 Found 1282 hits with Last Name = 'kay' and Initial = 'j'
Found 1282 hits with Last Name = 'kay' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-3
(Homo sapiens (Human)) | BDBM50160957
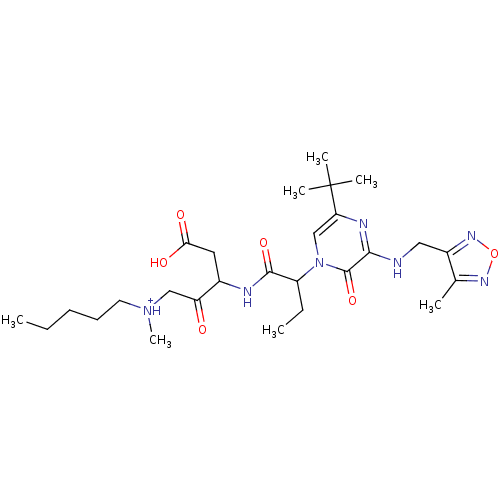
(CHEMBL179503 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...)Show SMILES CCCCC[NH+](C)CC(=O)C(CC(O)=O)NC(=O)C(CC)n1cc(nc(NCc2nonc2C)c1=O)C(C)(C)C Show InChI InChI=1S/C27H43N7O6/c1-8-10-11-12-33(7)15-21(35)18(13-23(36)37)29-25(38)20(9-2)34-16-22(27(4,5)6)30-24(26(34)39)28-14-19-17(3)31-40-32-19/h16,18,20H,8-15H2,1-7H3,(H,28,30)(H,29,38)(H,36,37)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human caspase-3 in neuronal precursor (NT2) cells |
Bioorg Med Chem Lett 15: 1173-80 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.006
BindingDB Entry DOI: 10.7270/Q2D50MGS |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50160974
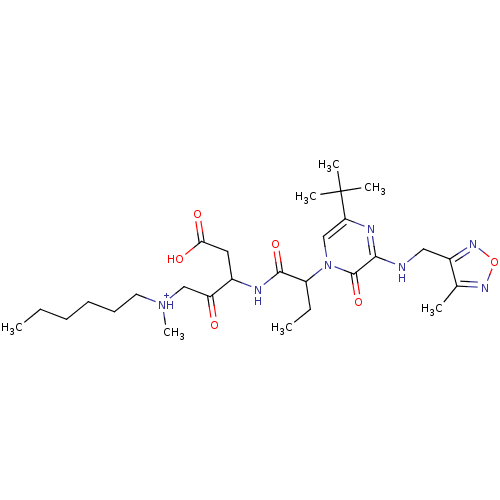
(CHEMBL366927 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...)Show SMILES CCCCCC[NH+](C)CC(=O)C(CC(O)=O)NC(=O)C(CC)n1cc(nc(NCc2nonc2C)c1=O)C(C)(C)C Show InChI InChI=1S/C28H45N7O6/c1-8-10-11-12-13-34(7)16-22(36)19(14-24(37)38)30-26(39)21(9-2)35-17-23(28(4,5)6)31-25(27(35)40)29-15-20-18(3)32-41-33-20/h17,19,21H,8-16H2,1-7H3,(H,29,31)(H,30,39)(H,37,38)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human caspase-3 in neuronal precursor (NT2) cells |
Bioorg Med Chem Lett 15: 1173-80 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.006
BindingDB Entry DOI: 10.7270/Q2D50MGS |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay |
Bioorg Med Chem Lett 29: 2565-2570 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.006
BindingDB Entry DOI: 10.7270/Q2JM2F1C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334454

(CHEMBL1643895 | Ramosetron | US9045501, Ramosetron)Show SMILES Cn1cc(C(=O)[C@@H]2CCc3nc[nH]c3C2)c2ccccc12 |r| Show InChI InChI=1S/C17H17N3O/c1-20-9-13(12-4-2-3-5-16(12)20)17(21)11-6-7-14-15(8-11)19-10-18-14/h2-5,9-11H,6-8H2,1H3,(H,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50045284
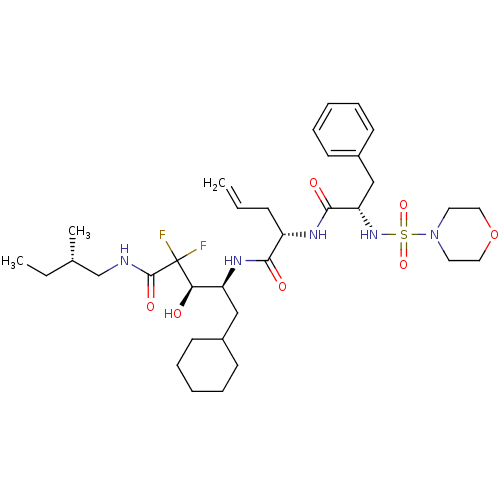
(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES CC[C@H](C)CNC(=O)C(F)(F)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C34H53F2N5O7S/c1-4-12-27(38-32(44)29(22-26-15-10-7-11-16-26)40-49(46,47)41-17-19-48-20-18-41)31(43)39-28(21-25-13-8-6-9-14-25)30(42)34(35,36)33(45)37-23-24(3)5-2/h4,7,10-11,15-16,24-25,27-30,40,42H,1,5-6,8-9,12-14,17-23H2,2-3H3,(H,37,45)(H,38,44)(H,39,43)/t24-,27-,28-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin E |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50045281

(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES CC(C)[C@@H](O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C32H52N4O7S/c1-4-11-26(31(39)34-27(20-24-12-7-5-8-13-24)30(38)22-29(37)23(2)3)33-32(40)28(21-25-14-9-6-10-15-25)35-44(41,42)36-16-18-43-19-17-36/h4,6,9-10,14-15,23-24,26-30,35,37-38H,1,5,7-8,11-13,16-22H2,2-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498806
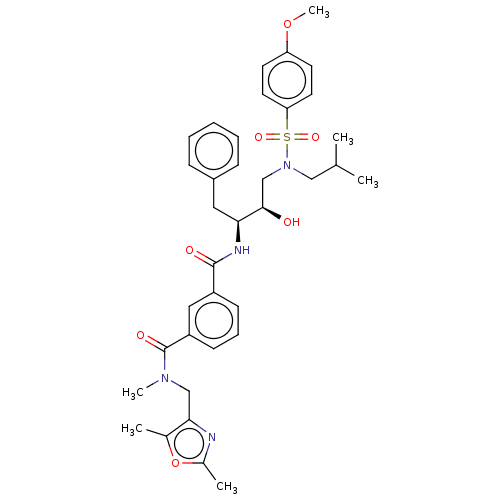
(CHEMBL3627879)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)C(=O)N(C)Cc1nc(C)oc1C |r| Show InChI InChI=1S/C36H44N4O7S/c1-24(2)21-40(48(44,45)31-17-15-30(46-6)16-18-31)23-34(41)32(19-27-11-8-7-9-12-27)38-35(42)28-13-10-14-29(20-28)36(43)39(5)22-33-25(3)47-26(4)37-33/h7-18,20,24,32,34,41H,19,21-23H2,1-6H3,(H,38,42)/t32-,34+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014549
(CHEMBL3261480 | US9045501, 4)Show SMILES Cc1cn2CCN([C@@H]3CN4CCC3CC4)C(=O)c3cc(Cl)cc1c23 |r,wD:7.6,(8.92,-16.19,;8.45,-14.73,;9.36,-13.48,;8.42,-12.13,;8.87,-10.66,;7.99,-9.38,;6.46,-9.26,;5.89,-7.83,;4.36,-7.61,;3.8,-6.17,;4.77,-4.97,;6.29,-5.2,;6.84,-6.63,;5.89,-5.6,;5.13,-6.93,;5.4,-10.41,;3.93,-9.96,;5.64,-11.94,;4.31,-12.71,;4.31,-14.25,;2.98,-15.02,;5.64,-15.02,;6.97,-14.25,;6.98,-12.7,)| Show InChI InChI=1S/C19H22ClN3O/c1-12-10-22-6-7-23(17-11-21-4-2-13(17)3-5-21)19(24)16-9-14(20)8-15(12)18(16)22/h8-10,13,17H,2-7,11H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM171372
(US9085571, Table 1, Compound 20)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc(C)cc(c1)C(=O)N(C)Cc1nc(C)oc1C |r| Show InChI InChI=1S/C37H46N4O7S/c1-24(2)21-41(49(45,46)32-15-13-31(47-7)14-16-32)23-35(42)33(19-28-11-9-8-10-12-28)39-36(43)29-17-25(3)18-30(20-29)37(44)40(6)22-34-26(4)48-27(5)38-34/h8-18,20,24,33,35,42H,19,21-23H2,1-7H3,(H,39,43)/t33-,35+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498814
(CHEMBL3627876)Show SMILES COC[C@H]1C[C@@H](O)CN1C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C35H45N3O8S/c1-24(2)20-37(47(43,44)31-15-13-30(46-4)14-16-31)22-33(40)32(17-25-9-6-5-7-10-25)36-34(41)26-11-8-12-27(18-26)35(42)38-21-29(39)19-28(38)23-45-3/h5-16,18,24,28-29,32-33,39-40H,17,19-23H2,1-4H3,(H,36,41)/t28-,29-,32+,33-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498808
(CHEMBL3627874)Show SMILES COC[C@H]1C[C@H](O)CN1C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C35H45N3O8S/c1-24(2)20-37(47(43,44)31-15-13-30(46-4)14-16-31)22-33(40)32(17-25-9-6-5-7-10-25)36-34(41)26-11-8-12-27(18-26)35(42)38-21-29(39)19-28(38)23-45-3/h5-16,18,24,28-29,32-33,39-40H,17,19-23H2,1-4H3,(H,36,41)/t28-,29+,32+,33-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM171370
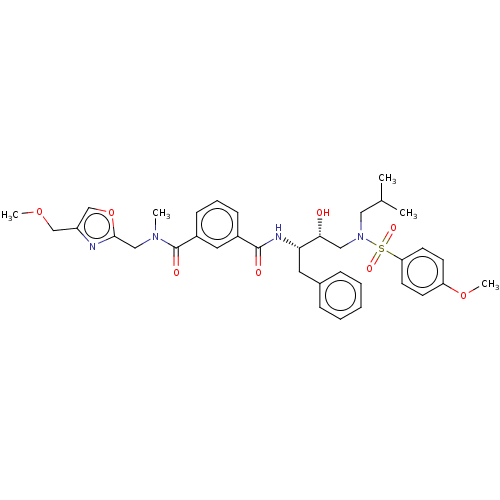
(US9085571, Table 1, Compound 18)Show SMILES COCc1coc(CN(C)C(=O)c2cccc(c2)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)CN(CC(C)C)S(=O)(=O)c2ccc(OC)cc2)n1 |r| Show InChI InChI=1S/C36H44N4O8S/c1-25(2)20-40(49(44,45)31-16-14-30(47-5)15-17-31)21-33(41)32(18-26-10-7-6-8-11-26)38-35(42)27-12-9-13-28(19-27)36(43)39(3)22-34-37-29(23-46-4)24-48-34/h6-17,19,24-25,32-33,41H,18,20-23H2,1-5H3,(H,38,42)/t32-,33+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50045290

(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES CC[C@H](C)CNC(=O)C(F)(F)C(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C34H51F2N5O7S/c1-4-12-27(38-32(44)29(22-26-15-10-7-11-16-26)40-49(46,47)41-17-19-48-20-18-41)31(43)39-28(21-25-13-8-6-9-14-25)30(42)34(35,36)33(45)37-23-24(3)5-2/h4,7,10-11,15-16,24-25,27-29,40H,1,5-6,8-9,12-14,17-23H2,2-3H3,(H,37,45)(H,38,44)(H,39,43)/t24-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498812
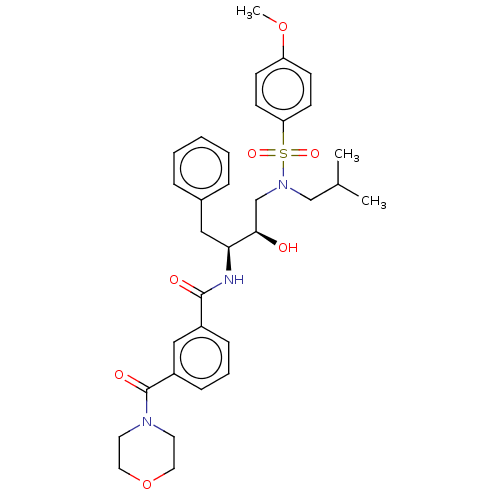
(CHEMBL3627855)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C33H41N3O7S/c1-24(2)22-36(44(40,41)29-14-12-28(42-3)13-15-29)23-31(37)30(20-25-8-5-4-6-9-25)34-32(38)26-10-7-11-27(21-26)33(39)35-16-18-43-19-17-35/h4-15,21,24,30-31,37H,16-20,22-23H2,1-3H3,(H,34,38)/t30-,31+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014558
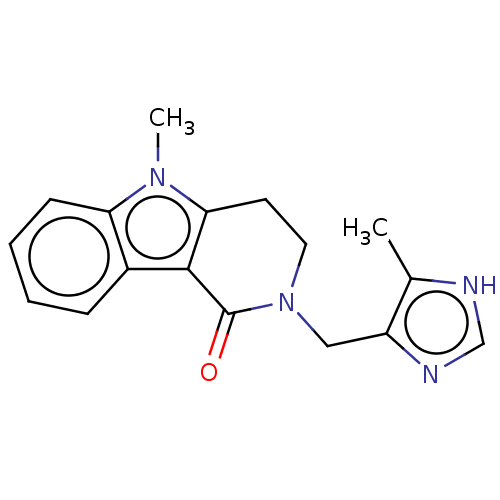
(ALOSETRON | CHEBI:253342 | Lotronex | US9045501, A...)Show InChI InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014552
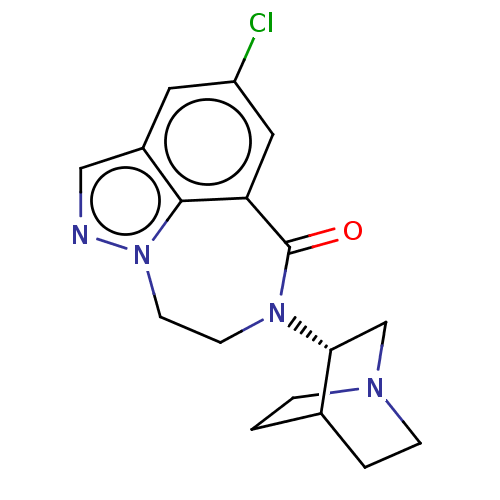
(CHEMBL3261483 | US9045501, 8)Show SMILES Clc1cc2C(=O)N(CCn3ncc(c1)c23)[C@@H]1CN2CCC1CC2 |r,wD:15.17,(22.97,-13.82,;24.31,-13.05,;24.31,-11.51,;25.64,-10.74,;25.4,-9.21,;23.93,-8.77,;26.45,-8.07,;27.99,-8.19,;28.86,-9.46,;28.42,-10.93,;29.36,-12.28,;28.45,-13.53,;26.97,-13.05,;25.64,-13.82,;26.98,-11.51,;25.89,-6.64,;24.36,-6.41,;23.8,-4.97,;24.76,-3.77,;26.29,-4,;26.84,-5.43,;25.89,-4.4,;25.13,-5.74,)| Show InChI InChI=1S/C17H19ClN4O/c18-13-7-12-9-19-22-6-5-21(17(23)14(8-13)16(12)22)15-10-20-3-1-11(15)2-4-20/h7-9,11,15H,1-6,10H2/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50008139
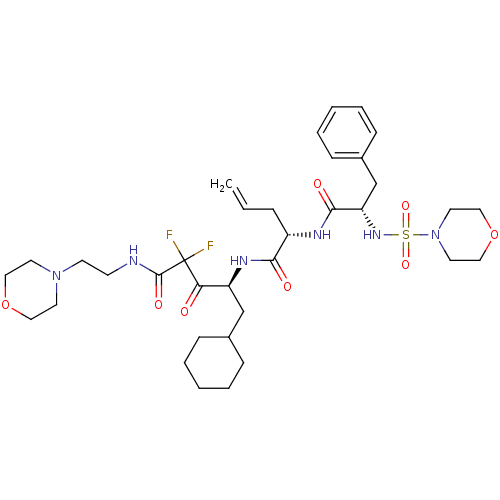
(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES FC(F)(C(=O)NCCN1CCOCC1)C(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C35H52F2N6O8S/c1-2-9-28(39-33(46)30(25-27-12-7-4-8-13-27)41-52(48,49)43-18-22-51-23-19-43)32(45)40-29(24-26-10-5-3-6-11-26)31(44)35(36,37)34(47)38-14-15-42-16-20-50-21-17-42/h2,4,7-8,12-13,26,28-30,41H,1,3,5-6,9-11,14-25H2,(H,38,47)(H,39,46)(H,40,45)/t28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin E |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM93624
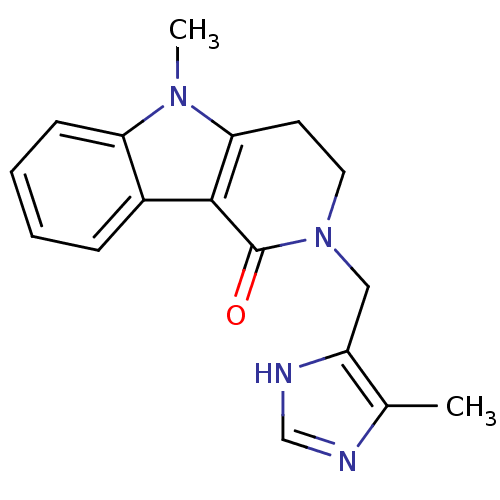
(5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4...)Show InChI InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin E
(Homo sapiens (Human)) | BDBM50045290

(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES CC[C@H](C)CNC(=O)C(F)(F)C(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C34H51F2N5O7S/c1-4-12-27(38-32(44)29(22-26-15-10-7-11-16-26)40-49(46,47)41-17-19-48-20-18-41)31(43)39-28(21-25-13-8-6-9-14-25)30(42)34(35,36)33(45)37-23-24(3)5-2/h4,7,10-11,15-16,24-25,27-29,40H,1,5-6,8-9,12-14,17-23H2,2-3H3,(H,37,45)(H,38,44)(H,39,43)/t24-,27-,28-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin E |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50522184

(CHEMBL4444017)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)Nc1c(N(C)[C@@H]2CCOC2)c(=O)c1=O |r| Show InChI InChI=1S/C30H39N3O7S/c1-20(2)17-33(41(37,38)24-12-10-23(39-4)11-13-24)18-26(34)25(16-21-8-6-5-7-9-21)31-27-28(30(36)29(27)35)32(3)22-14-15-40-19-22/h5-13,20,22,25-26,31,34H,14-19H2,1-4H3/t22-,25+,26-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay |
Bioorg Med Chem Lett 29: 2565-2570 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.006
BindingDB Entry DOI: 10.7270/Q2JM2F1C |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498807
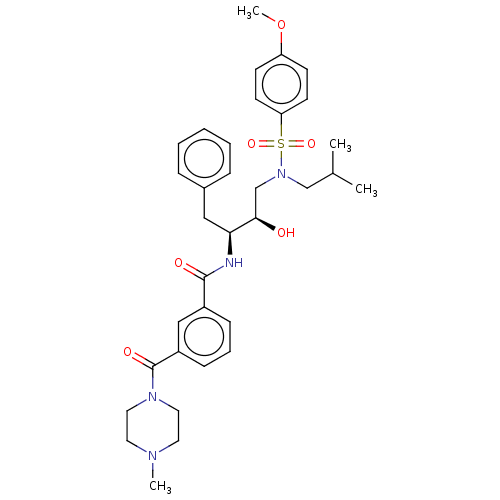
(CHEMBL3627856)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)C(=O)N1CCN(C)CC1 |r| Show InChI InChI=1S/C34H44N4O6S/c1-25(2)23-38(45(42,43)30-15-13-29(44-4)14-16-30)24-32(39)31(21-26-9-6-5-7-10-26)35-33(40)27-11-8-12-28(22-27)34(41)37-19-17-36(3)18-20-37/h5-16,22,25,31-32,39H,17-21,23-24H2,1-4H3,(H,35,40)/t31-,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50045285
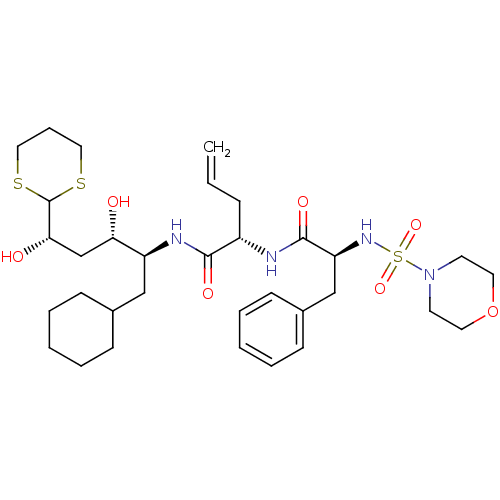
(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES O[C@@H](C[C@H](O)C1SCCCS1)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N4O7S3/c1-2-10-26(34-32(41)28(22-25-13-7-4-8-14-25)36-47(42,43)37-15-17-44-18-16-37)31(40)35-27(21-24-11-5-3-6-12-24)29(38)23-30(39)33-45-19-9-20-46-33/h2,4,7-8,13-14,24,26-30,33,36,38-39H,1,3,5-6,9-12,15-23H2,(H,34,41)(H,35,40)/t26-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM171371
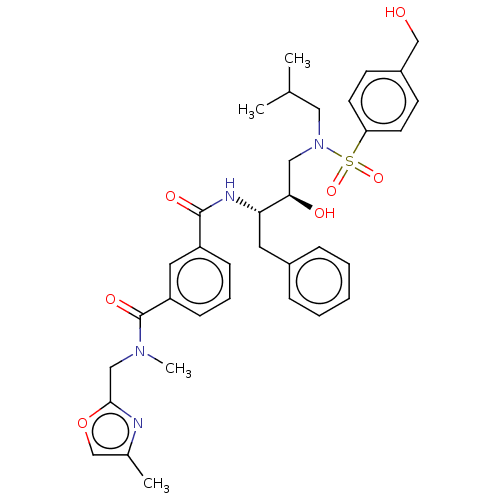
(US9085571, Table 1, Compound 19)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)C(=O)N(C)Cc1nc(C)co1)S(=O)(=O)c1ccc(CO)cc1 |r| Show InChI InChI=1S/C35H42N4O7S/c1-24(2)19-39(47(44,45)30-15-13-27(22-40)14-16-30)20-32(41)31(17-26-9-6-5-7-10-26)37-34(42)28-11-8-12-29(18-28)35(43)38(4)21-33-36-25(3)23-46-33/h5-16,18,23-24,31-32,40-41H,17,19-22H2,1-4H3,(H,37,42)/t31-,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334442
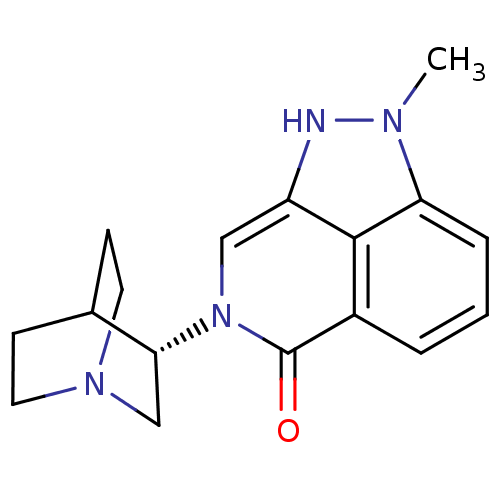
((S)-2-methyl-7-(quinuclidin-3-yl)-7,8-dihydropyraz...)Show SMILES Cn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:6.5,(21.53,-4.9,;20.98,-3.46,;22.01,-1.72,;20.85,-.68,;20.86,.86,;19.52,1.63,;19.51,3.17,;18.18,3.93,;18.18,5.47,;19.52,6.24,;20.85,5.47,;20.85,3.94,;19.34,4.44,;19.66,5.08,;18.19,.86,;16.85,1.65,;18.19,-.68,;16.86,-1.45,;16.85,-2.99,;18.19,-3.76,;19.52,-2.99,;19.52,-1.45,)| Show InChI InChI=1S/C17H20N4O/c1-19-14-4-2-3-12-16(14)13(18-19)9-21(17(12)22)15-10-20-7-5-11(15)6-8-20/h2-4,9,11,15,18H,5-8,10H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334453

((S)-5-methyl-2-(quinuclidin-3-yl)-2,3-dihydropyrro...)Show SMILES Cn1cc2CN([C@@H]3CN4CCC3CC4)C(=O)c3cccc1c23 |r,wD:6.5,(4.39,-34,;3.85,-32.56,;4.88,-30.82,;3.72,-29.78,;3.73,-28.24,;2.39,-27.47,;2.38,-25.93,;1.05,-25.17,;1.05,-23.63,;2.39,-22.86,;3.72,-23.63,;3.72,-25.16,;2.21,-24.66,;2.53,-24.02,;1.06,-28.24,;-.28,-27.45,;1.06,-29.78,;-.27,-30.55,;-.28,-32.09,;1.06,-32.86,;2.39,-32.09,;2.39,-30.55,)| Show InChI InChI=1S/C18H21N3O/c1-19-9-13-10-21(16-11-20-7-5-12(16)6-8-20)18(22)14-3-2-4-15(19)17(13)14/h2-4,9,12,16H,5-8,10-11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50045291
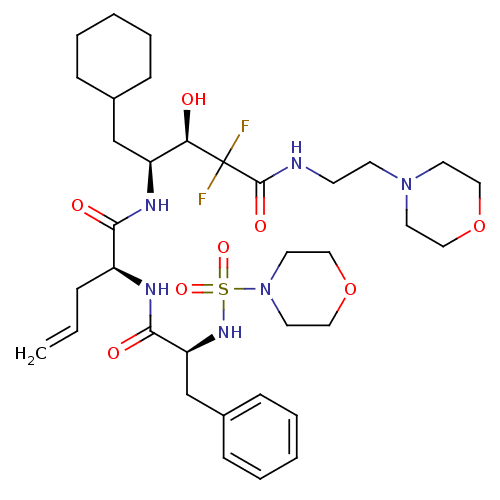
(CHEMBL88340 | i2-[2-(Morpholine-4-sulfonylamino)-3...)Show SMILES O[C@H]([C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(F)(F)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C35H54F2N6O8S/c1-2-9-28(39-33(46)30(25-27-12-7-4-8-13-27)41-52(48,49)43-18-22-51-23-19-43)32(45)40-29(24-26-10-5-3-6-11-26)31(44)35(36,37)34(47)38-14-15-42-16-20-50-21-17-42/h2,4,7-8,12-13,26,28-31,41,44H,1,3,5-6,9-11,14-25H2,(H,38,47)(H,39,46)(H,40,45)/t28-,29-,30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014553

(CHEMBL3261484)Show SMILES Cc1cc2C(=O)N(CCn3ncc(c1)c23)[C@@H]1CN2CCC1CC2 |r,wD:15.17,(32.02,-14.44,;33.35,-13.67,;33.36,-12.13,;34.68,-11.36,;34.44,-9.83,;32.97,-9.38,;35.5,-8.68,;37.04,-8.8,;37.91,-10.07,;37.47,-11.55,;38.4,-12.89,;37.49,-14.15,;36.02,-13.67,;34.69,-14.44,;36.02,-12.12,;34.94,-7.25,;33.41,-7.03,;32.85,-5.59,;33.81,-4.38,;35.33,-4.62,;35.89,-6.05,;34.94,-5.01,;34.17,-6.35,)| Show InChI InChI=1S/C18H22N4O/c1-12-8-14-10-19-22-7-6-21(18(23)15(9-12)17(14)22)16-11-20-4-2-13(16)3-5-20/h8-10,13,16H,2-7,11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
Gastricsin
(Homo sapiens (Human)) | BDBM50008139

(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES FC(F)(C(=O)NCCN1CCOCC1)C(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C35H52F2N6O8S/c1-2-9-28(39-33(46)30(25-27-12-7-4-8-13-27)41-52(48,49)43-18-22-51-23-19-43)32(45)40-29(24-26-10-5-3-6-11-26)31(44)35(36,37)34(47)38-14-15-42-16-20-50-21-17-42/h2,4,7-8,12-13,26,28-30,41H,1,3,5-6,9-11,14-25H2,(H,38,47)(H,39,46)(H,40,45)/t28-,29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human gastricsin |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334452
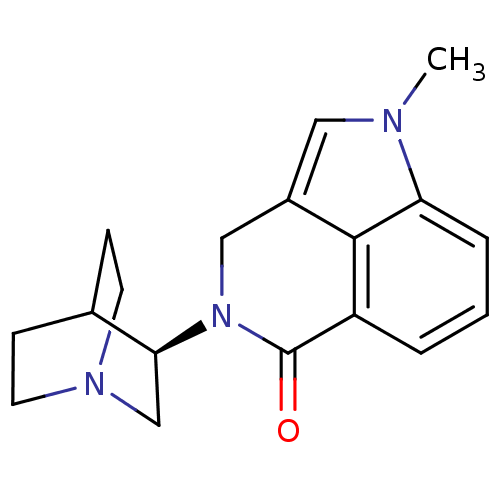
((R)-5-methyl-2-(quinuclidin-3-yl)-2,3-dihydropyrro...)Show SMILES Cn1cc2CN([C@H]3CN4CCC3CC4)C(=O)c3cccc1c23 |r,wU:6.5,(-3.65,-33.57,;-4.2,-32.13,;-3.17,-30.38,;-4.32,-29.35,;-4.32,-27.8,;-5.66,-27.04,;-5.66,-25.5,;-7,-24.74,;-6.99,-23.19,;-5.66,-22.42,;-4.33,-23.2,;-4.33,-24.73,;-5.83,-24.22,;-5.51,-23.59,;-6.98,-27.8,;-8.33,-27.02,;-6.99,-29.35,;-8.32,-30.12,;-8.32,-31.66,;-6.98,-32.43,;-5.66,-31.66,;-5.65,-30.12,)| Show InChI InChI=1S/C18H21N3O/c1-19-9-13-10-21(16-11-20-7-5-12(16)6-8-20)18(22)14-3-2-4-15(19)17(13)14/h2-4,9,12,16H,5-8,10-11H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498813
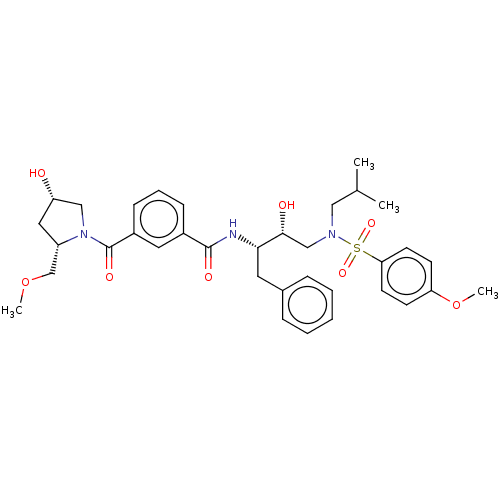
(CHEMBL3627875)Show SMILES COC[C@@H]1C[C@H](O)CN1C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C35H45N3O8S/c1-24(2)20-37(47(43,44)31-15-13-30(46-4)14-16-31)22-33(40)32(17-25-9-6-5-7-10-25)36-34(41)26-11-8-12-27(18-26)35(42)38-21-29(39)19-28(38)23-45-3/h5-16,18,24,28-29,32-33,39-40H,17,19-23H2,1-4H3,(H,36,41)/t28-,29-,32-,33+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334445
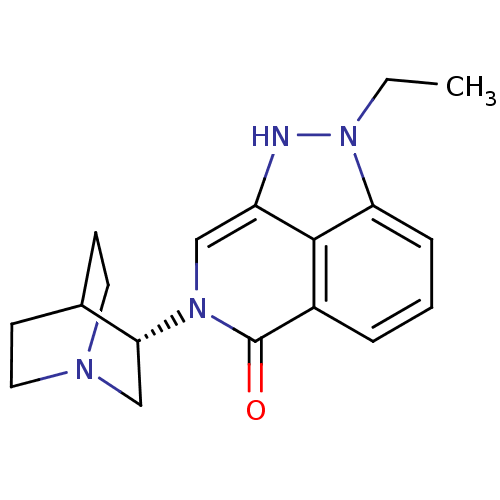
((S)-2-ethyl-7-(quinuclidin-3-yl)-7,8-dihydropyrazo...)Show SMILES CCn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:7.6,(47.4,-4.88,;48.37,-3.68,;47.82,-2.25,;48.85,-.5,;47.7,.54,;47.7,2.08,;46.36,2.85,;46.36,4.39,;45.02,5.15,;45.03,6.69,;46.36,7.46,;47.69,6.69,;47.69,5.16,;46.19,5.66,;46.5,6.3,;45.04,2.08,;43.69,2.87,;45.03,.54,;43.7,-.23,;43.7,-1.78,;45.04,-2.55,;46.36,-1.78,;46.36,-.23,)| Show InChI InChI=1S/C18H22N4O/c1-2-22-15-5-3-4-13-17(15)14(19-22)10-21(18(13)23)16-11-20-8-6-12(16)7-9-20/h3-5,10,12,16,19H,2,6-9,11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334439
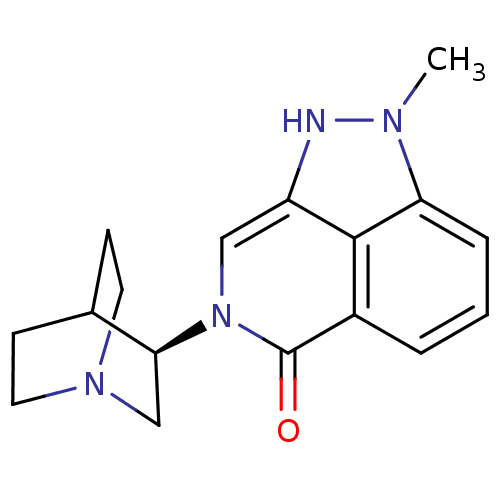
((R)-2-methyl-7-(quinuclidin-3-yl)-7,8-dihydropyraz...)Show SMILES Cn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:6.5,(12.29,-5.2,;11.75,-3.77,;12.77,-2.02,;11.62,-.98,;11.62,.56,;10.28,1.33,;10.28,2.87,;8.95,3.63,;8.95,5.17,;10.28,5.94,;11.61,5.17,;11.61,3.64,;10.11,4.14,;10.43,4.78,;8.96,.56,;7.61,1.35,;8.96,-.99,;7.62,-1.76,;7.62,-3.3,;8.96,-4.07,;10.29,-3.3,;10.29,-1.75,)| Show InChI InChI=1S/C17H20N4O/c1-19-14-4-2-3-12-16(14)13(18-19)9-21(17(12)22)15-10-20-7-5-11(15)6-8-20/h2-4,9,11,15,18H,5-8,10H2,1H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50045281

(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES CC(C)[C@@H](O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C32H52N4O7S/c1-4-11-26(31(39)34-27(20-24-12-7-5-8-13-24)30(38)22-29(37)23(2)3)33-32(40)28(21-25-14-9-6-10-15-25)35-44(41,42)36-16-18-43-19-17-36/h4,6,9-10,14-15,23-24,26-30,35,37-38H,1,5,7-8,11-13,16-22H2,2-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin E |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334444
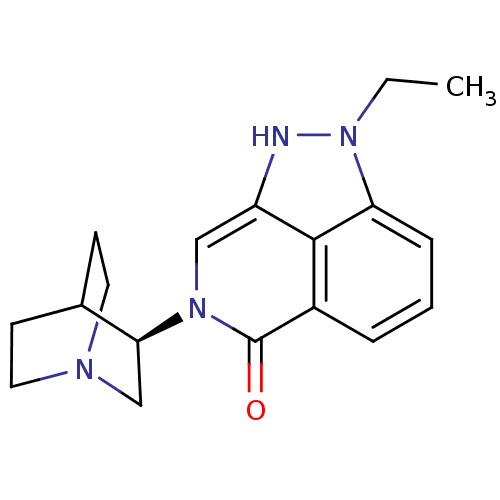
((R)-2-ethyl-7-(quinuclidin-3-yl)-7,8-dihydropyrazo...)Show SMILES CCn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:7.6,(39.2,-5.65,;40.17,-4.46,;39.63,-3.02,;40.65,-1.27,;39.5,-.24,;39.5,1.31,;38.16,2.07,;38.16,3.61,;36.83,4.37,;36.83,5.92,;38.16,6.69,;39.49,5.91,;39.49,4.38,;37.99,4.89,;38.31,5.52,;36.84,1.31,;35.49,2.09,;36.84,-.24,;35.5,-1.01,;35.5,-2.55,;36.84,-3.32,;38.16,-2.55,;38.17,-1.01,)| Show InChI InChI=1S/C18H22N4O/c1-2-22-15-5-3-4-13-17(15)14(19-22)10-21(18(13)23)16-11-20-8-6-12(16)7-9-20/h3-5,10,12,16,19H,2,6-9,11H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(HAMSTER) | BDBM82262
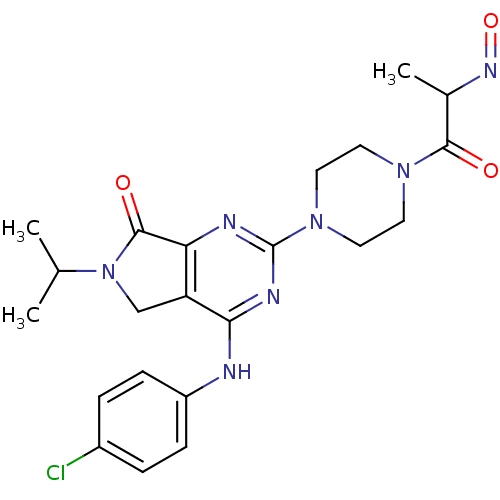
(ZM253,270)Show SMILES CC(C)N1Cc2c(nc(nc2Nc2ccc(Cl)cc2)N2CCN(CC2)C(=O)C(C)N=O)C1=O Show InChI InChI=1S/C22H26ClN7O3/c1-13(2)30-12-17-18(21(30)32)25-22(26-19(17)24-16-6-4-15(23)5-7-16)29-10-8-28(9-11-29)20(31)14(3)27-33/h4-7,13-14H,8-12H2,1-3H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1216-21 (1995)
BindingDB Entry DOI: 10.7270/Q2VQ316V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334443

((R)-4-fluoro-2-methyl-7-(quinuclidin-3-yl)-7,8-dih...)Show SMILES Cn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cc(F)cc1c23 |r,wU:6.5,(31.37,-5.57,;30.82,-4.13,;31.85,-2.38,;30.7,-1.35,;30.7,.2,;29.36,.96,;29.36,2.5,;28.02,3.26,;28.03,4.81,;29.36,5.58,;30.69,4.8,;30.69,3.27,;29.19,3.77,;29.51,4.41,;28.04,.2,;26.69,.98,;28.03,-1.35,;26.7,-2.12,;26.7,-3.66,;25.37,-4.43,;28.04,-4.43,;29.36,-3.66,;29.37,-2.12,)| Show InChI InChI=1S/C17H19FN4O/c1-20-14-7-11(18)6-12-16(14)13(19-20)8-22(17(12)23)15-9-21-4-2-10(15)3-5-21/h6-8,10,15,19H,2-5,9H2,1H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50045285

(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES O[C@@H](C[C@H](O)C1SCCCS1)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N4O7S3/c1-2-10-26(34-32(41)28(22-25-13-7-4-8-14-25)36-47(42,43)37-15-17-44-18-16-37)31(40)35-27(21-24-11-5-3-6-12-24)29(38)23-30(39)33-45-19-9-20-46-33/h2,4,7-8,13-14,24,26-30,33,36,38-39H,1,3,5-6,9-12,15-23H2,(H,34,41)(H,35,40)/t26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin E |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(HAMSTER) | BDBM82262

(ZM253,270)Show SMILES CC(C)N1Cc2c(nc(nc2Nc2ccc(Cl)cc2)N2CCN(CC2)C(=O)C(C)N=O)C1=O Show InChI InChI=1S/C22H26ClN7O3/c1-13(2)30-12-17-18(21(30)32)25-22(26-19(17)24-16-6-4-15(23)5-7-16)29-10-8-28(9-11-29)20(31)14(3)27-33/h4-7,13-14H,8-12H2,1-3H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1216-21 (1995)
BindingDB Entry DOI: 10.7270/Q2VQ316V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334447
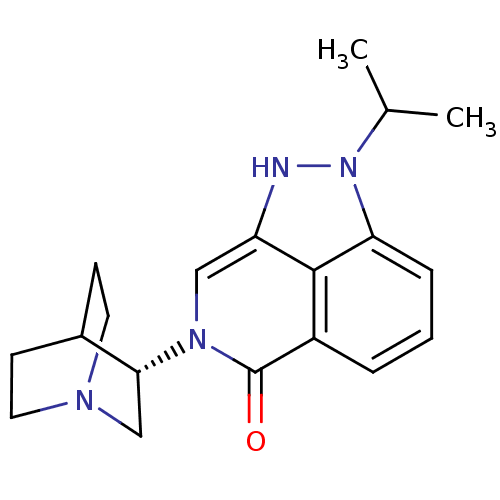
((S)-2-isopropyl-7-(quinuclidin-3-yl)-7,8-dihydropy...)Show SMILES CC(C)n1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:8.7,(5.55,-19.44,;6.52,-18.25,;8.03,-18.49,;5.97,-16.81,;7,-15.06,;5.85,-14.02,;5.85,-12.48,;4.51,-11.71,;4.5,-10.18,;3.17,-9.41,;3.17,-7.87,;4.51,-7.1,;5.84,-7.87,;5.84,-9.4,;4.33,-8.9,;4.65,-8.27,;3.18,-12.48,;1.84,-11.7,;3.18,-14.03,;1.85,-14.8,;1.84,-16.34,;3.18,-17.11,;4.51,-16.34,;4.51,-14.8,)| Show InChI InChI=1S/C19H24N4O/c1-12(2)23-16-5-3-4-14-18(16)15(20-23)10-22(19(14)24)17-11-21-8-6-13(17)7-9-21/h3-5,10,12-13,17,20H,6-9,11H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334448

((S)-2-isobutyl-7-(quinuclidin-3-yl)-7,8-dihydropyr...)Show SMILES CC(C)Cn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:9.8,(15.89,-21.07,;15.34,-19.64,;13.82,-19.39,;16.31,-18.44,;15.77,-17.01,;16.79,-15.26,;15.64,-14.22,;15.64,-12.68,;14.3,-11.91,;14.3,-10.37,;12.97,-9.61,;12.97,-8.07,;14.3,-7.3,;15.63,-8.07,;15.63,-9.6,;14.13,-9.1,;14.45,-8.46,;12.98,-12.68,;11.63,-11.89,;12.98,-14.22,;11.64,-14.99,;11.64,-16.54,;12.98,-17.31,;14.31,-16.54,;14.31,-14.99,)| Show InChI InChI=1S/C20H26N4O/c1-13(2)10-24-17-5-3-4-15-19(17)16(21-24)11-23(20(15)25)18-12-22-8-6-14(18)7-9-22/h3-5,11,13-14,18,21H,6-10,12H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50045292
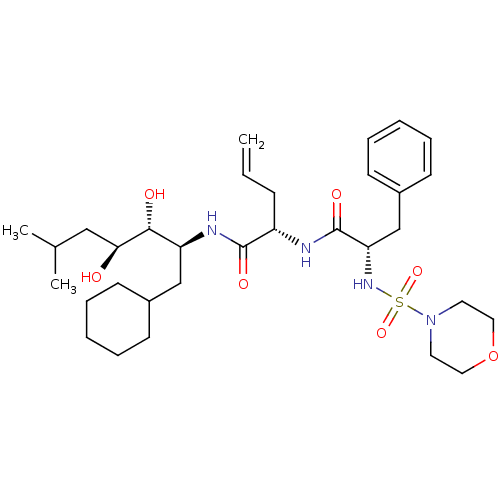
((S)-2-[(S)-2-(Morpholine-4-sulfonylamino)-3-phenyl...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C32H52N4O7S/c1-4-11-26(31(39)34-27(21-24-12-7-5-8-13-24)30(38)29(37)20-23(2)3)33-32(40)28(22-25-14-9-6-10-15-25)35-44(41,42)36-16-18-43-19-17-36/h4,6,9-10,14-15,23-24,26-30,35,37-38H,1,5,7-8,11-13,16-22H2,2-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498810
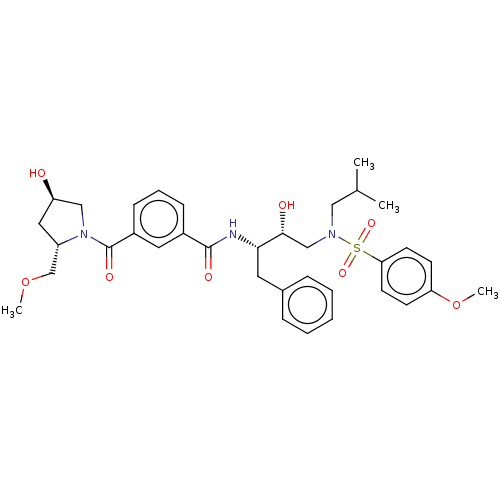
(CHEMBL3627857)Show SMILES COC[C@@H]1C[C@@H](O)CN1C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C35H45N3O8S/c1-24(2)20-37(47(43,44)31-15-13-30(46-4)14-16-31)22-33(40)32(17-25-9-6-5-7-10-25)36-34(41)26-11-8-12-27(18-26)35(42)38-21-29(39)19-28(38)23-45-3/h5-16,18,24,28-29,32-33,39-40H,17,19-23H2,1-4H3,(H,36,41)/t28-,29+,32-,33+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50045287
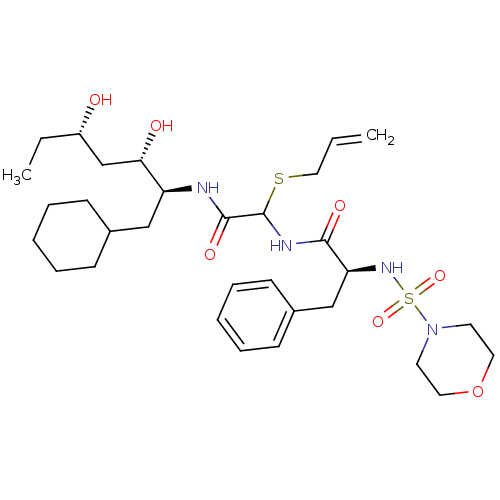
(CHEMBL88526 | N-[Allylsulfanyl-(1-cyclohexylmethyl...)Show SMILES CC[C@H](O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)SCC=C Show InChI InChI=1S/C31H50N4O7S2/c1-3-19-43-31(30(39)32-26(28(37)22-25(36)4-2)20-23-11-7-5-8-12-23)33-29(38)27(21-24-13-9-6-10-14-24)34-44(40,41)35-15-17-42-18-16-35/h3,6,9-10,13-14,23,25-28,31,34,36-37H,1,4-5,7-8,11-12,15-22H2,2H3,(H,32,39)(H,33,38)/t25-,26-,27-,28-,31?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin E |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014548

(CHEMBL3261479 | US9045501, 2)Show SMILES O=C1N(CCn2ccc3cccc1c23)[C@@H]1CN2CCC1CC2 |r,wD:14.16,(3.92,-9.95,;5.4,-10.4,;6.45,-9.26,;7.99,-9.38,;8.86,-10.65,;8.41,-12.12,;9.35,-13.46,;8.44,-14.72,;6.97,-14.24,;5.64,-15.01,;4.3,-14.24,;4.31,-12.7,;5.63,-11.93,;6.97,-12.69,;5.89,-7.83,;4.36,-7.6,;3.8,-6.16,;4.76,-4.96,;6.28,-5.2,;6.84,-6.62,;5.89,-5.59,;5.13,-6.93,)| Show InChI InChI=1S/C18H21N3O/c22-18-15-3-1-2-14-6-9-20(17(14)15)10-11-21(18)16-12-19-7-4-13(16)5-8-19/h1-3,6,9,13,16H,4-5,7-8,10-12H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50045284

(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES CC[C@H](C)CNC(=O)C(F)(F)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C34H53F2N5O7S/c1-4-12-27(38-32(44)29(22-26-15-10-7-11-16-26)40-49(46,47)41-17-19-48-20-18-41)31(43)39-28(21-25-13-8-6-9-14-25)30(42)34(35,36)33(45)37-23-24(3)5-2/h4,7,10-11,15-16,24-25,27-30,40,42H,1,5-6,8-9,12-14,17-23H2,2-3H3,(H,37,45)(H,38,44)(H,39,43)/t24-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50045283
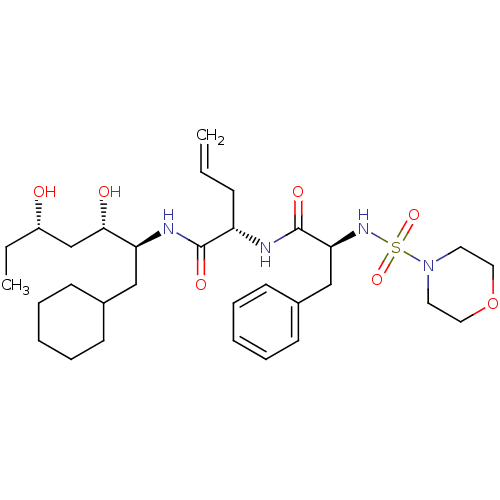
(2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...)Show SMILES CC[C@H](O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC=C)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C31H50N4O7S/c1-3-11-26(30(38)33-27(29(37)22-25(36)4-2)20-23-12-7-5-8-13-23)32-31(39)28(21-24-14-9-6-10-15-24)34-43(40,41)35-16-18-42-19-17-35/h3,6,9-10,14-15,23,25-29,34,36-37H,1,4-5,7-8,11-13,16-22H2,2H3,(H,32,39)(H,33,38)/t25-,26-,27-,28-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin E |
J Med Chem 36: 2614-20 (1993)
BindingDB Entry DOI: 10.7270/Q2X0664J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334446

((R)-2-isopropyl-7-(quinuclidin-3-yl)-7,8-dihydropy...)Show SMILES CC(C)n1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:8.7,(-3.99,-20.22,;-3.02,-19.03,;-1.5,-19.27,;-3.56,-17.59,;-2.53,-15.84,;-3.69,-14.8,;-3.68,-13.26,;-5.02,-12.49,;-5.03,-10.95,;-6.36,-10.19,;-6.36,-8.65,;-5.02,-7.88,;-3.69,-8.65,;-3.69,-10.18,;-5.2,-9.68,;-4.88,-9.04,;-6.35,-13.26,;-7.7,-12.48,;-6.35,-14.81,;-7.69,-15.58,;-7.69,-17.12,;-6.35,-17.89,;-5.02,-17.12,;-5.02,-15.58,)| Show InChI InChI=1S/C19H24N4O/c1-12(2)23-16-5-3-4-14-18(16)15(20-23)10-22(19(14)24)17-11-21-8-6-13(17)7-9-21/h3-5,10,12-13,17,20H,6-9,11H2,1-2H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334440

((R)-7-(quinuclidin-3-yl)-7,8-dihydropyrazolo[3,4,5...)Show SMILES O=c1n(cc2[nH][nH]c3cccc1c23)[C@H]1CN2CCC1CC2 |r,wU:13.15,(-7.86,1.16,;-6.52,.38,;-5.19,1.14,;-3.85,.37,;-3.86,-1.17,;-2.7,-2.21,;-3.73,-3.95,;-5.19,-3.48,;-6.52,-4.25,;-7.86,-3.48,;-7.85,-1.94,;-6.52,-1.17,;-5.19,-1.94,;-5.2,2.68,;-6.53,3.44,;-6.53,4.98,;-5.19,5.75,;-3.86,4.98,;-3.86,3.45,;-5.37,3.95,;-5.05,4.59,)| Show InChI InChI=1S/C16H18N4O/c21-16-11-2-1-3-12-15(11)13(18-17-12)8-20(16)14-9-19-6-4-10(14)5-7-19/h1-3,8,10,14,17-18H,4-7,9H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50522181

(CHEMBL4456725)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2N(C)c1c(N[C@@H](Cc2ccccc2)[C@H](O)CN(CC(C)C)S(=O)(=O)c2ccc(OC)cc2)c(=O)c1=O |r| Show InChI InChI=1S/C32H41N3O8S/c1-20(2)17-35(44(39,40)23-12-10-22(41-4)11-13-23)18-27(36)25(16-21-8-6-5-7-9-21)33-28-29(31(38)30(28)37)34(3)26-19-43-32-24(26)14-15-42-32/h5-13,20,24-27,32-33,36H,14-19H2,1-4H3/t24-,25-,26-,27+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay |
Bioorg Med Chem Lett 29: 2565-2570 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.006
BindingDB Entry DOI: 10.7270/Q2JM2F1C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data