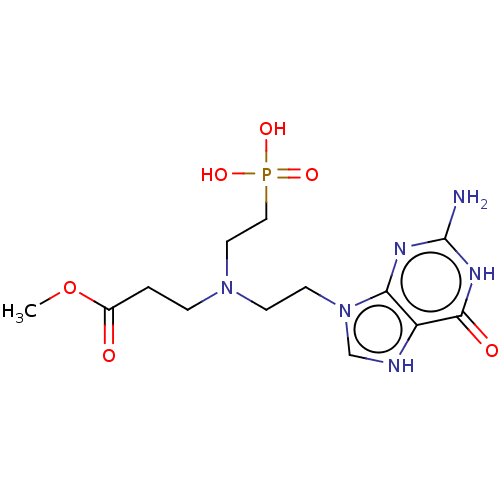 (US9200020, Table 3 compound 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194500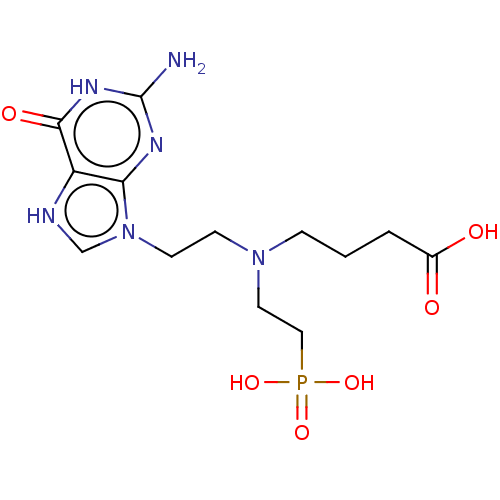 (US9200020, Table 3 compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392266 (CHEMBL2153484) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194494 (US9200020, Table 3 compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194492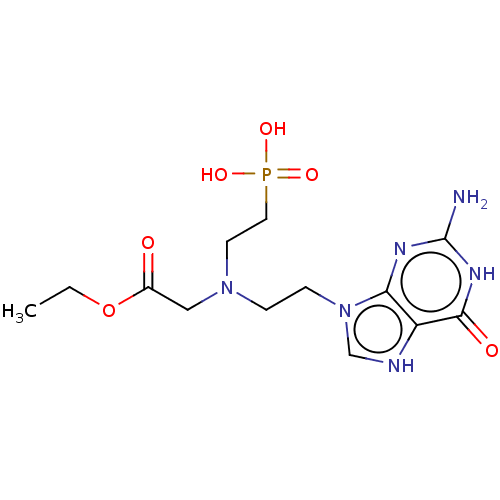 (US9200020, Table 3 compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392260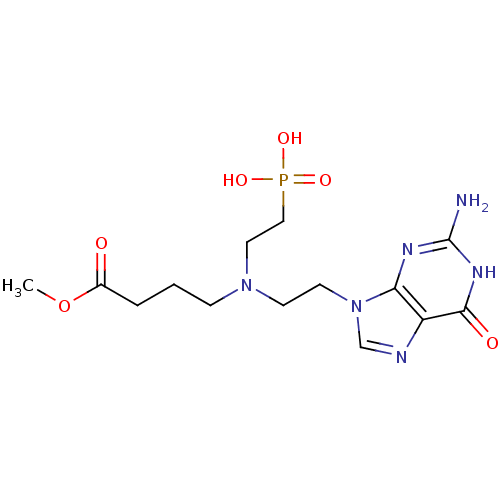 (CHEMBL2153478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50304140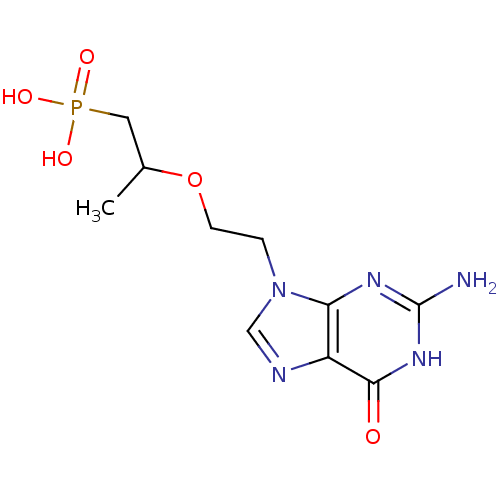 (9-[2-(1-Phosphonopropan-2-yloxy)ethyl]guanine | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant HGPRT expressed in Escherichia coli by spectrophotometric assay | Bioorg Med Chem 17: 6218-32 (2009) Article DOI: 10.1016/j.bmc.2009.07.044 BindingDB Entry DOI: 10.7270/Q23778TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392258 (CHEMBL2153476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM60887 (US9200020, Table 3, Compound 2B) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | -41.6 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitr... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194490 (US9200020, Table 1, Compound 1) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194500 (US9200020, Table 3 compound 9) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194499 (US9200020, Table 3 compound 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 150 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392265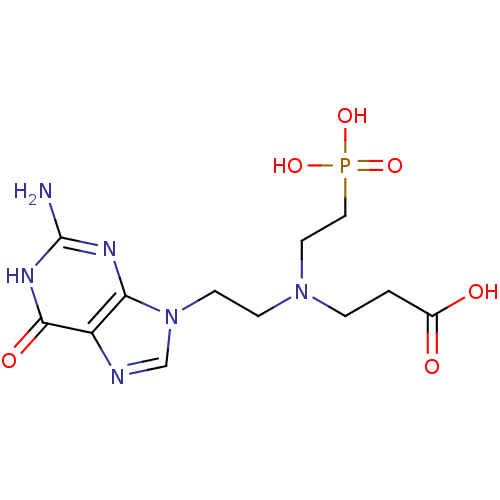 (CHEMBL2153483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM60890 (US9200020, Table 3 compound 5B) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 200 | -39.8 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitr... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194496 (US9200020, Table 3 compound 5) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194502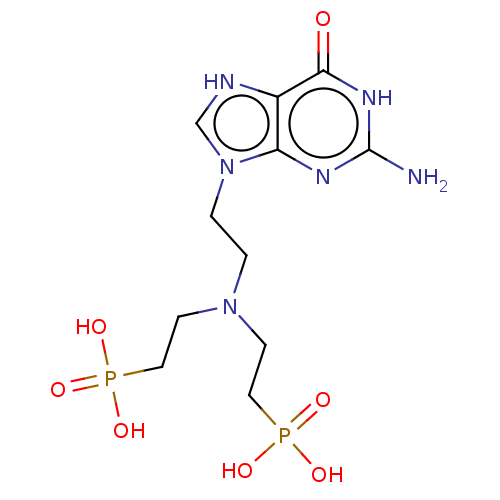 (US9200020, Table 3 compound 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194501 (US9200020, Table 3 compound 10) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392267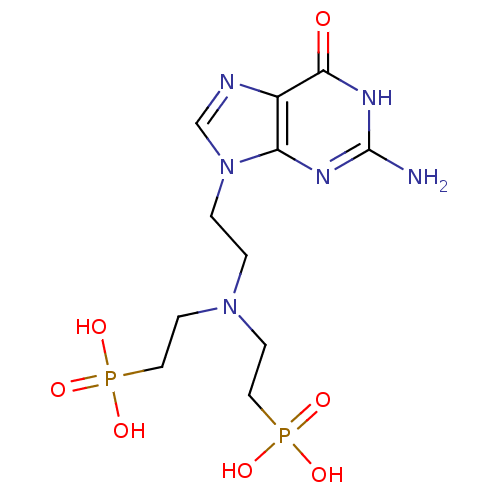 (CHEMBL2153485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194499 (US9200020, Table 3 compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine phosphoribosyltransferase (Plasmodium vivax) | BDBM194496 (US9200020, Table 3 compound 5) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194502 (US9200020, Table 3 compound 11) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM60880 (US9200020, Table 1, Compound 1B) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 300 | -38.7 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitr... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50304141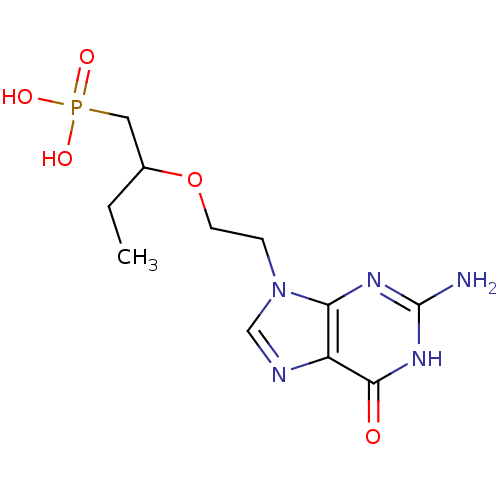 (9-[2-(1-Phosphonobutan-2-yloxy)ethyl]guanine | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant HGPRT expressed in Escherichia coli by spectrophotometric assay | Bioorg Med Chem 17: 6218-32 (2009) Article DOI: 10.1016/j.bmc.2009.07.044 BindingDB Entry DOI: 10.7270/Q23778TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50049966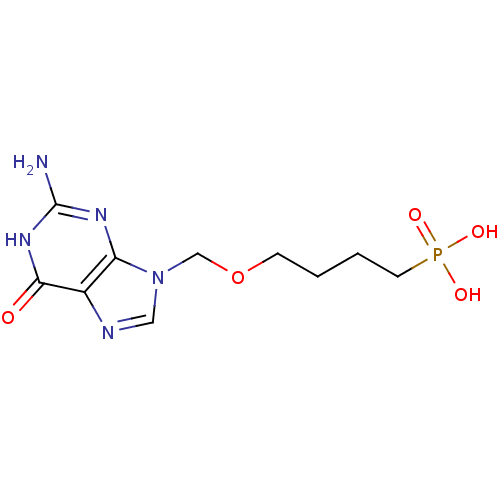 (CHEMBL177948 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT | Bioorg Med Chem 20: 1076-89 (2012) Article DOI: 10.1016/j.bmc.2011.11.034 BindingDB Entry DOI: 10.7270/Q2RV0P44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM60897 (US9200020, Table 3 compound 11B) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 400 | -38.0 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitr... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM60893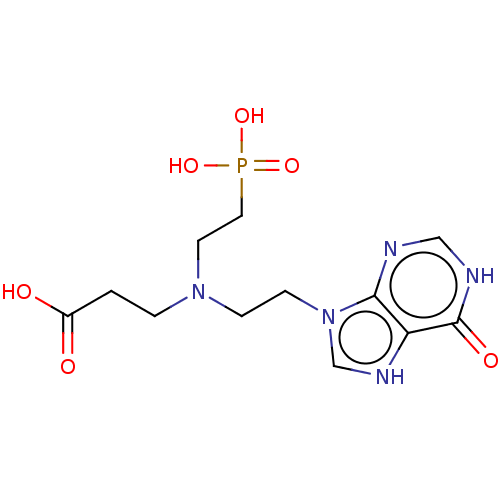 (US9200020, Table 3 compound 8B) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 400 | -38.0 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitr... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine-guanine phosphoribosyltransferase (Escherichia coli (strain K12)) | BDBM194503 (US9200020, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194493 (US9200020, Table 3 compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50039559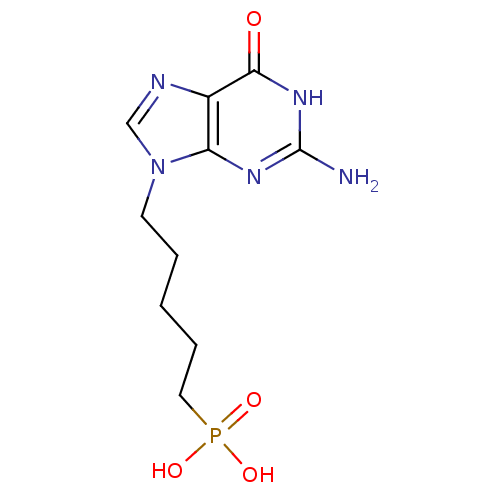 (CHEMBL267803 | [5-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT | Bioorg Med Chem 20: 1076-89 (2012) Article DOI: 10.1016/j.bmc.2011.11.034 BindingDB Entry DOI: 10.7270/Q2RV0P44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50360117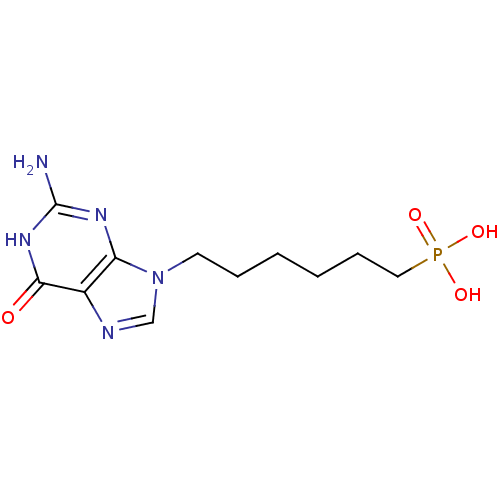 (CHEMBL1928784) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT | Bioorg Med Chem 20: 1076-89 (2012) Article DOI: 10.1016/j.bmc.2011.11.034 BindingDB Entry DOI: 10.7270/Q2RV0P44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392259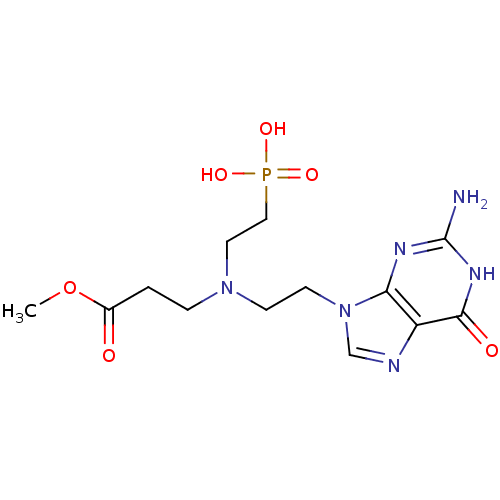 (CHEMBL2153477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392262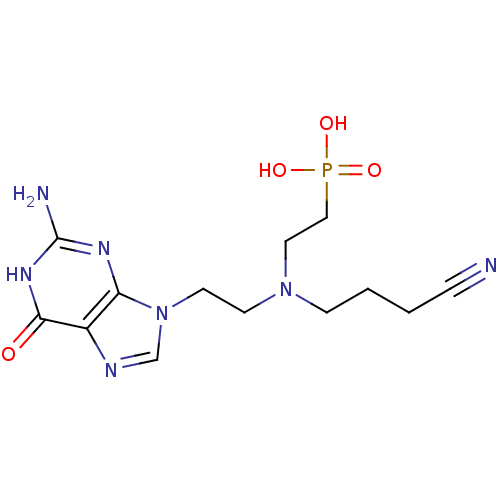 (CHEMBL2153481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50427808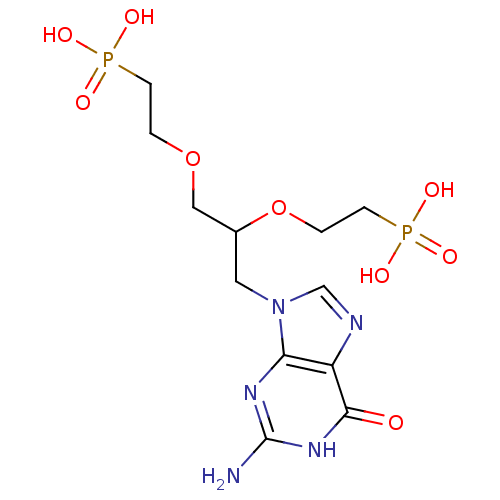 (CHEMBL2325754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human N-terminal hexahistidine-tagged HGPRT | J Med Chem 56: 2513-26 (2013) Article DOI: 10.1021/jm301893b BindingDB Entry DOI: 10.7270/Q2MW2JGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM60892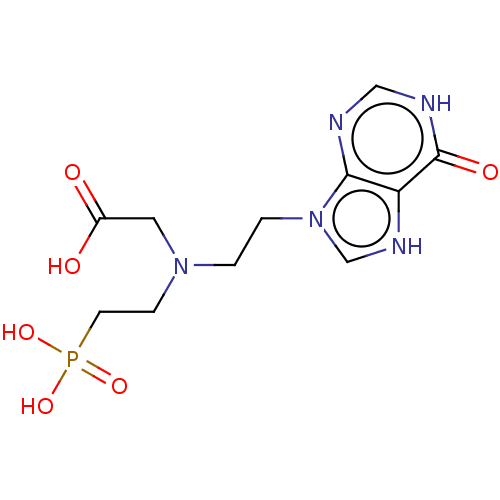 (US9200020, Table 3 compound 7B) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 600 | -36.9 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitr... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194497 (US9200020, Table 3 compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine phosphoribosyltransferase (Plasmodium vivax) | BDBM60890 (US9200020, Table 3 compound 5B) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 700 | -36.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitr... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194498 (US9200020, Table 3 compound 7) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine phosphoribosyltransferase (Plasmodium vivax) | BDBM194502 (US9200020, Table 3 compound 11) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194490 (US9200020, Table 1, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50293820 (2-(2-(2-amino-6-oxo-1,6,7,8-tetrahydropurin-9-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay | J Med Chem 52: 4391-9 (2009) Article DOI: 10.1021/jm900267n BindingDB Entry DOI: 10.7270/Q27M0809 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50427809 (CHEMBL2325753) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human N-terminal hexahistidine-tagged HGPRT | J Med Chem 56: 2513-26 (2013) Article DOI: 10.1021/jm301893b BindingDB Entry DOI: 10.7270/Q2MW2JGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM194494 (US9200020, Table 3 compound 3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50392264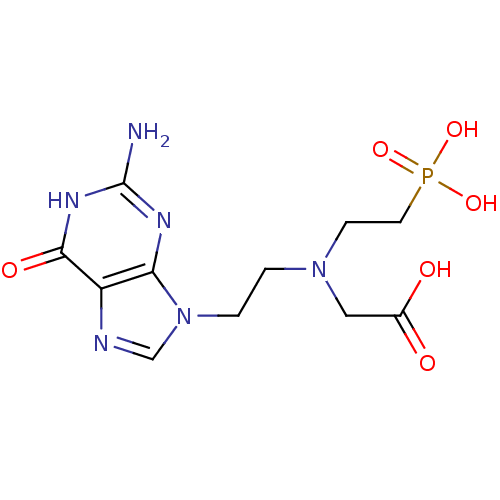 (CHEMBL2153482) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine | J Med Chem 55: 6209-23 (2012) Article DOI: 10.1021/jm300662d BindingDB Entry DOI: 10.7270/Q2VX0HND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM194498 (US9200020, Table 3 compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I. US Patent | Assay Description The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... | US Patent US9200020 (2015) BindingDB Entry DOI: 10.7270/Q2TM78XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |