 Found 1347 hits with Last Name = 'joossens' and Initial = 'j'
Found 1347 hits with Last Name = 'joossens' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prolyl endopeptidase FAP
(Mus musculus (Mouse)) | BDBM50434188

(CHEMBL2385281 | US9346814, Cmpd No 2 Example 3)Show SMILES O=C(CNC(=O)c1ccnc2ccccc12)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H16N4O2/c18-10-12-4-3-9-21(12)16(22)11-20-17(23)14-7-8-19-15-6-2-1-5-13(14)15/h1-2,5-8,12H,3-4,9,11H2,(H,20,23)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... |
ACS Med Chem Lett 4: 491-6 (2013)
Article DOI: 10.1021/ml300410d
BindingDB Entry DOI: 10.7270/Q2KP83J7 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Mus musculus (Mouse)) | BDBM50434169

(CHEMBL2385300 | US9346814, Cmpd No 22 Example 26)Show SMILES Brc1cccc2nccc(C(=O)NCC(=O)N3CCC[C@H]3C#N)c12 |r| Show InChI InChI=1S/C17H15BrN4O2/c18-13-4-1-5-14-16(13)12(6-7-20-14)17(24)21-10-15(23)22-8-2-3-11(22)9-19/h1,4-7,11H,2-3,8,10H2,(H,21,24)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... |
ACS Med Chem Lett 4: 491-6 (2013)
Article DOI: 10.1021/ml300410d
BindingDB Entry DOI: 10.7270/Q2KP83J7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50350167
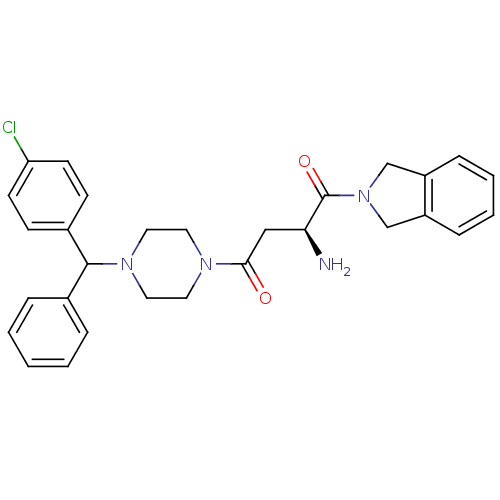
(CHEMBL1814741)Show SMILES N[C@@H](CC(=O)N1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1)C(=O)N1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H31ClN4O2/c30-25-12-10-22(11-13-25)28(21-6-2-1-3-7-21)33-16-14-32(15-17-33)27(35)18-26(31)29(36)34-19-23-8-4-5-9-24(23)20-34/h1-13,26,28H,14-20,31H2/t26-,28?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human DPP8 assessed as pNA release from Ala-Pro- p-nitroanilide substrate pre-incubated with enzyme for 15 min ... |
J Med Chem 54: 5737-46 (2011)
Article DOI: 10.1021/jm200383j
BindingDB Entry DOI: 10.7270/Q2G73F37 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110025
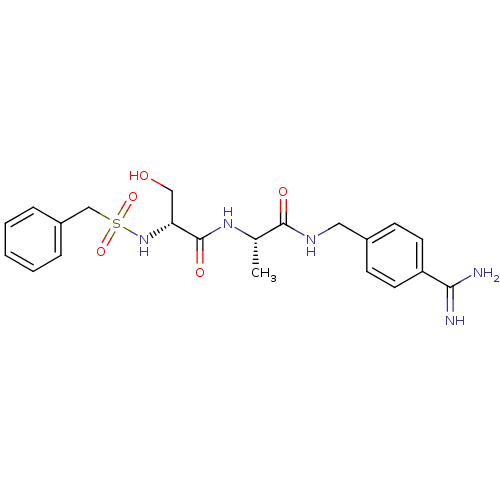
(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by spectrop... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16152
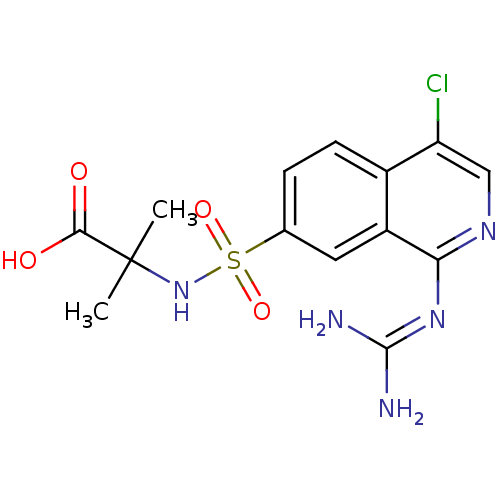
(2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#6]C([#6])([#7]S(=O)(=O)c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1)[#6](-[#8])=O Show InChI InChI=1S/C14H16ClN5O4S/c1-14(2,12(21)22)20-25(23,24)7-3-4-8-9(5-7)11(19-13(16)17)18-6-10(8)15/h3-6,20H,1-2H3,(H,21,22)(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using S-2444 as substrate |
J Med Chem 58: 9238-57 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01171
BindingDB Entry DOI: 10.7270/Q241713M |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228427
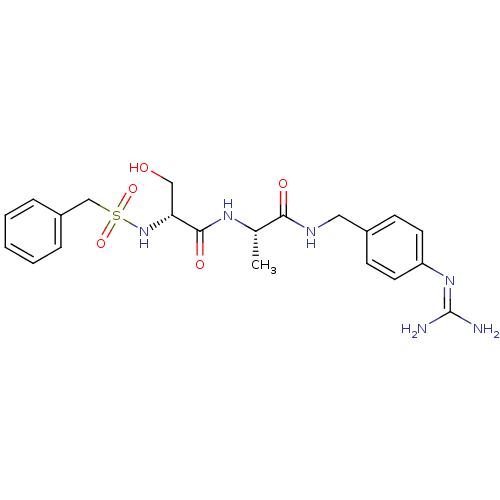
((R)-N-[(S)-1-(4-guanidino-benzylcarbamoyl)-ethyl]-...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C21H28N6O5S/c1-14(19(29)24-11-15-7-9-17(10-8-15)26-21(22)23)25-20(30)18(12-28)27-33(31,32)13-16-5-3-2-4-6-16/h2-10,14,18,27-28H,11-13H2,1H3,(H,24,29)(H,25,30)(H4,22,23,26)/t14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50231520
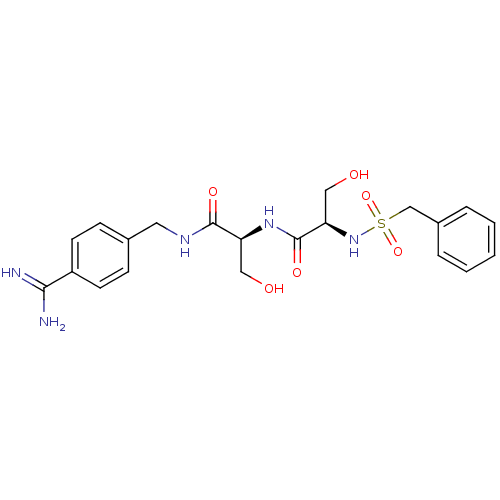
((R)-N-[(S)-1-(4-carbamimidoyl-benzylcarbamoyl)-2-h...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CO)NC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C21H27N5O6S/c22-19(23)16-8-6-14(7-9-16)10-24-20(29)17(11-27)25-21(30)18(12-28)26-33(31,32)13-15-4-2-1-3-5-15/h1-9,17-18,26-28H,10-13H2,(H3,22,23)(H,24,29)(H,25,30)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 58: 9238-57 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01171
BindingDB Entry DOI: 10.7270/Q241713M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50350175
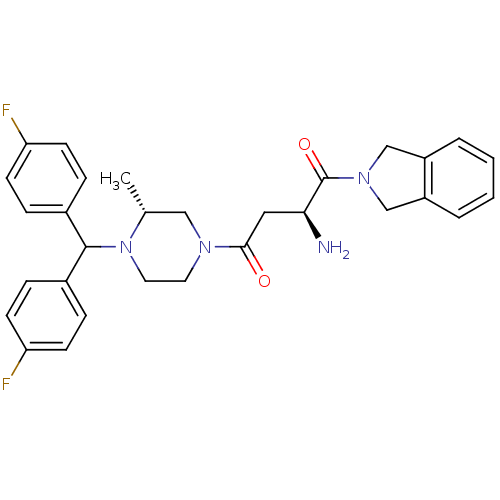
(CHEMBL1814749)Show SMILES C[C@@H]1CN(CCN1C(c1ccc(F)cc1)c1ccc(F)cc1)C(=O)C[C@H](N)C(=O)N1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H32F2N4O2/c1-20-17-34(28(37)16-27(33)30(38)35-18-23-4-2-3-5-24(23)19-35)14-15-36(20)29(21-6-10-25(31)11-7-21)22-8-12-26(32)13-9-22/h2-13,20,27,29H,14-19,33H2,1H3/t20-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human DPP8 assessed as pNA release from Ala-Pro- p-nitroanilide substrate pre-incubated with enzyme for 15 min ... |
J Med Chem 54: 5737-46 (2011)
Article DOI: 10.1021/jm200383j
BindingDB Entry DOI: 10.7270/Q2G73F37 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147422
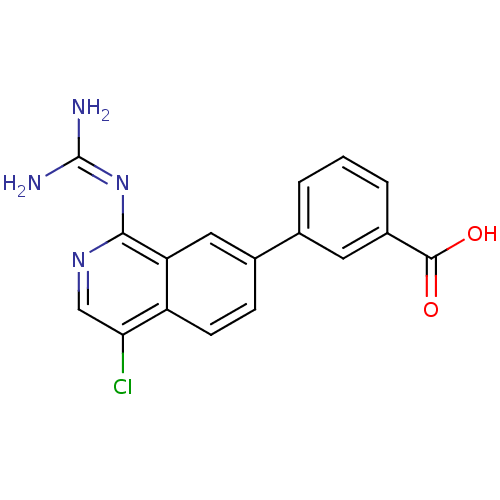
(3-(4-Chloro-1-guanidino-isoquinolin-7-yl)-benzoic ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ncc(Cl)c2ccc(cc12)-c1cccc(c1)-[#6](-[#8])=O Show InChI InChI=1S/C17H13ClN4O2/c18-14-8-21-15(22-17(19)20)13-7-10(4-5-12(13)14)9-2-1-3-11(6-9)16(23)24/h1-8H,(H,23,24)(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using S-2444 as substrate |
J Med Chem 58: 9238-57 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01171
BindingDB Entry DOI: 10.7270/Q241713M |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138662
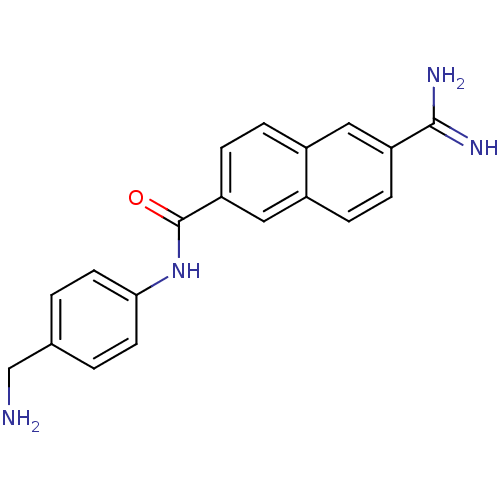
(6-Carbamimidoyl-naphthalene-2-carboxylic acid (4-a...)Show InChI InChI=1S/C19H18N4O/c20-11-12-1-7-17(8-2-12)23-19(24)16-6-4-13-9-15(18(21)22)5-3-14(13)10-16/h1-10H,11,20H2,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by spectrop... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50110025

(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasma thrombin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138678
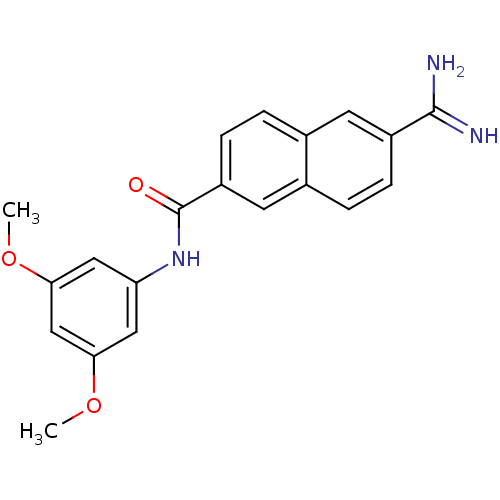
(6-Carbamimidoyl-naphthalene-2-carboxylic acid (3,5...)Show SMILES COc1cc(NC(=O)c2ccc3cc(ccc3c2)C(N)=N)cc(OC)c1 Show InChI InChI=1S/C20H19N3O3/c1-25-17-9-16(10-18(11-17)26-2)23-20(24)15-6-4-12-7-14(19(21)22)5-3-13(12)8-15/h3-11H,1-2H3,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by spectrop... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM23891
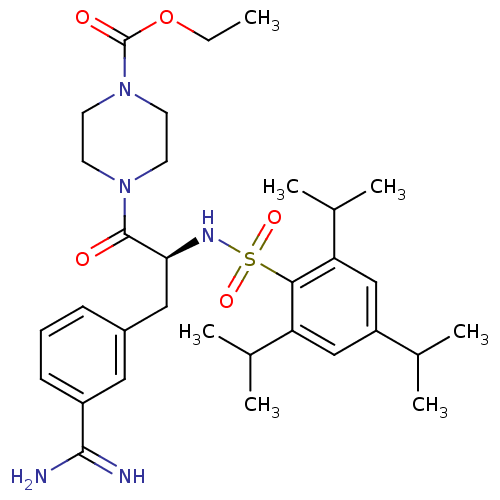
(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasma plasmin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins ... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by spectrop... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of uPA (unknown origin) |
J Med Chem 58: 9238-57 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01171
BindingDB Entry DOI: 10.7270/Q241713M |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasma thrombin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50110025

(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasma plasmin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins ... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138670

(6-Carbamimidoyl-naphthalene-2-carboxylic acid phen...)Show InChI InChI=1S/C18H15N3O/c19-17(20)14-8-6-13-11-15(9-7-12(13)10-14)18(22)21-16-4-2-1-3-5-16/h1-11H,(H3,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by spectrop... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138670

(6-Carbamimidoyl-naphthalene-2-carboxylic acid phen...)Show InChI InChI=1S/C18H15N3O/c19-17(20)14-8-6-13-11-15(9-7-12(13)10-14)18(22)21-16-4-2-1-3-5-16/h1-11H,(H3,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Competitive inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50138670

(6-Carbamimidoyl-naphthalene-2-carboxylic acid phen...)Show InChI InChI=1S/C18H15N3O/c19-17(20)14-8-6-13-11-15(9-7-12(13)10-14)18(22)21-16-4-2-1-3-5-16/h1-11H,(H3,19,20)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasma plasmin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins ... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228427

((R)-N-[(S)-1-(4-guanidino-benzylcarbamoyl)-ethyl]-...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C21H28N6O5S/c1-14(19(29)24-11-15-7-9-17(10-8-15)26-21(22)23)25-20(30)18(12-28)27-33(31,32)13-16-5-3-2-4-6-16/h2-10,14,18,27-28H,11-13H2,1H3,(H,24,29)(H,25,30)(H4,22,23,26)/t14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of recombinant tPA using H-D-Ile-Pro-Arg-pNA.2HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by sp... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50138670

(6-Carbamimidoyl-naphthalene-2-carboxylic acid phen...)Show InChI InChI=1S/C18H15N3O/c19-17(20)14-8-6-13-11-15(9-7-12(13)10-14)18(22)21-16-4-2-1-3-5-16/h1-11H,(H3,19,20)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasma thrombin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110025

(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of recombinant tPA using H-D-Ile-Pro-Arg-pNA.2HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by sp... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228422
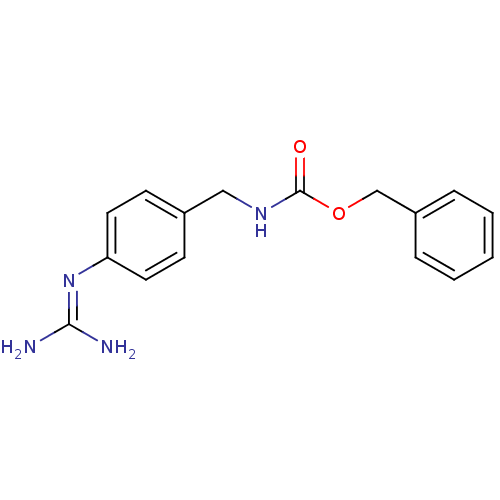
((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50138662

(6-Carbamimidoyl-naphthalene-2-carboxylic acid (4-a...)Show InChI InChI=1S/C19H18N4O/c20-11-12-1-7-17(8-2-12)23-19(24)16-6-4-13-9-15(18(21)22)5-3-14(13)10-16/h1-10H,11,20H2,(H3,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasma plasmin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins ... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138670

(6-Carbamimidoyl-naphthalene-2-carboxylic acid phen...)Show InChI InChI=1S/C18H15N3O/c19-17(20)14-8-6-13-11-15(9-7-12(13)10-14)18(22)21-16-4-2-1-3-5-16/h1-11H,(H3,19,20)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of recombinant tPA using H-D-Ile-Pro-Arg-pNA.2HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by sp... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228427

((R)-N-[(S)-1-(4-guanidino-benzylcarbamoyl)-ethyl]-...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C21H28N6O5S/c1-14(19(29)24-11-15-7-9-17(10-8-15)26-21(22)23)25-20(30)18(12-28)27-33(31,32)13-16-5-3-2-4-6-16/h2-10,14,18,27-28H,11-13H2,1H3,(H,24,29)(H,25,30)(H4,22,23,26)/t14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138662

(6-Carbamimidoyl-naphthalene-2-carboxylic acid (4-a...)Show InChI InChI=1S/C19H18N4O/c20-11-12-1-7-17(8-2-12)23-19(24)16-6-4-13-9-15(18(21)22)5-3-14(13)10-16/h1-10H,11,20H2,(H3,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of recombinant tPA using H-D-Ile-Pro-Arg-pNA.2HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by sp... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50138662

(6-Carbamimidoyl-naphthalene-2-carboxylic acid (4-a...)Show InChI InChI=1S/C19H18N4O/c20-11-12-1-7-17(8-2-12)23-19(24)16-6-4-13-9-15(18(21)22)5-3-14(13)10-16/h1-10H,11,20H2,(H3,21,22)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasma thrombin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228422

((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228422

((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228422

((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228422

((4-guanidino-benzyl)-carbamic acid benzyl ester | ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C16H18N4O2/c17-15(18)20-14-8-6-12(7-9-14)10-19-16(21)22-11-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,19,21)(H4,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50146972
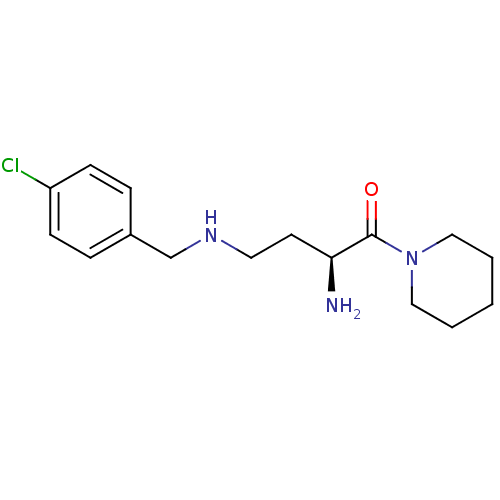
((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...)Show InChI InChI=1S/C16H24ClN3O/c17-14-6-4-13(5-7-14)12-19-9-8-15(18)16(21)20-10-2-1-3-11-20/h4-7,15,19H,1-3,8-12,18H2/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 |
J Med Chem 54: 5737-46 (2011)
Article DOI: 10.1021/jm200383j
BindingDB Entry DOI: 10.7270/Q2G73F37 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50225074

((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |TLB:1:12:15:20.18.17,THB:13:14:17:22.12.21,13:12:15.14.20:17,21:12:15:20.18.17,21:18:15:22.13.12,19:18:15:22.13.12| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 54: 5737-46 (2011)
Article DOI: 10.1021/jm200383j
BindingDB Entry DOI: 10.7270/Q2G73F37 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50194745
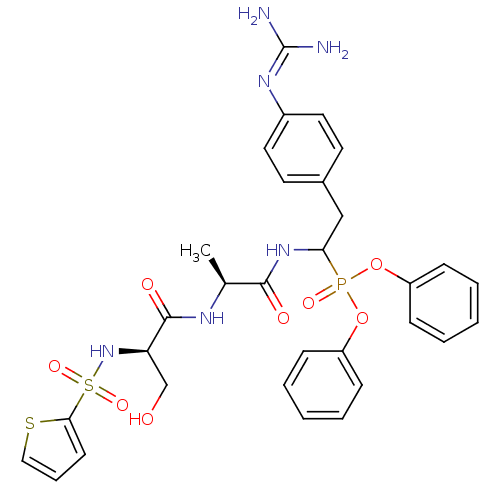
(CHEMBL385900 | diphenyl 1-[(N-2-thiophenesulfonyl-...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)c1cccs1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C31H35N6O8PS2/c1-21(34-30(40)26(20-38)37-48(42,43)28-13-8-18-47-28)29(39)36-27(19-22-14-16-23(17-15-22)35-31(32)33)46(41,44-24-9-4-2-5-10-24)45-25-11-6-3-7-12-25/h2-18,21,26-27,37-38H,19-20H2,1H3,(H,34,40)(H,36,39)(H4,32,33,35)/t21-,26+,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 49: 5785-93 (2006)
Article DOI: 10.1021/jm060622g
BindingDB Entry DOI: 10.7270/Q2SF2VT3 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 purified from human seminal plasma using Gly-Pro-p-nitroanilide as substrate by spectrophotometry |
J Med Chem 57: 3053-74 (2014)
Article DOI: 10.1021/jm500031w
BindingDB Entry DOI: 10.7270/Q2P84DFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50194741
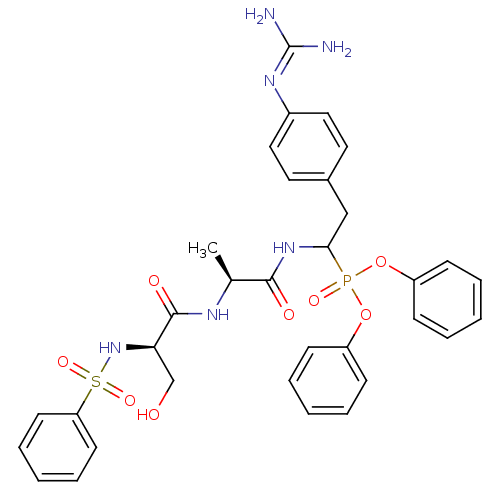
(CHEMBL385158 | diphenyl 1-[(N-benzenesulfonyl-D-se...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C33H37N6O8PS/c1-23(36-32(42)29(22-40)39-49(44,45)28-15-9-4-10-16-28)31(41)38-30(21-24-17-19-25(20-18-24)37-33(34)35)48(43,46-26-11-5-2-6-12-26)47-27-13-7-3-8-14-27/h2-20,23,29-30,39-40H,21-22H2,1H3,(H,36,42)(H,38,41)(H4,34,35,37)/t23-,29+,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 49: 5785-93 (2006)
Article DOI: 10.1021/jm060622g
BindingDB Entry DOI: 10.7270/Q2SF2VT3 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50399718

(CHEMBL2178958)Show SMILES [N-]=[N+]=N[C@H]1C[C@H](N(C1)C(=O)CCCc1ccccc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H24N6O2/c21-13-17-9-5-11-25(17)20(28)18-12-16(23-24-22)14-26(18)19(27)10-4-8-15-6-2-1-3-7-15/h1-3,6-7,16-18H,4-5,8-12,14H2/t16-,17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of pig PREP expressed in Escherichia coli using Z-Gly-Pro-p-nitroanilide substrate |
J Med Chem 55: 9856-67 (2012)
Article DOI: 10.1021/jm301060g
BindingDB Entry DOI: 10.7270/Q2SN0B3S |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228418
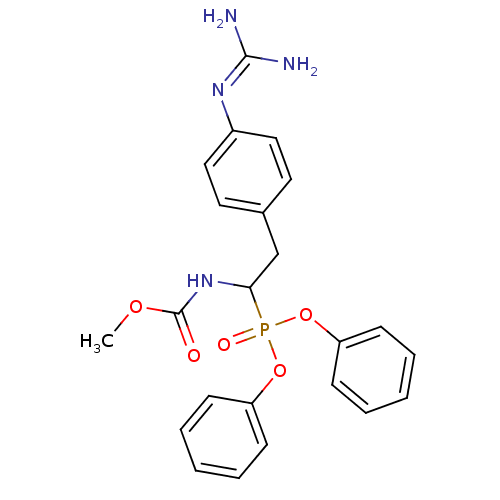
(CHEMBL393591 | methyl 1-(diphenoxyphosphoryl)-2-(4...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C23H25N4O5P/c1-30-23(28)27-21(16-17-12-14-18(15-13-17)26-22(24)25)33(29,31-19-8-4-2-5-9-19)32-20-10-6-3-7-11-20/h2-15,21H,16H2,1H3,(H,27,28)(H4,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Mus musculus (Mouse)) | BDBM50009366

(CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1ccnc2ccccc12 |r| Show InChI InChI=1S/C17H14F2N4O2/c18-17(19)7-11(8-20)23(10-17)15(24)9-22-16(25)13-5-6-21-14-4-2-1-3-12(13)14/h1-6,11H,7,9-10H2,(H,22,25)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center
US Patent
| Assay Description
Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... |
US Patent US9346814 (2016)
BindingDB Entry DOI: 10.7270/Q2W37V6D |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Mus musculus (Mouse)) | BDBM50009366

(CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1ccnc2ccccc12 |r| Show InChI InChI=1S/C17H14F2N4O2/c18-17(19)7-11(8-20)23(10-17)15(24)9-22-16(25)13-5-6-21-14-4-2-1-3-12(13)14/h1-6,11H,7,9-10H2,(H,22,25)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry |
J Med Chem 57: 3053-74 (2014)
Article DOI: 10.1021/jm500031w
BindingDB Entry DOI: 10.7270/Q2P84DFW |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Mus musculus (Mouse)) | BDBM50009364
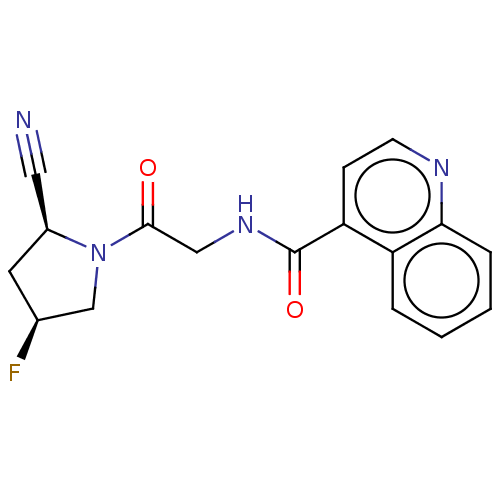
(CHEMBL3233840)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1ccnc2ccccc12 |r| Show InChI InChI=1S/C17H15FN4O2/c18-11-7-12(8-19)22(10-11)16(23)9-21-17(24)14-5-6-20-15-4-2-1-3-13(14)15/h1-6,11-12H,7,9-10H2,(H,21,24)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry |
J Med Chem 57: 3053-74 (2014)
Article DOI: 10.1021/jm500031w
BindingDB Entry DOI: 10.7270/Q2P84DFW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228412

(CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...)Show SMILES [#6]-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1)[#8]-c1ccc(-[#7]-[#6](-[#6])=O)cc1 Show InChI InChI=1S/C27H31N6O7P/c1-17(34)30-20-8-12-23(13-9-20)39-41(37,40-24-14-10-21(11-15-24)31-18(2)35)25(33-27(36)38-3)16-19-4-6-22(7-5-19)32-26(28)29/h4-15,25H,16H2,1-3H3,(H,30,34)(H,31,35)(H,33,36)(H4,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50194743
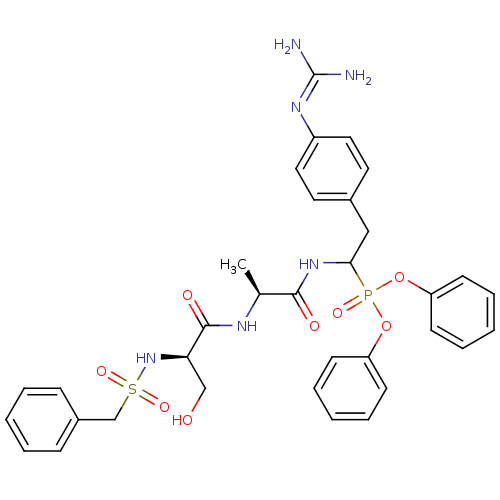
(CHEMBL214814 | diphenyl 1-[(N-alpha-toluenesulfony...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C34H39N6O8PS/c1-24(37-33(43)30(22-41)40-50(45,46)23-26-11-5-2-6-12-26)32(42)39-31(21-25-17-19-27(20-18-25)38-34(35)36)49(44,47-28-13-7-3-8-14-28)48-29-15-9-4-10-16-29/h2-20,24,30-31,40-41H,21-23H2,1H3,(H,37,43)(H,39,42)(H4,35,36,38)/t24-,30+,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 49: 5785-93 (2006)
Article DOI: 10.1021/jm060622g
BindingDB Entry DOI: 10.7270/Q2SF2VT3 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228425

(CHEMBL239118 | di-(4-acetamidophenyl) 1-(methylsul...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]S([#6])(=O)=O)cc1 Show InChI InChI=1S/C26H31N6O7PS/c1-17(33)29-20-8-12-23(13-9-20)38-40(35,39-24-14-10-21(11-15-24)30-18(2)34)25(32-41(3,36)37)16-19-4-6-22(7-5-19)31-26(27)28/h4-15,25,32H,16H2,1-3H3,(H,29,33)(H,30,34)(H4,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Mus musculus (Mouse)) | BDBM50009370

(CHEMBL3233847)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC(=O)c1ccnc2ccccc12 |r| Show InChI InChI=1S/C16H18BN3O4/c21-15(20-9-3-6-14(20)17(23)24)10-19-16(22)12-7-8-18-13-5-2-1-4-11(12)13/h1-2,4-5,7-8,14,23-24H,3,6,9-10H2,(H,19,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry |
J Med Chem 57: 3053-74 (2014)
Article DOI: 10.1021/jm500031w
BindingDB Entry DOI: 10.7270/Q2P84DFW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50194743

(CHEMBL214814 | diphenyl 1-[(N-alpha-toluenesulfony...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C34H39N6O8PS/c1-24(37-33(43)30(22-41)40-50(45,46)23-26-11-5-2-6-12-26)32(42)39-31(21-25-17-19-27(20-18-25)38-34(35)36)49(44,47-28-13-7-3-8-14-28)48-29-15-9-4-10-16-29/h2-20,24,30-31,40-41H,21-23H2,1H3,(H,37,43)(H,39,42)(H4,35,36,38)/t24-,30+,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228420
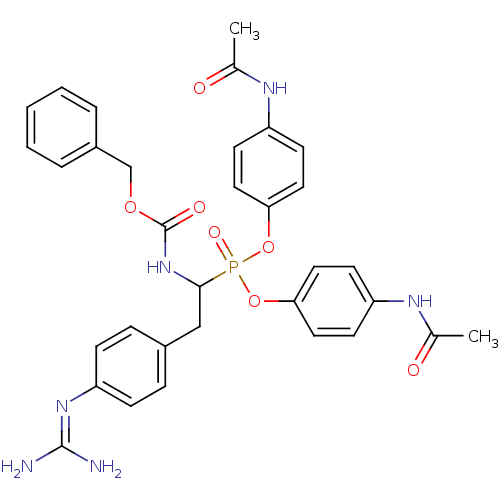
(CHEMBL391968 | di-(4-acetamidophenyl) 1-(benzyloxy...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#7]-[#6](-[#6])=O)cc2)[#6](-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C33H35N6O7P/c1-22(40)36-26-12-16-29(17-13-26)45-47(43,46-30-18-14-27(15-19-30)37-23(2)41)31(20-24-8-10-28(11-9-24)38-32(34)35)39-33(42)44-21-25-6-4-3-5-7-25/h3-19,31H,20-21H2,1-2H3,(H,36,40)(H,37,41)(H,39,42)(H4,34,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data