

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human P2Y14 receptor expressed in CHO cells by fluorescence assay | Eur J Med Chem 175: 34-39 (2019) Article DOI: 10.1016/j.ejmech.2019.04.068 BindingDB Entry DOI: 10.7270/Q29C71R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50004813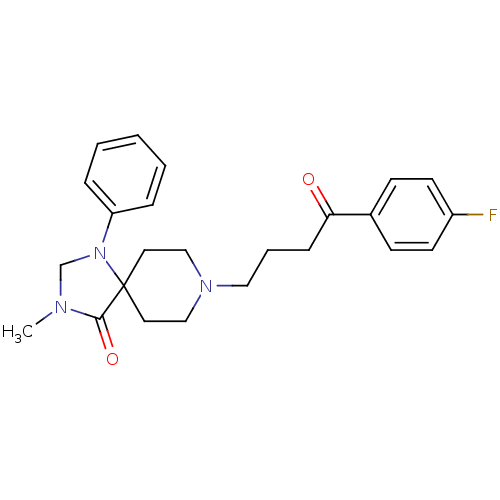 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-methyl-1-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153088 (CHEMBL365416 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153085 (CHEMBL364836 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50004813 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-methyl-1-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153083 (CHEMBL441502 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50400512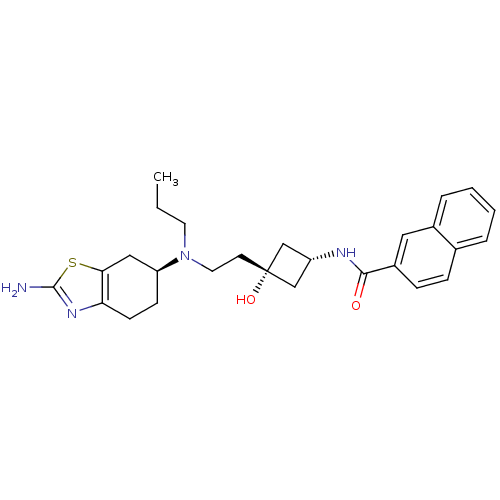 (CHEMBL2203405) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50119380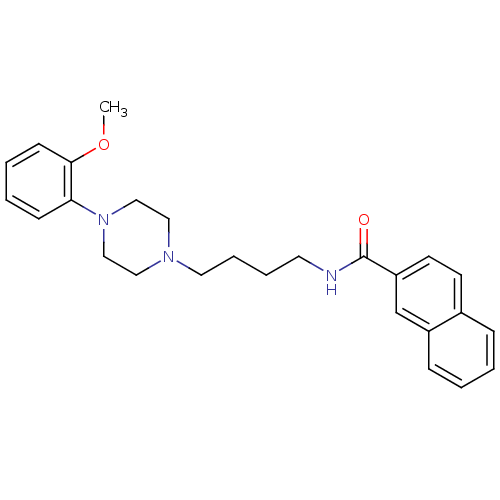 (CHEMBL25236 | CHEMBL540612 | N-(4-(4-(2-methoxyphe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM123850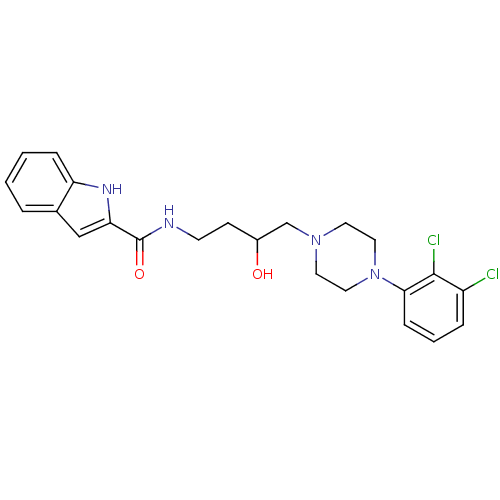 (US8748608, 34 | US8748608, 34 Enantiomer A | US874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50016453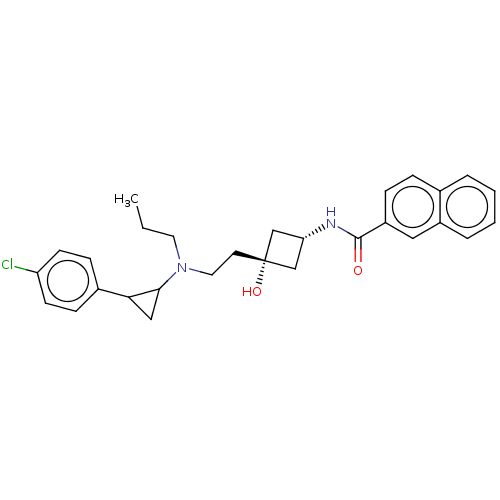 (CHEMBL3265063) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50366495 ((+)butaclamol | CHEMBL1255588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50016462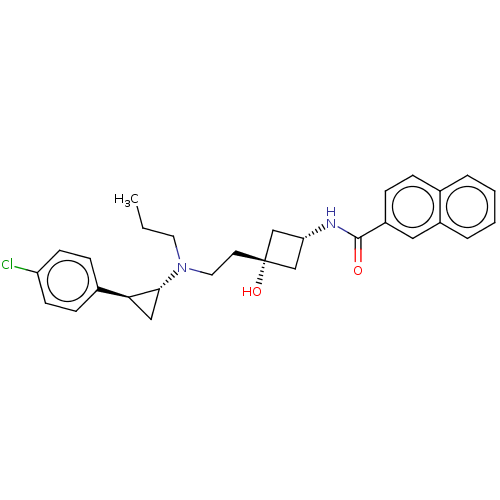 (CHEMBL3265067) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50016462 (CHEMBL3265067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153086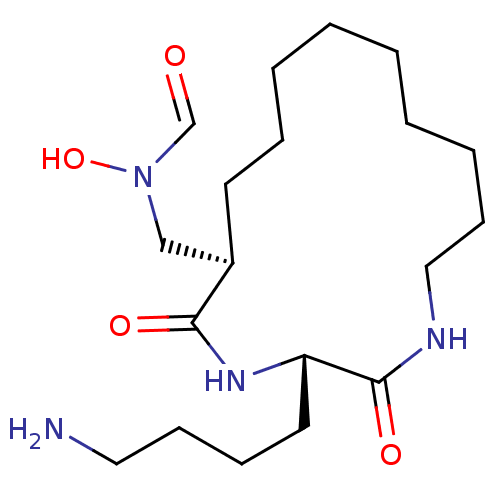 (CHEMBL188671 | N-[(1R,2S)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343888 ((R)-4-(4-(2,2-difluoro-1-hydroxyethyl)phenyl)-7-(4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of mouse P2Y14 receptor | Eur J Med Chem 175: 34-39 (2019) Article DOI: 10.1016/j.ejmech.2019.04.068 BindingDB Entry DOI: 10.7270/Q29C71R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50016453 (CHEMBL3265063) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50366495 ((+)butaclamol | CHEMBL1255588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50219105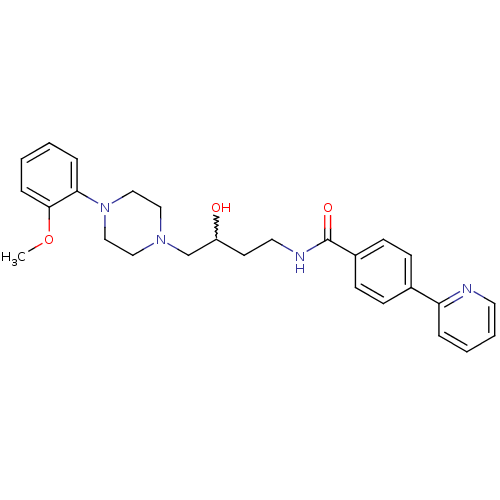 (CHEMBL242644 | N-(3-hydroxy-4-(4-(2-methoxyphenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50104501 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153084 (CHEMBL361449 | N-(4-{(3R,14S)-14-[(Formyl-hydroxy-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50005118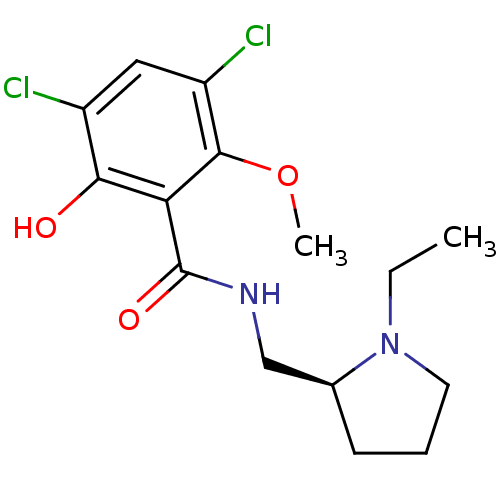 ((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50005118 ((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153087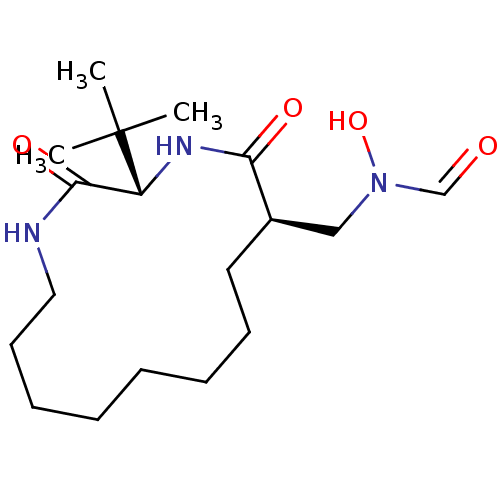 (CHEMBL188894 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50016447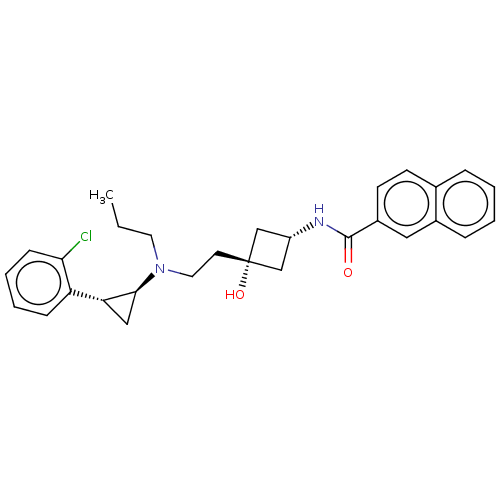 (CHEMBL3265064) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50016447 (CHEMBL3265064) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153080 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153081 (CHEMBL365910 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50016444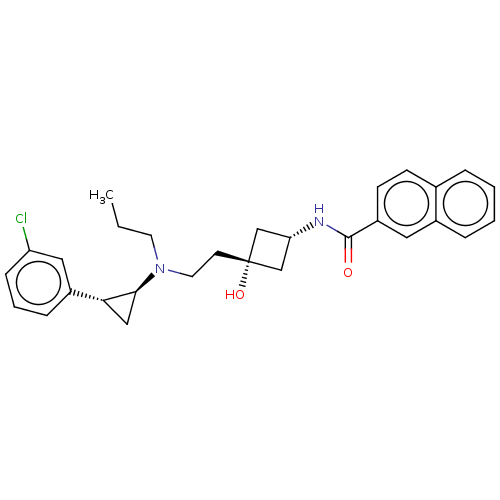 (CHEMBL3265065) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of mouse P2Y14 receptor in presence of 2% HSA | Eur J Med Chem 175: 34-39 (2019) Article DOI: 10.1016/j.ejmech.2019.04.068 BindingDB Entry DOI: 10.7270/Q29C71R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153079 (CHEMBL364979 | N-[(3S,6R)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50016444 (CHEMBL3265065) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50016464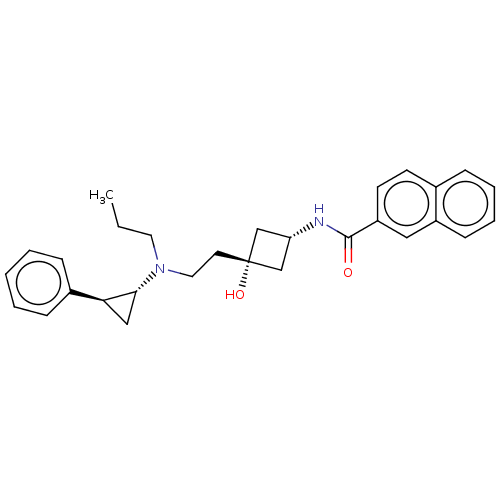 (CHEMBL3265062) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50312273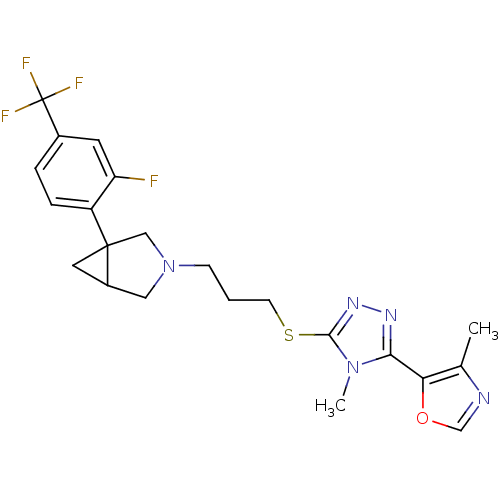 ((1R,5S/1S,5R)-1-[2-Fluoro-4-(trifluoromethyl)pheny...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153087 (CHEMBL188894 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153082 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-heptanoic ac...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153088 (CHEMBL365416 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153086 (CHEMBL188671 | N-[(1R,2S)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153085 (CHEMBL364836 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50016465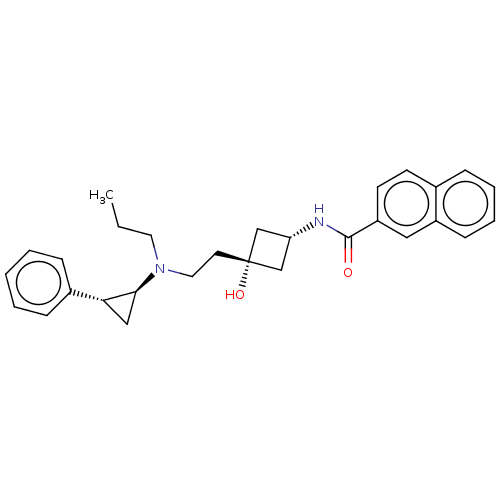 (CHEMBL3265061) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153083 (CHEMBL441502 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153079 (CHEMBL364979 | N-[(3S,6R)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl diphosphate synthase (Trypanosoma cruzi) | BDBM25298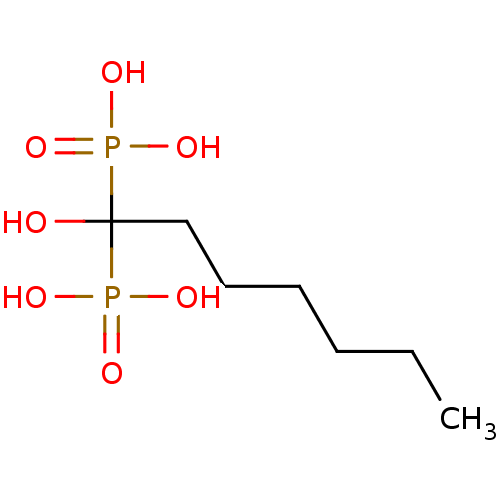 ((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi FPPS | Bioorg Med Chem 16: 3283-90 (2008) Article DOI: 10.1016/j.bmc.2007.12.010 BindingDB Entry DOI: 10.7270/Q2JD4XPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50016463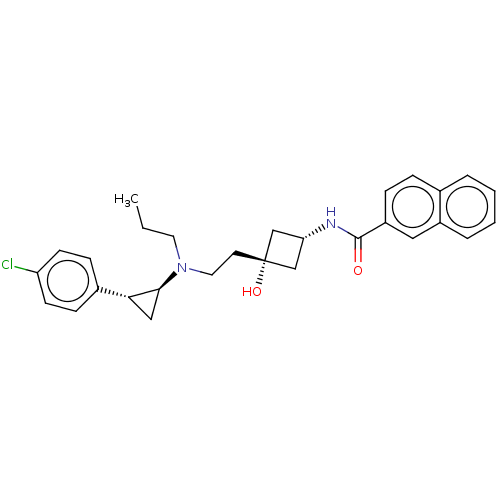 (CHEMBL3265066) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]R-(+)-7-OHDPAT from dopamine D3 receptor in Sprague-Dawley rat ventral striatum after 90 mins | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50016462 (CHEMBL3265067) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 623 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50016453 (CHEMBL3265063) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 667 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153084 (CHEMBL361449 | N-(4-{(3R,14S)-14-[(Formyl-hydroxy-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM123850 (US8748608, 34 | US8748608, 34 Enantiomer A | US874...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 746 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50016447 (CHEMBL3265064) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 923 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 4962-8 (2014) Article DOI: 10.1021/jm401798r BindingDB Entry DOI: 10.7270/Q2BV7J5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1385 total ) | Next | Last >> |