

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043393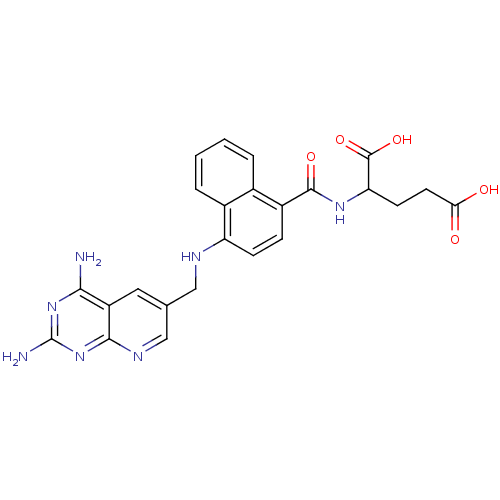 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043396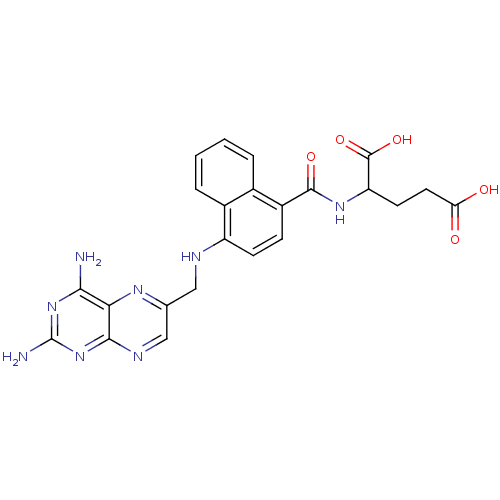 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043399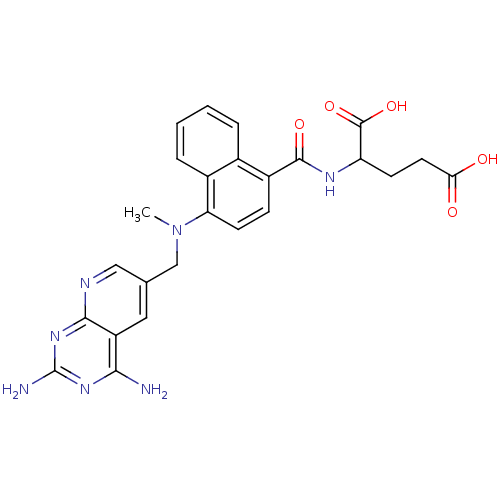 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of dihydrofolate reductase (DHFR) in L1210 cells | J Med Chem 35: 3002-6 (1992) BindingDB Entry DOI: 10.7270/Q2MP527Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043395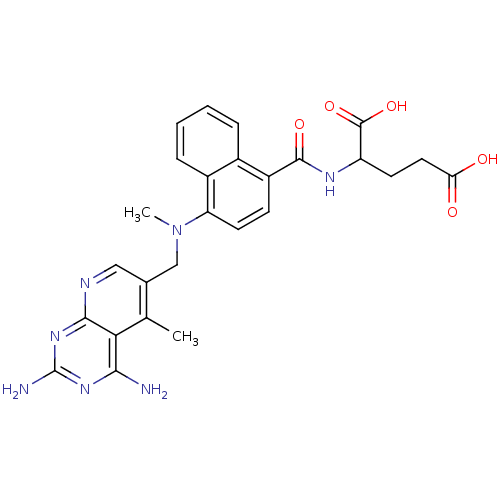 (2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043400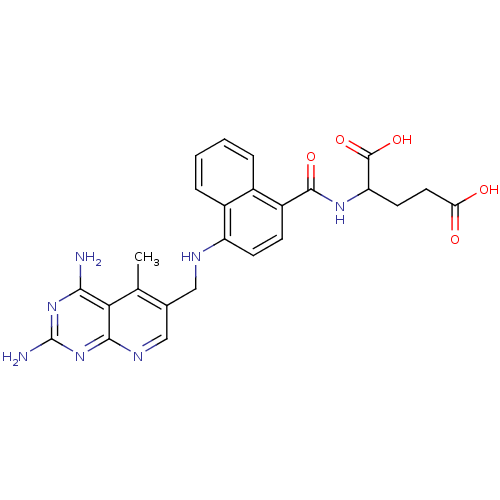 (2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043398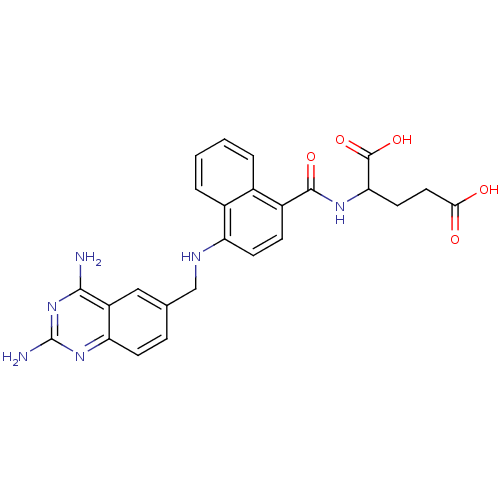 (2-({4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043394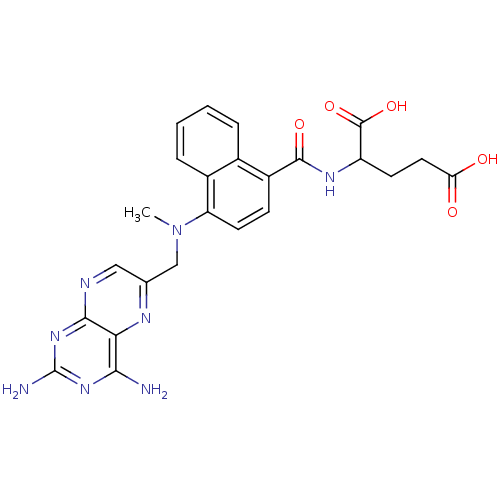 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029704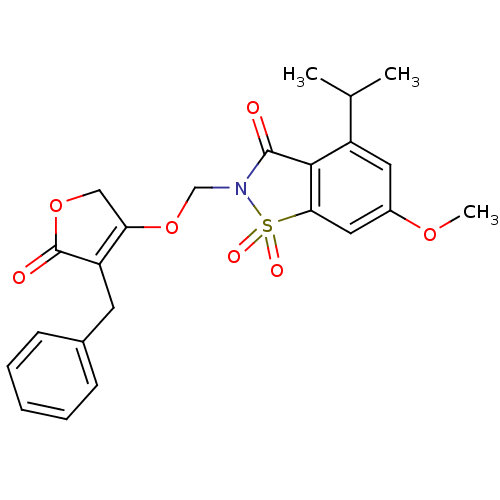 (2-(4-Benzyl-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043397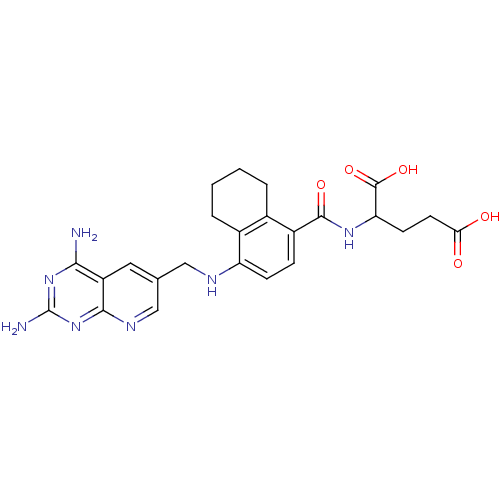 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029709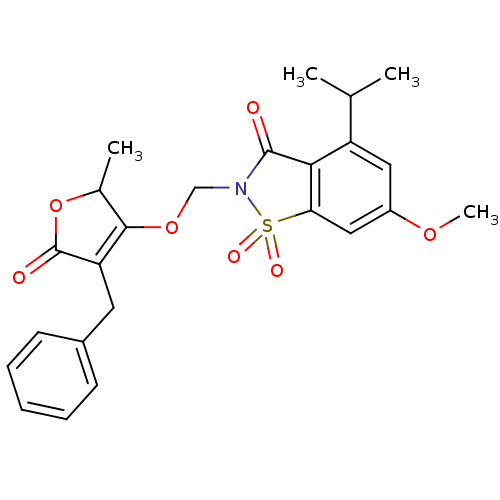 (2-(4-Benzyl-2-methyl-5-oxo-2,5-dihydro-furan-3-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189451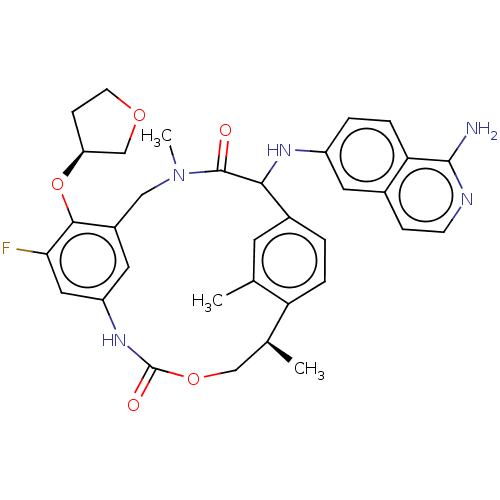 (US9174974, Example 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029710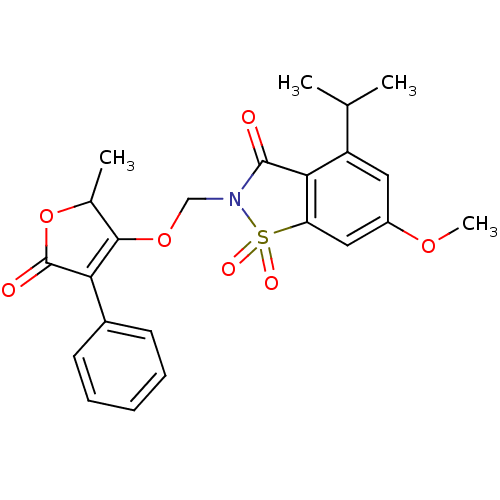 (4-Isopropyl-6-methoxy-2-(2-methyl-5-oxo-4-phenyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699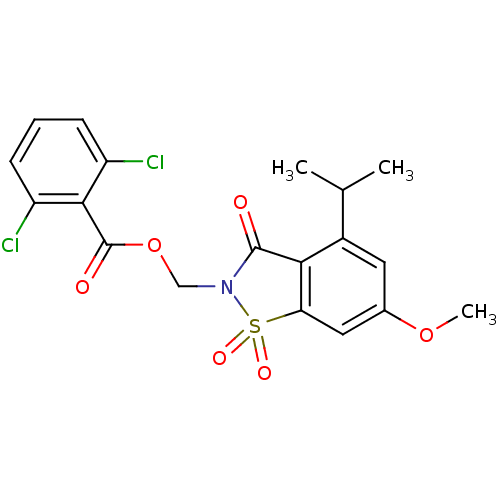 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029698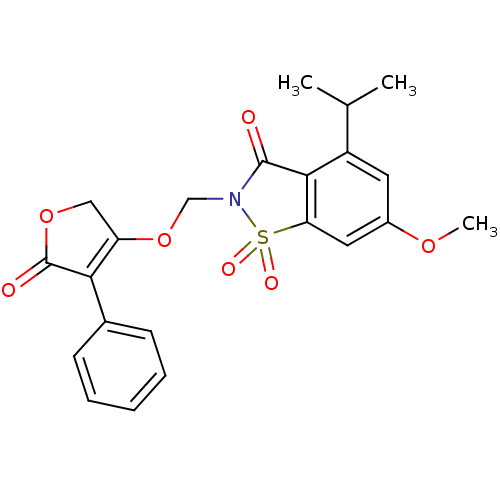 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(5-oxo-4-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029717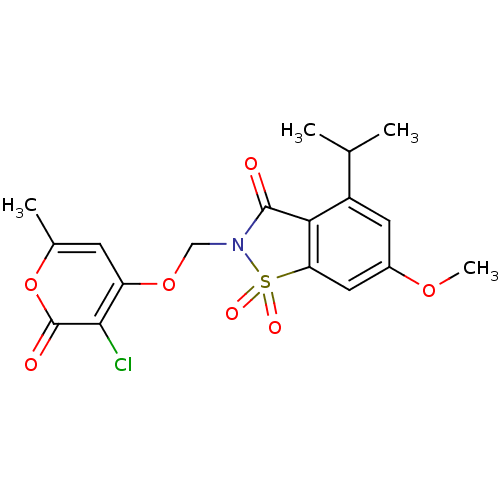 (2-(3-Chloro-6-methyl-2-oxo-2H-pyran-4-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029696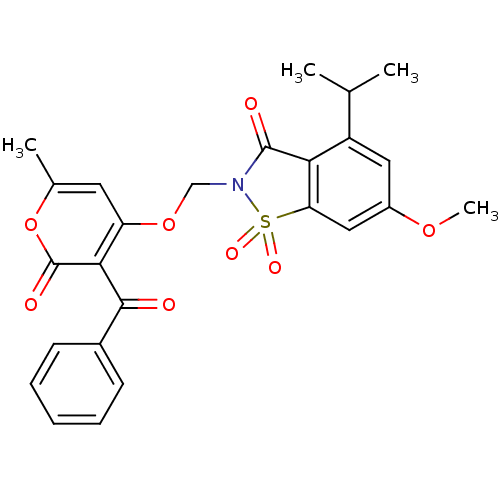 (2-(3-Benzoyl-6-methyl-2-oxo-2H-pyran-4-yloxymethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029712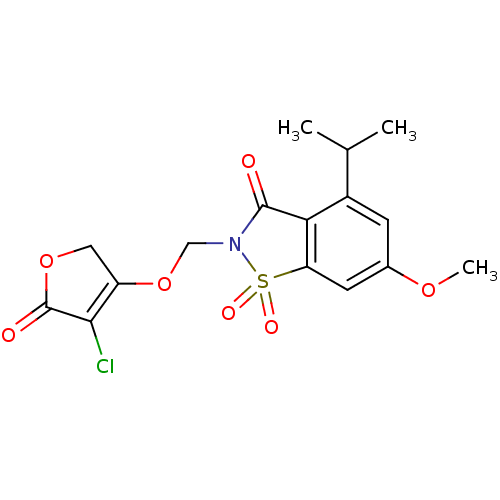 (2-(4-Chloro-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029713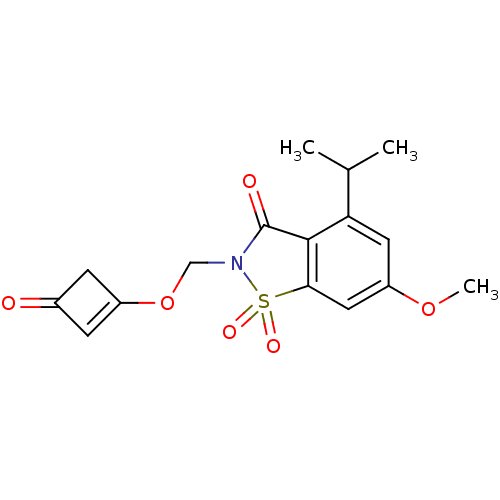 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(3-oxo-cyclobut-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029691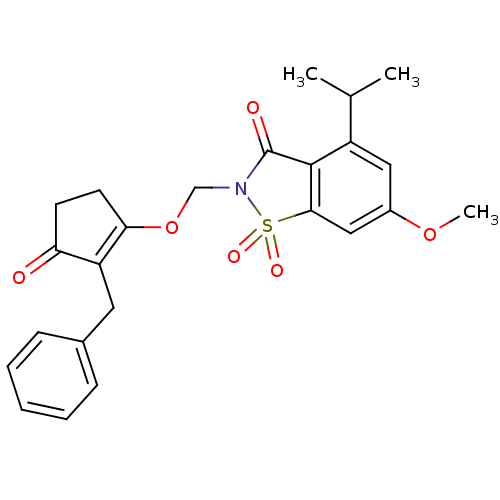 (2-(2-Benzyl-3-oxo-cyclopent-1-enyloxymethyl)-4-iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379543 (CHEMBL2012752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545960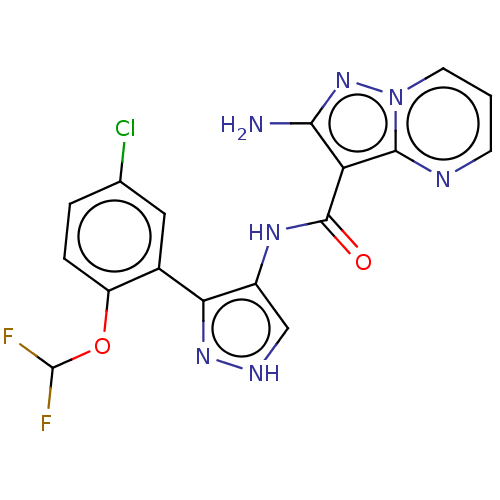 (CHEMBL4740778 | US11649241, Example 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545980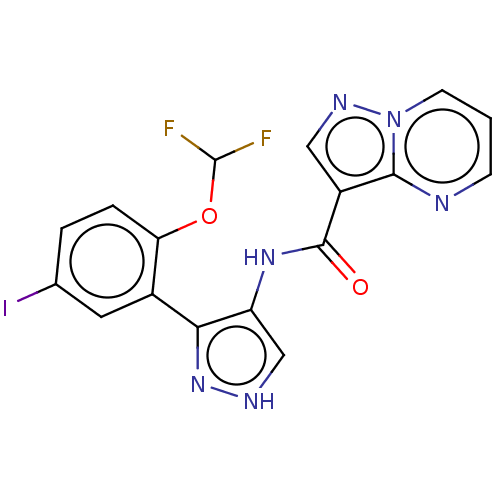 (CHEMBL4764019) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545979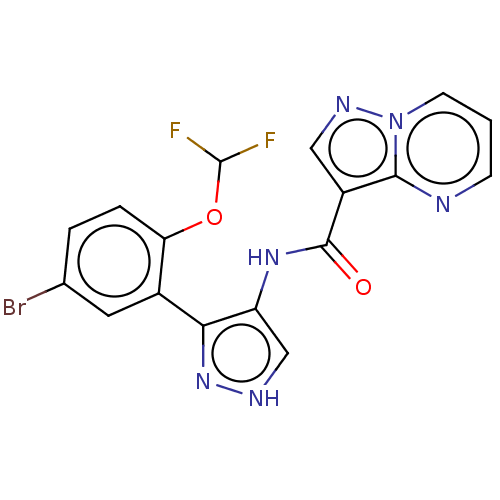 (CHEMBL4742159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189454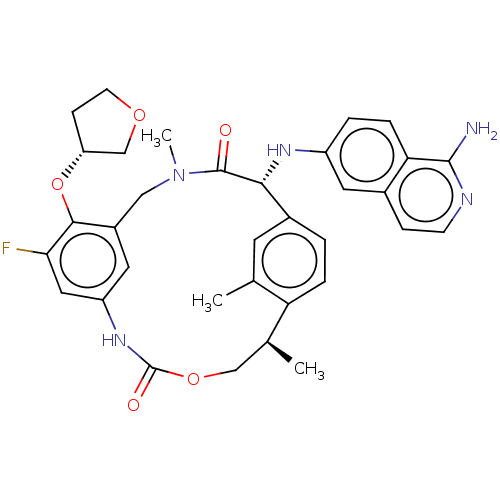 (US9174974, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029692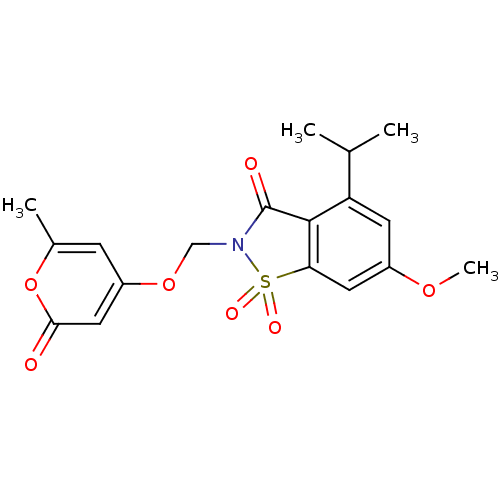 (4-Isopropyl-6-methoxy-2-(6-methyl-2-oxo-2H-pyran-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329261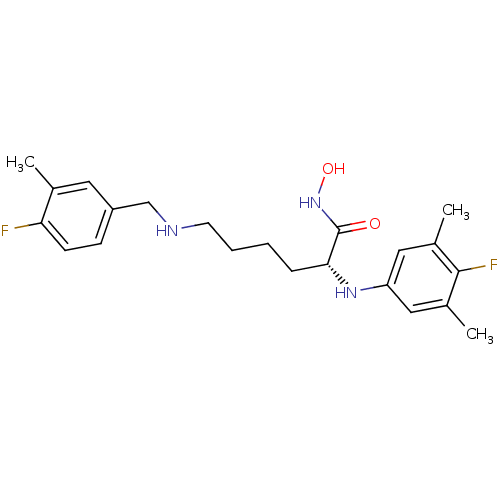 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329261 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate by FRET assay | Bioorg Med Chem Lett 20: 6850-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.058 BindingDB Entry DOI: 10.7270/Q2222V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029695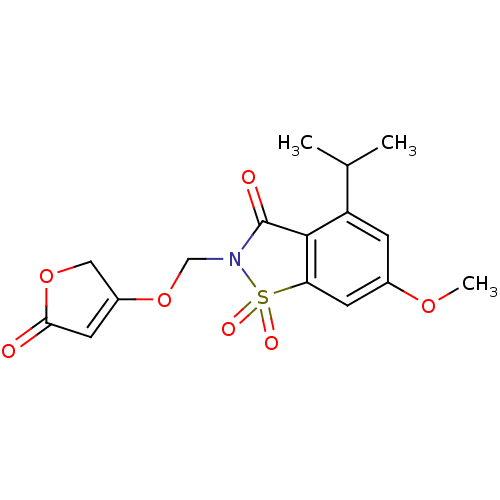 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(5-oxo-2,5-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545987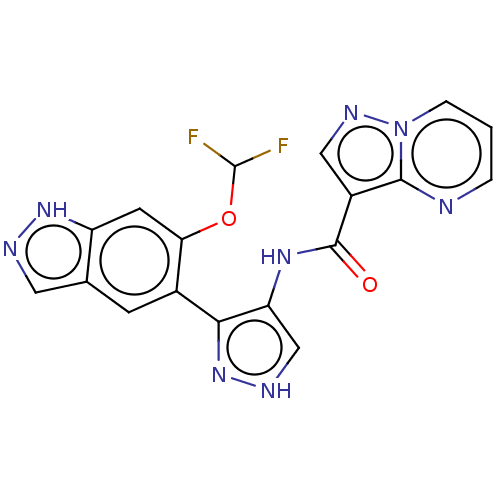 (CHEMBL4746726) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545986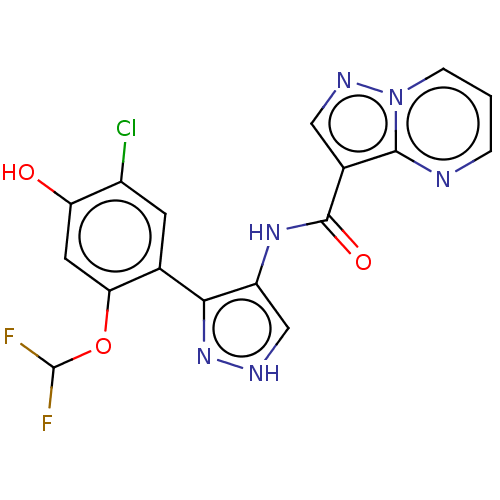 (CHEMBL4789075) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029711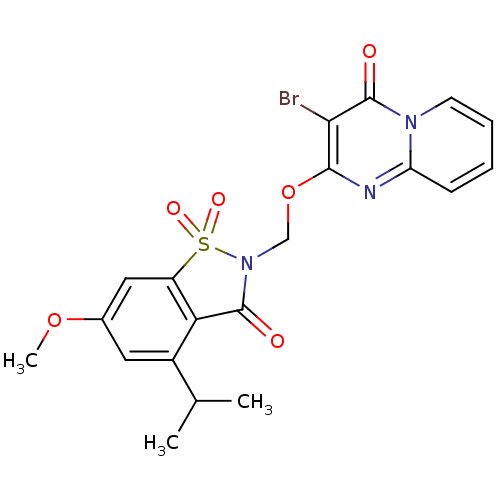 (3-Bromo-2-(4-isopropyl-6-methoxy-1,1,3-trioxo-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029720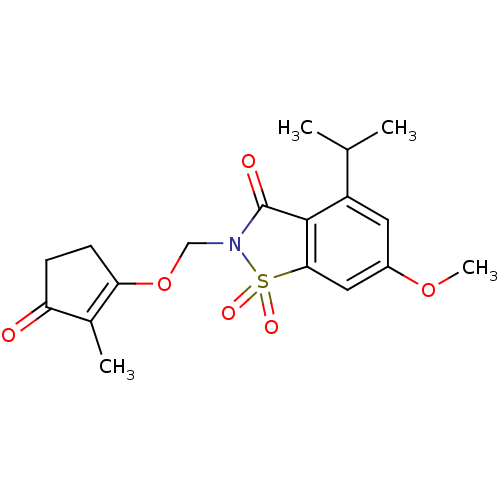 (4-Isopropyl-6-methoxy-2-(2-methyl-3-oxo-cyclopent-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029707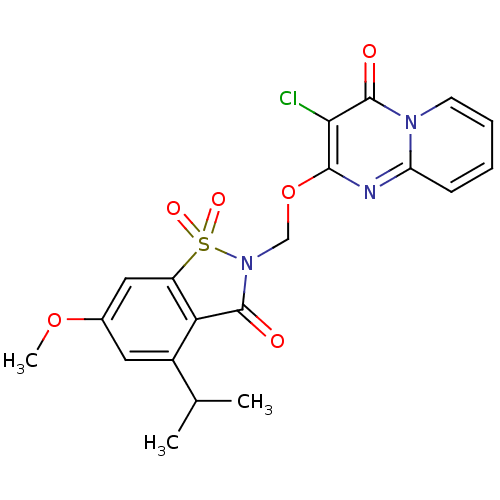 (3-Chloro-2-(4-isopropyl-6-methoxy-1,1,3-trioxo-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545961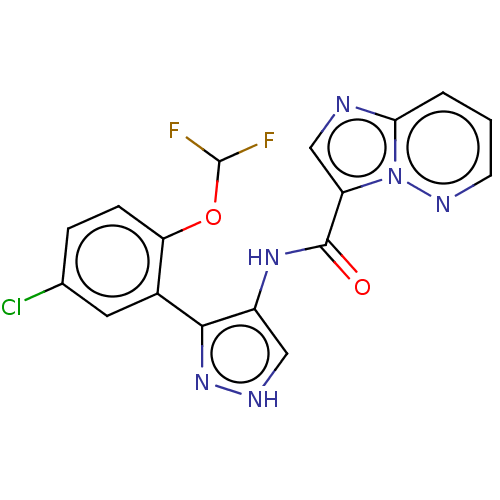 (CHEMBL4793262) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545944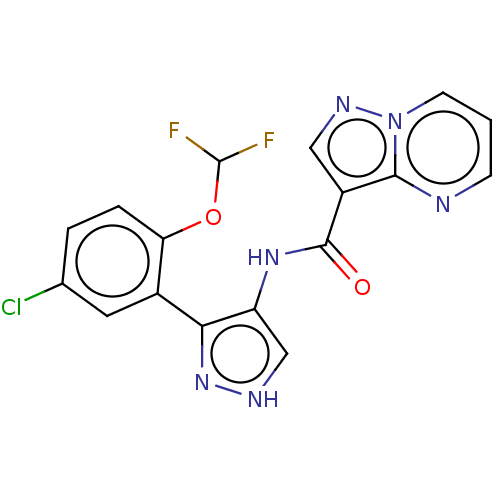 (CHEMBL4744172) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50567169 (CHEMBL4873876) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Apelin-13 from human APJ receptor stably expressed in human HEK293 cell membrane incubated for 120 mins by TopCount scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01878 BindingDB Entry DOI: 10.7270/Q20G3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50545987 (CHEMBL4746726) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK1 (854 to 1154 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545964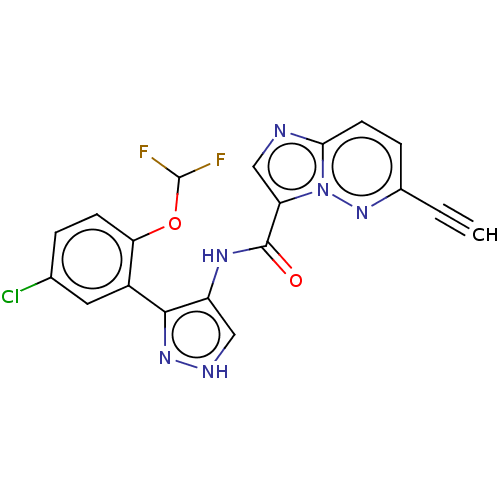 (CHEMBL4746416) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029721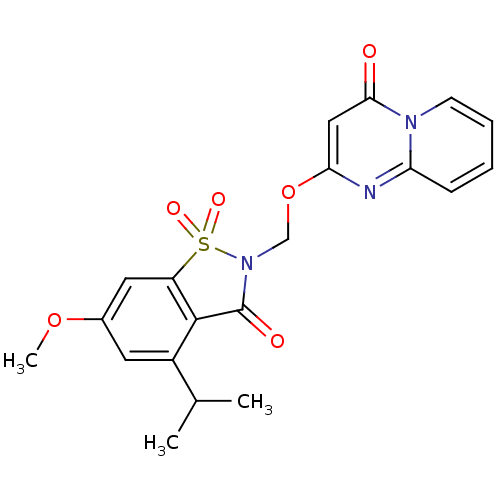 (2-(4-Isopropyl-6-methoxy-1,1,3-trioxo-1,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029715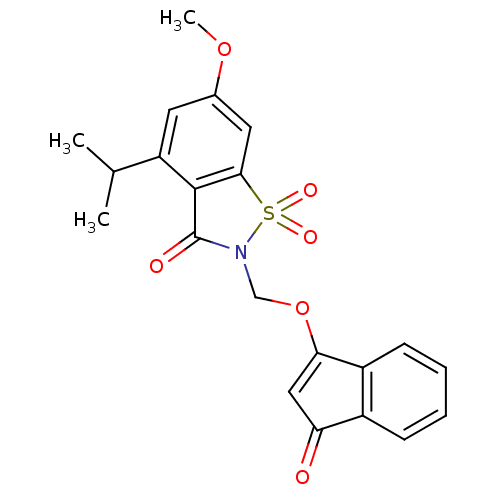 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(3-oxo-3H-inden-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545965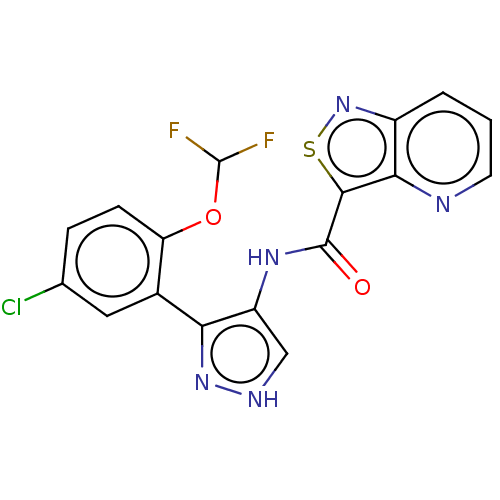 (CHEMBL4788860 | US11649241, Example 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545945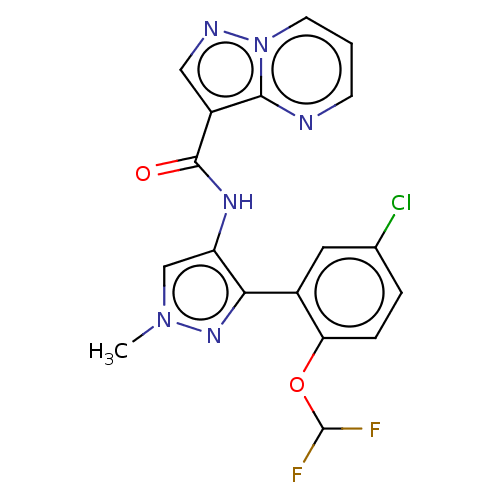 (CHEMBL4777342) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029718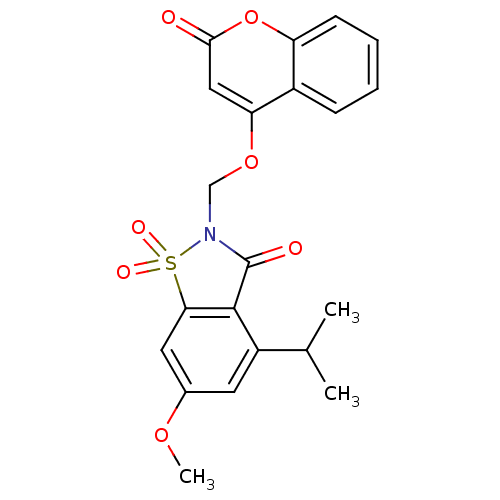 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(2-oxo-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029700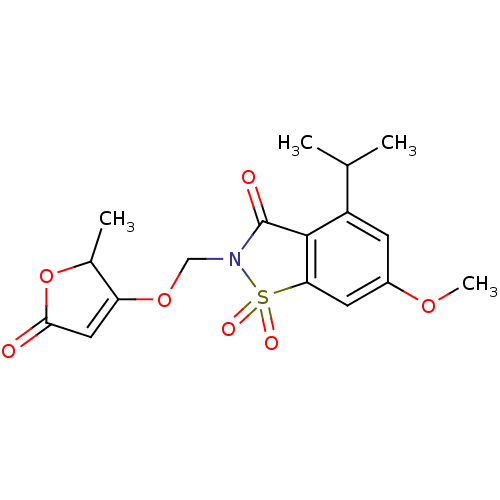 (4-Isopropyl-6-methoxy-2-(2-methyl-5-oxo-2,5-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015266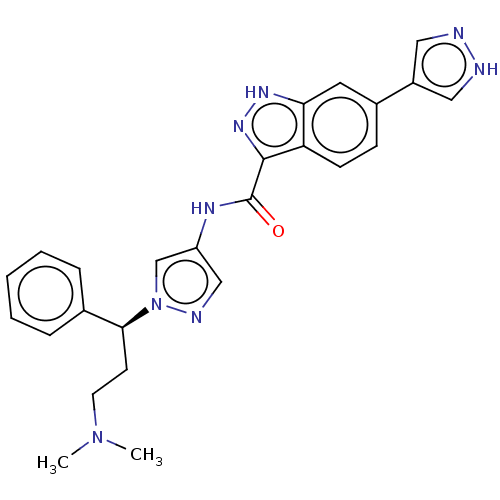 (CHEMBL3263053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015266 (CHEMBL3263053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50004544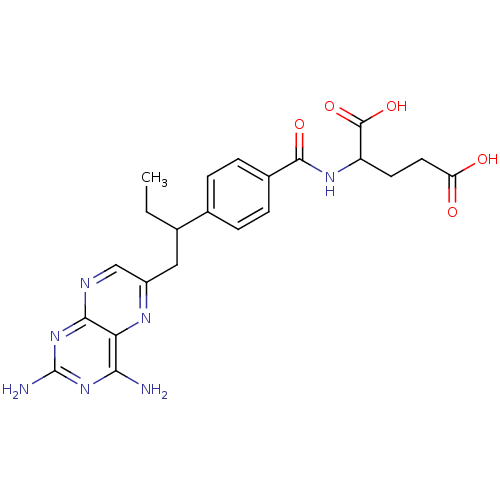 (2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of dihydrofolate reductase (DHFR) in L1210 cells | J Med Chem 35: 3002-6 (1992) BindingDB Entry DOI: 10.7270/Q2MP527Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50434787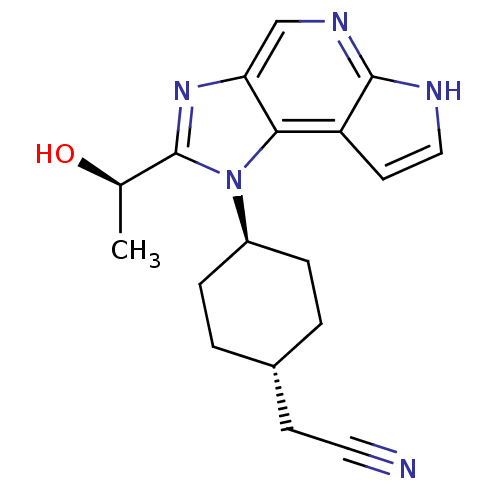 (CHEMBL2386635 | US10487083, Example C | US10703751...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant JAK1 (unknown origin) using Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 mins in presence of ATP | J Med Chem 56: 4764-85 (2013) Article DOI: 10.1021/jm4004895 BindingDB Entry DOI: 10.7270/Q2CV4K40 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 20995 total ) | Next | Last >> |