 Found 98 hits with Last Name = 'kelly' and Initial = 'k'
Found 98 hits with Last Name = 'kelly' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor 3
(RAT) | BDBM50096326
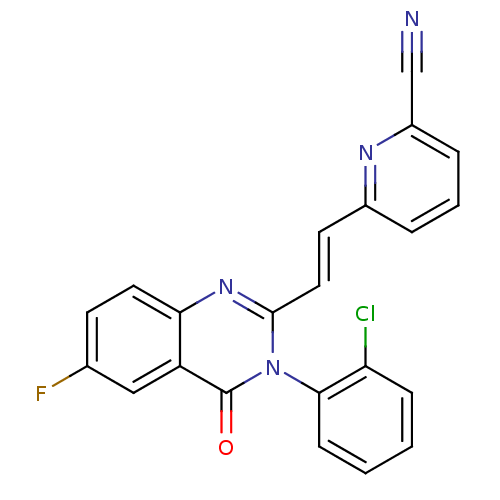
(6-{(E)-2-[3-(2-Chloro-phenyl)-6-fluoro-4-oxo-3,4-d...)Show SMILES Fc1ccc2nc(\C=C\c3cccc(n3)C#N)n(-c3ccccc3Cl)c(=O)c2c1 |(1.2,-4.46,;2.55,-5.21,;2.55,-6.75,;3.88,-7.52,;5.21,-6.75,;6.56,-7.52,;7.91,-6.73,;9.25,-7.5,;9.25,-9.04,;10.6,-9.81,;10.6,-11.35,;11.93,-12.12,;13.26,-11.35,;13.26,-9.78,;11.92,-9.04,;14.58,-8.99,;15.91,-8.22,;7.91,-5.18,;9.25,-4.41,;9.22,-2.88,;10.53,-2.1,;11.88,-2.85,;11.91,-4.39,;10.57,-5.18,;10.57,-6.72,;6.56,-4.41,;6.54,-2.87,;5.21,-5.19,;3.88,-4.42,)| Show InChI InChI=1S/C22H12ClFN4O/c23-18-6-1-2-7-20(18)28-21(11-9-15-4-3-5-16(13-25)26-15)27-19-10-8-14(24)12-17(19)22(28)29/h1-12H/b11-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50096327
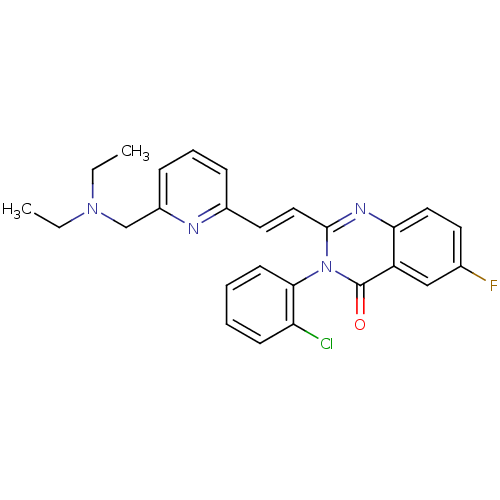
((R)-3-(2-Chloro-phenyl)-2-[(E)-2-(6-diethylaminome...)Show SMILES CCN(CC)Cc1cccc(\C=C\c2nc3ccc(F)cc3c(=O)n2-c2ccccc2Cl)n1 |(13.07,-4.53,;11.73,-3.76,;10.41,-4.56,;10.42,-6.09,;9.1,-6.86,;9.06,-3.81,;7.73,-4.59,;7.75,-6.13,;6.41,-6.9,;5.09,-6.14,;5.09,-4.6,;3.74,-3.86,;3.74,-2.32,;2.4,-1.55,;1.06,-2.32,;-.3,-1.55,;-1.63,-2.32,;-2.95,-1.55,;-2.95,-.01,;-4.31,.76,;-1.63,.76,;-.3,-.01,;1.05,.81,;1.03,2.35,;2.4,.04,;3.72,.81,;3.72,2.32,;5.04,3.12,;6.37,2.35,;6.37,.81,;5.07,.04,;5.06,-1.52,;6.4,-3.82,)| Show InChI InChI=1S/C26H24ClFN4O/c1-3-31(4-2)17-20-9-7-8-19(29-20)13-15-25-30-23-14-12-18(28)16-21(23)26(33)32(25)24-11-6-5-10-22(24)27/h5-16H,3-4,17H2,1-2H3/b15-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85710
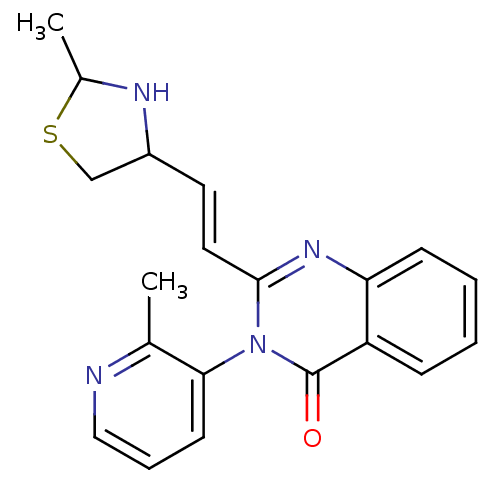
(CP-471236)Show SMILES CC1NC(CS1)\C=C\c1nc2ccccc2c(=O)n1-c1cccnc1C |(16.26,-2.66,;14.78,-3.06,;13.53,-2.16,;12.28,-3.06,;12.76,-4.52,;14.3,-4.52,;11.08,-2.44,;11.08,-.9,;9.75,-.13,;8.41,-.9,;7.08,-.13,;5.75,-.9,;4.41,-.13,;4.41,1.41,;5.75,2.18,;7.08,1.41,;8.41,2.18,;8.41,3.72,;9.75,1.41,;11.08,2.18,;12.42,1.41,;13.75,2.18,;13.75,3.72,;12.42,4.49,;11.08,3.72,;9.75,4.49,)| Show InChI InChI=1S/C20H20N4OS/c1-13-18(8-5-11-21-13)24-19(10-9-15-12-26-14(2)22-15)23-17-7-4-3-6-16(17)20(24)25/h3-11,14-15,22H,12H2,1-2H3/b10-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85711
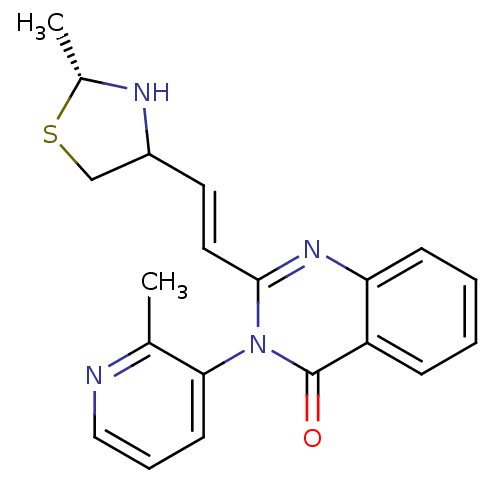
(CP-471237)Show SMILES C[C@@H]1NC(CS1)\C=C\c1nc2ccccc2c(=O)n1-c1cccnc1C |r,wU:1.0,(16.26,-2.66,;14.78,-3.06,;13.53,-2.16,;12.28,-3.06,;12.76,-4.52,;14.3,-4.52,;11.08,-2.44,;11.08,-.9,;9.75,-.13,;8.41,-.9,;7.08,-.13,;5.75,-.9,;4.41,-.13,;4.41,1.41,;5.75,2.18,;7.08,1.41,;8.41,2.18,;8.41,3.72,;9.75,1.41,;11.08,2.18,;12.42,1.41,;13.75,2.18,;13.75,3.72,;12.42,4.49,;11.08,3.72,;9.75,4.49,)| Show InChI InChI=1S/C20H20N4OS/c1-13-18(8-5-11-21-13)24-19(10-9-15-12-26-14(2)22-15)23-17-7-4-3-6-16(17)20(24)25/h3-11,14-15,22H,12H2,1-2H3/b10-9+/t14-,15?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50048389
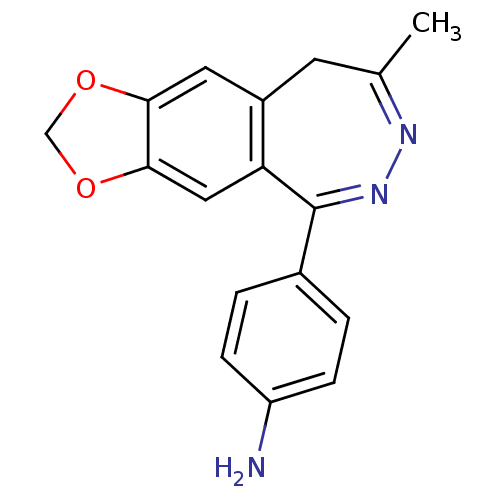
(4-(8-Methyl-9H-1,3-dioxa-6,7-diaza-cyclohepta[f]in...)Show SMILES CC1=NN=C(c2ccc(N)cc2)c2cc3OCOc3cc2C1 |t:1,3| Show InChI InChI=1S/C17H15N3O2/c1-10-6-12-7-15-16(22-9-21-15)8-14(12)17(20-19-10)11-2-4-13(18)5-3-11/h2-5,7-8H,6,9,18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155213
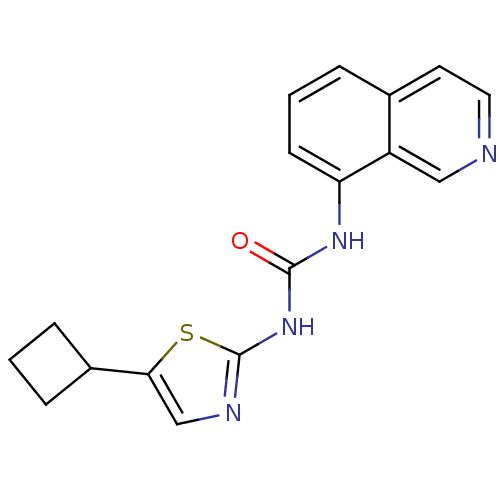
(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-8-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)12-4-1-5-12)20-14-6-2-3-11-7-8-18-9-13(11)14/h2-3,6-10,12H,1,4-5H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415046

(CHEMBL583658)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:10.10,12.15,(14.65,-10.99,;14.63,-9.45,;13.28,-8.7,;13.27,-7.16,;14.59,-6.38,;15.92,-7.14,;15.95,-8.67,;17.25,-6.35,;17.24,-4.81,;18.59,-7.11,;19.92,-6.33,;20.6,-4.95,;21.99,-5.63,;21.3,-7.01,;23.45,-5.14,;23.91,-3.67,;25.45,-3.66,;25.94,-5.12,;27.27,-5.89,;27.27,-7.43,;25.93,-8.2,;28.6,-8.21,;28.59,-9.75,;29.92,-10.51,;29.92,-12.05,;28.58,-12.82,;27.25,-12.05,;25.93,-12.81,;24.6,-12.05,;24.6,-10.51,;25.93,-9.74,;27.25,-10.51,;24.7,-6.03,)| Show InChI InChI=1S/C26H25N5O2/c1-17-6-4-11-23(28-17)26(33)29-20-13-21(14-20)31-15-24(27-16-31)30-25(32)12-19-9-5-8-18-7-2-3-10-22(18)19/h2-11,15-16,20-21H,12-14H2,1H3,(H,29,33)(H,30,32)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415045

(CHEMBL571782)Show SMILES Clc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:10.10,12.15,(-7.08,-11.69,;-7.1,-10.15,;-8.45,-9.39,;-8.47,-7.85,;-7.14,-7.07,;-5.81,-7.83,;-5.78,-9.36,;-4.48,-7.05,;-4.5,-5.51,;-3.14,-7.8,;-1.81,-7.02,;-1.13,-5.64,;.26,-6.32,;-.44,-7.71,;1.72,-5.83,;2.18,-4.37,;3.72,-4.35,;4.21,-5.81,;5.54,-6.58,;5.54,-8.13,;4.2,-8.89,;6.87,-8.9,;6.86,-10.44,;8.19,-11.21,;8.19,-12.74,;6.85,-13.52,;5.52,-12.74,;4.19,-13.5,;2.87,-12.74,;2.87,-11.2,;4.2,-10.44,;5.52,-11.21,;2.97,-6.73,)| Show InChI InChI=1S/C25H22ClN5O2/c26-22-10-4-9-21(29-22)25(33)28-18-12-19(13-18)31-14-23(27-15-31)30-24(32)11-17-7-3-6-16-5-1-2-8-20(16)17/h1-10,14-15,18-19H,11-13H2,(H,28,33)(H,30,32)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155231
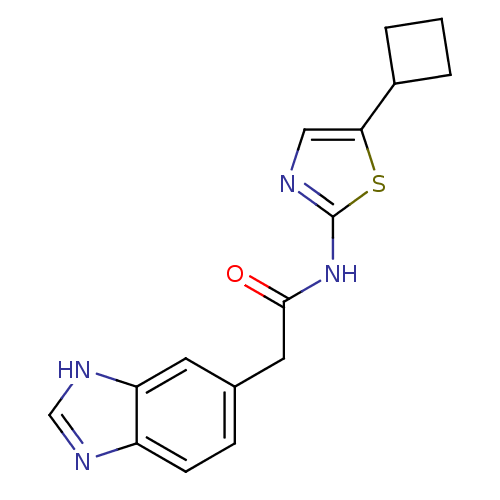
(2-(1H-Benzoimidazol-5-yl)-N-(5-cyclobutyl-thiazol-...)Show InChI InChI=1S/C16H16N4OS/c21-15(7-10-4-5-12-13(6-10)19-9-18-12)20-16-17-8-14(22-16)11-2-1-3-11/h4-6,8-9,11H,1-3,7H2,(H,18,19)(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155209

(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-5-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-3-1-4-11)20-14-6-2-5-12-9-18-8-7-13(12)14/h2,5-11H,1,3-4H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415039

(CHEMBL571780)Show SMILES CC(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:6.8,4.3,(6.38,-30.85,;7.71,-30.06,;9.05,-30.82,;7.69,-28.52,;9.02,-27.74,;9.7,-26.36,;11.09,-27.05,;10.4,-28.43,;12.55,-26.56,;13.01,-25.09,;14.55,-25.07,;15.04,-26.53,;16.37,-27.31,;16.37,-28.85,;15.03,-29.61,;17.7,-29.62,;17.69,-31.16,;19.02,-31.93,;19.02,-33.46,;17.68,-34.24,;16.35,-33.46,;15.03,-34.22,;13.7,-33.46,;13.7,-31.92,;15.03,-31.16,;16.35,-31.93,;13.81,-27.45,)| Show InChI InChI=1S/C21H22N4O2/c1-14(26)23-17-10-18(11-17)25-12-20(22-13-25)24-21(27)9-16-7-4-6-15-5-2-3-8-19(15)16/h2-8,12-13,17-18H,9-11H2,1H3,(H,23,26)(H,24,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415037

(CHEMBL576502)Show SMILES COc1ccc(CC(=O)Nc2cn(cn2)[C@@H]2C[C@@H](C2)NC(C)=O)cc1 |r,wU:15.15,17.20,(2.53,9.19,;1,9.03,;.37,7.63,;-1.16,7.46,;-1.78,6.06,;-.88,4.81,;-1.51,3.41,;-.6,2.16,;.93,2.32,;-1.23,.75,;-.32,-.49,;-.8,-1.96,;.45,-2.86,;1.69,-1.96,;1.22,-.49,;.45,-4.4,;-.64,-5.49,;.45,-6.58,;1.54,-5.49,;.45,-8.12,;-.89,-8.89,;-.89,-10.43,;-2.22,-8.12,;.65,4.97,;1.28,6.38,)| Show InChI InChI=1S/C18H22N4O3/c1-12(23)20-14-8-15(9-14)22-10-17(19-11-22)21-18(24)7-13-3-5-16(25-2)6-4-13/h3-6,10-11,14-15H,7-9H2,1-2H3,(H,20,23)(H,21,24)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155236
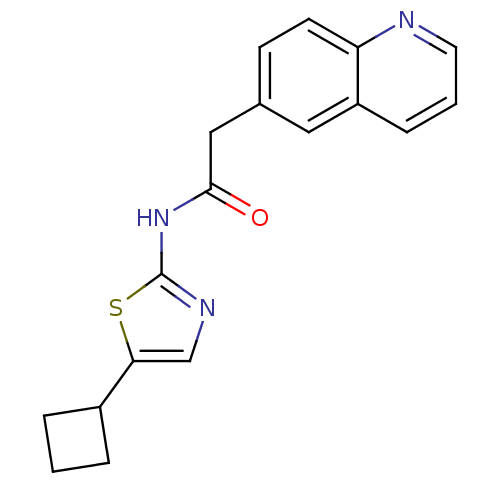
(CHEMBL363954 | N-(5-Cyclobutyl-thiazol-2-yl)-2-qui...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)13-3-1-4-13)10-12-6-7-15-14(9-12)5-2-8-19-15/h2,5-9,11,13H,1,3-4,10H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155235
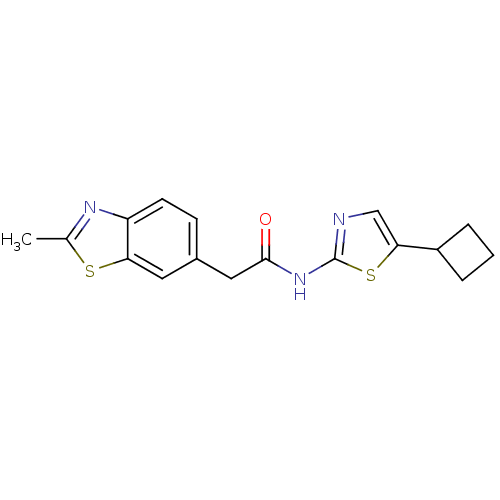
(CHEMBL186470 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(2-...)Show InChI InChI=1S/C17H17N3OS2/c1-10-19-13-6-5-11(7-14(13)22-10)8-16(21)20-17-18-9-15(23-17)12-3-2-4-12/h5-7,9,12H,2-4,8H2,1H3,(H,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155230
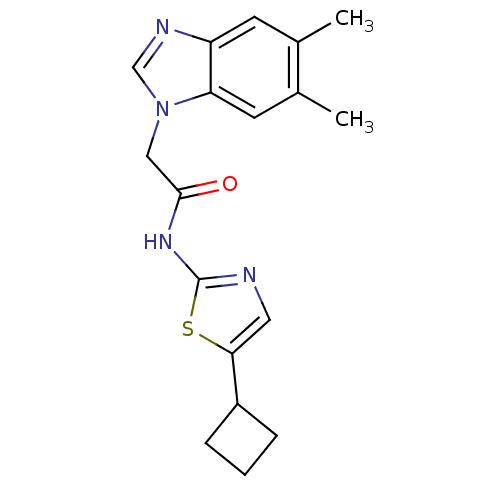
(CHEMBL187903 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(5,...)Show InChI InChI=1S/C18H20N4OS/c1-11-6-14-15(7-12(11)2)22(10-20-14)9-17(23)21-18-19-8-16(24-18)13-4-3-5-13/h6-8,10,13H,3-5,9H2,1-2H3,(H,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155237

(CHEMBL184404 | N-(5-Cyclobutyl-thiazol-2-yl)-2-iso...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)12-3-1-4-12)9-13-5-2-6-14-10-19-8-7-15(13)14/h2,5-8,10-12H,1,3-4,9H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155207

(1-(5-Cyclobutyl-thiazol-2-yl)-3-quinolin-5-yl-urea...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-4-1-5-11)20-14-8-2-7-13-12(14)6-3-9-18-13/h2-3,6-11H,1,4-5H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415044

(CHEMBL569587)Show SMILES O=C(Cc1cccc2ccccc12)Nc1cn(cn1)[C@@H]1C[C@@H](C1)NC(=O)c1ccccn1 |r,wU:21.26,19.21,(28.29,2.47,;29.63,3.24,;30.96,2.46,;30.96,.92,;32.28,.16,;32.28,-1.38,;30.94,-2.15,;29.61,-1.38,;28.29,-2.14,;26.96,-1.38,;26.96,.16,;28.29,.93,;29.62,.16,;29.63,4.78,;28.3,5.55,;27.07,4.63,;25.81,5.53,;26.27,7,;27.81,7.01,;24.35,5.04,;22.96,5.72,;22.28,4.34,;23.66,3.66,;20.95,3.56,;19.61,4.32,;19.6,5.86,;18.29,3.53,;16.95,4.29,;15.63,3.51,;15.64,1.97,;16.99,1.21,;18.31,2,)| Show InChI InChI=1S/C25H23N5O2/c31-24(12-18-8-5-7-17-6-1-2-9-21(17)18)29-23-15-30(16-27-23)20-13-19(14-20)28-25(32)22-10-3-4-11-26-22/h1-11,15-16,19-20H,12-14H2,(H,28,32)(H,29,31)/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155214
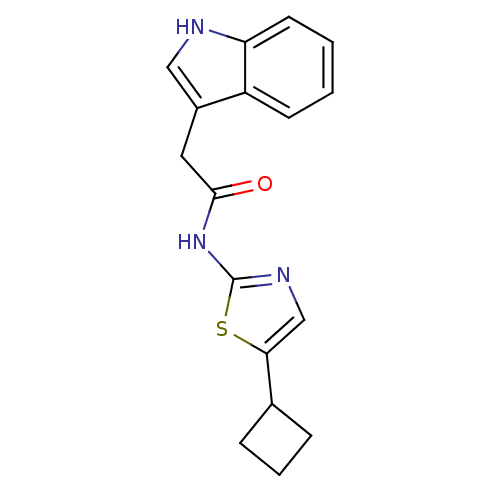
(CHEMBL186240 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(1H...)Show InChI InChI=1S/C17H17N3OS/c21-16(8-12-9-18-14-7-2-1-6-13(12)14)20-17-19-10-15(22-17)11-4-3-5-11/h1-2,6-7,9-11,18H,3-5,8H2,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155235

(CHEMBL186470 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(2-...)Show InChI InChI=1S/C17H17N3OS2/c1-10-19-13-6-5-11(7-14(13)22-10)8-16(21)20-17-18-9-15(23-17)12-3-2-4-12/h5-7,9,12H,2-4,8H2,1H3,(H,18,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155225
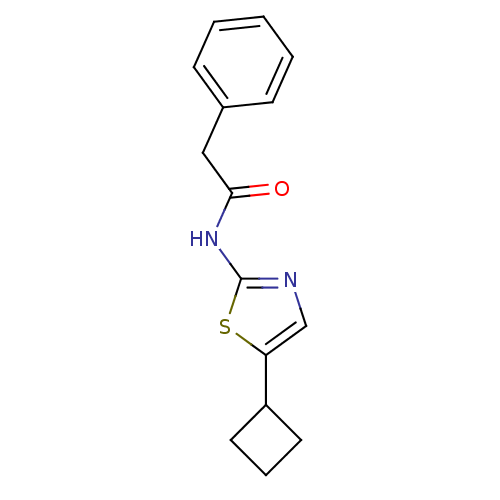
(CHEMBL365855 | N-(5-Cyclobutyl-thiazol-2-yl)-2-phe...)Show InChI InChI=1S/C15H16N2OS/c18-14(9-11-5-2-1-3-6-11)17-15-16-10-13(19-15)12-7-4-8-12/h1-3,5-6,10,12H,4,7-9H2,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155236

(CHEMBL363954 | N-(5-Cyclobutyl-thiazol-2-yl)-2-qui...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)13-3-1-4-13)10-12-6-7-15-14(9-12)5-2-8-19-15/h2,5-9,11,13H,1,3-4,10H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155230

(CHEMBL187903 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(5,...)Show InChI InChI=1S/C18H20N4OS/c1-11-6-14-15(7-12(11)2)22(10-20-14)9-17(23)21-18-19-8-16(24-18)13-4-3-5-13/h6-8,10,13H,3-5,9H2,1-2H3,(H,19,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415042

(CHEMBL582813)Show SMILES O=C(Cc1cccc2ccccc12)Nc1cn(cn1)[C@@H]1C[C@@H](C1)NC(=O)c1ccncc1 |r,wU:21.26,19.21,(31.19,-43.99,;32.52,-43.23,;33.86,-44,;33.85,-45.54,;35.18,-46.31,;35.18,-47.84,;33.84,-48.62,;32.5,-47.84,;31.18,-48.6,;29.86,-47.84,;29.86,-46.3,;31.19,-45.54,;32.51,-46.31,;32.53,-41.68,;31.2,-40.91,;29.96,-41.83,;28.71,-40.93,;29.17,-39.47,;30.71,-39.45,;27.25,-41.42,;25.86,-40.74,;25.17,-42.12,;26.55,-42.81,;23.85,-42.9,;22.51,-42.15,;22.49,-40.61,;21.18,-42.93,;19.85,-42.17,;18.52,-42.95,;18.54,-44.49,;19.89,-45.25,;21.21,-44.46,)| Show InChI InChI=1S/C25H23N5O2/c31-24(12-19-6-3-5-17-4-1-2-7-22(17)19)29-23-15-30(16-27-23)21-13-20(14-21)28-25(32)18-8-10-26-11-9-18/h1-11,15-16,20-21H,12-14H2,(H,28,32)(H,29,31)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155231

(2-(1H-Benzoimidazol-5-yl)-N-(5-cyclobutyl-thiazol-...)Show InChI InChI=1S/C16H16N4OS/c21-15(7-10-4-5-12-13(6-10)19-9-18-12)20-16-17-8-14(22-16)11-2-1-3-11/h4-6,8-9,11H,1-3,7H2,(H,18,19)(H,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155214

(CHEMBL186240 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(1H...)Show InChI InChI=1S/C17H17N3OS/c21-16(8-12-9-18-14-7-2-1-6-13(12)14)20-17-19-10-15(22-17)11-4-3-5-11/h1-2,6-7,9-11,18H,3-5,8H2,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155232
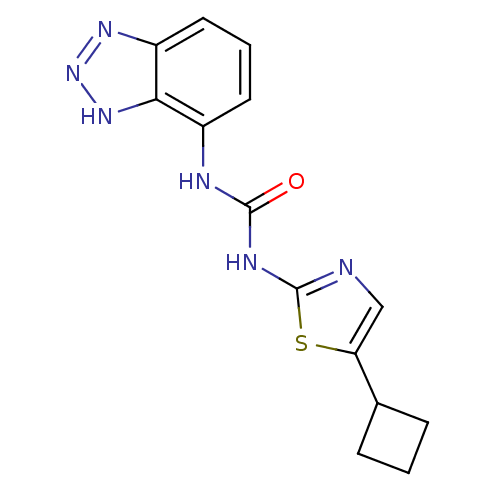
(1-(3H-Benzotriazol-4-yl)-3-(5-cyclobutyl-thiazol-2...)Show InChI InChI=1S/C14H14N6OS/c21-13(16-9-5-2-6-10-12(9)19-20-18-10)17-14-15-7-11(22-14)8-3-1-4-8/h2,5-8H,1,3-4H2,(H,18,19,20)(H2,15,16,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155207

(1-(5-Cyclobutyl-thiazol-2-yl)-3-quinolin-5-yl-urea...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-4-1-5-11)20-14-8-2-7-13-12(14)6-3-9-18-13/h2-3,6-11H,1,4-5H2,(H2,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155222

(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-6-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-2-1-3-11)20-14-5-4-13-9-18-7-6-12(13)8-14/h4-11H,1-3H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155221
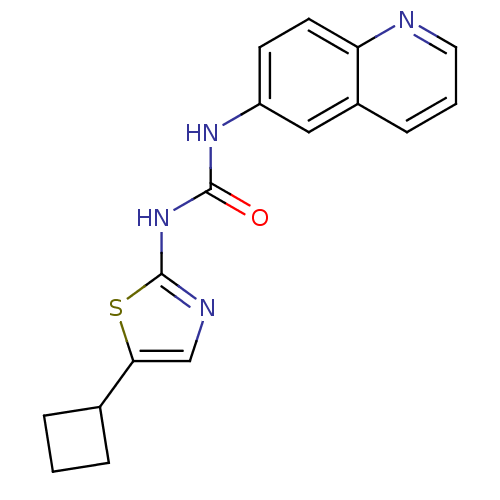
(1-(5-Cyclobutyl-thiazol-2-yl)-3-quinolin-6-yl-urea...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-3-1-4-11)20-13-6-7-14-12(9-13)5-2-8-18-14/h2,5-11H,1,3-4H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415026
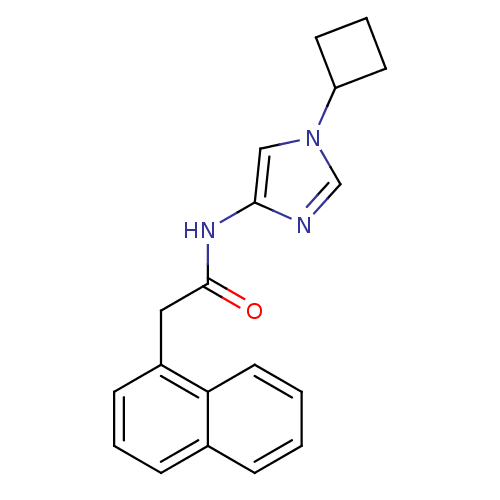
(CHEMBL571565)Show InChI InChI=1S/C19H19N3O/c23-19(21-18-12-22(13-20-18)16-8-4-9-16)11-15-7-3-6-14-5-1-2-10-17(14)15/h1-3,5-7,10,12-13,16H,4,8-9,11H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415043

(CHEMBL569586)Show SMILES O=C(Cc1cccc2ccccc12)Nc1cn(cn1)[C@@H]1C[C@@H](C1)NC(=O)c1cccnc1 |r,wU:21.26,19.21,(3.14,2.57,;4.48,3.33,;5.81,2.56,;5.8,1.02,;7.13,.25,;7.13,-1.29,;5.79,-2.06,;4.46,-1.28,;3.13,-2.05,;1.81,-1.28,;1.81,.26,;3.14,1.02,;4.46,.25,;4.48,4.87,;3.15,5.65,;1.91,4.73,;.66,5.62,;1.12,7.09,;2.66,7.11,;-.8,5.13,;-2.19,5.82,;-2.88,4.44,;-1.5,3.75,;-4.2,3.65,;-5.54,4.41,;-5.56,5.95,;-6.87,3.63,;-8.2,4.39,;-9.53,3.61,;-9.51,2.07,;-8.16,1.31,;-6.84,2.09,)| Show InChI InChI=1S/C25H23N5O2/c31-24(11-18-7-3-6-17-5-1-2-9-22(17)18)29-23-15-30(16-27-23)21-12-20(13-21)28-25(32)19-8-4-10-26-14-19/h1-10,14-16,20-21H,11-13H2,(H,28,32)(H,29,31)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155220

(CHEMBL364338 | N-(5-Cyclobutyl-thiazol-2-yl)-2-ind...)Show InChI InChI=1S/C17H17N3OS/c21-16(11-20-9-8-12-4-1-2-7-14(12)20)19-17-18-10-15(22-17)13-5-3-6-13/h1-2,4,7-10,13H,3,5-6,11H2,(H,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155213

(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-8-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)12-4-1-5-12)20-14-6-2-3-11-7-8-18-9-13(11)14/h2-3,6-10,12H,1,4-5H2,(H2,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155206

(CHEMBL186511 | N-(5-Cyclopentyl-thiazol-2-yl)-2-ph...)Show InChI InChI=1S/C16H18N2OS/c19-15(10-12-6-2-1-3-7-12)18-16-17-11-14(20-16)13-8-4-5-9-13/h1-3,6-7,11,13H,4-5,8-10H2,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50155225

(CHEMBL365855 | N-(5-Cyclobutyl-thiazol-2-yl)-2-phe...)Show InChI InChI=1S/C15H16N2OS/c18-14(9-11-5-2-1-3-6-11)17-15-16-10-13(19-15)12-7-4-8-12/h1-3,5-6,10,12H,4,7-9H2,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155210
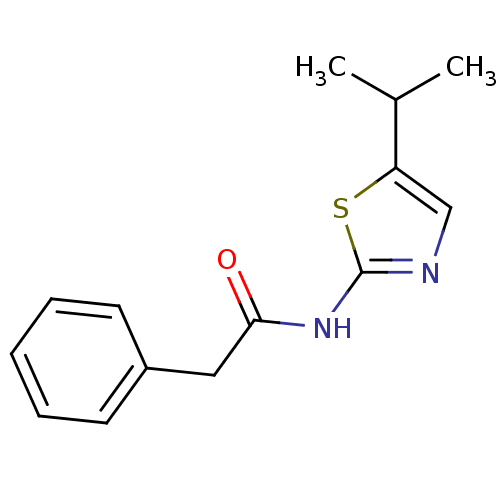
(CHEMBL426972 | N-(5-Isopropyl-thiazol-2-yl)-2-phen...)Show InChI InChI=1S/C14H16N2OS/c1-10(2)12-9-15-14(18-12)16-13(17)8-11-6-4-3-5-7-11/h3-7,9-10H,8H2,1-2H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155237

(CHEMBL184404 | N-(5-Cyclobutyl-thiazol-2-yl)-2-iso...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)12-3-1-4-12)9-13-5-2-6-14-10-19-8-7-15(13)14/h2,5-8,10-12H,1,3-4,9H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155225

(CHEMBL365855 | N-(5-Cyclobutyl-thiazol-2-yl)-2-phe...)Show InChI InChI=1S/C15H16N2OS/c18-14(9-11-5-2-1-3-6-11)17-15-16-10-13(19-15)12-7-4-8-12/h1-3,5-6,10,12H,4,7-9H2,(H,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155216

(CHEMBL185734 | N-(5-Methylsulfanyl-thiazol-2-yl)-2...)Show InChI InChI=1S/C12H12N2OS2/c1-16-11-8-13-12(17-11)14-10(15)7-9-5-3-2-4-6-9/h2-6,8H,7H2,1H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415033
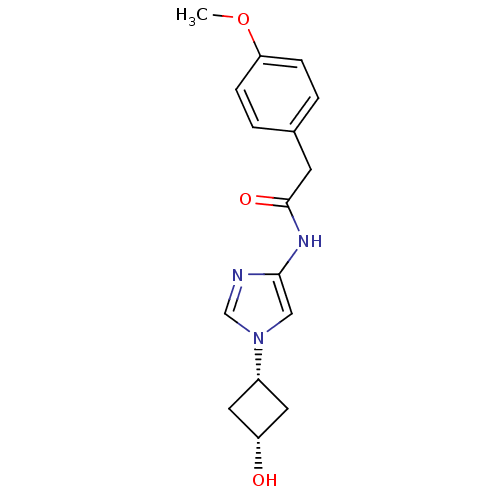
(CHEMBL571781)Show SMILES COc1ccc(CC(=O)Nc2cn(cn2)[C@@H]2C[C@H](O)C2)cc1 |r,wU:15.15,17.18,(-2.4,7.95,;-.87,7.79,;-.25,6.39,;1.29,6.22,;1.91,4.82,;1.01,3.57,;1.63,2.16,;.73,.92,;-.8,1.08,;1.35,-.49,;.45,-1.73,;.92,-3.2,;-.32,-4.1,;-1.57,-3.2,;-1.09,-1.73,;-.32,-5.64,;-1.41,-6.73,;-.32,-7.82,;-.32,-9.36,;.77,-6.73,;-.53,3.73,;-1.15,5.14,)| Show InChI InChI=1S/C16H19N3O3/c1-22-14-4-2-11(3-5-14)6-16(21)18-15-9-19(10-17-15)12-7-13(20)8-12/h2-5,9-10,12-13,20H,6-8H2,1H3,(H,18,21)/t12-,13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155209

(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-5-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-3-1-4-11)20-14-6-2-5-12-9-18-8-7-13(12)14/h2,5-11H,1,3-4H2,(H2,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155210

(CHEMBL426972 | N-(5-Isopropyl-thiazol-2-yl)-2-phen...)Show InChI InChI=1S/C14H16N2OS/c1-10(2)12-9-15-14(18-12)16-13(17)8-11-6-4-3-5-7-11/h3-7,9-10H,8H2,1-2H3,(H,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155220

(CHEMBL364338 | N-(5-Cyclobutyl-thiazol-2-yl)-2-ind...)Show InChI InChI=1S/C17H17N3OS/c21-16(11-20-9-8-12-4-1-2-7-14(12)20)19-17-18-10-15(22-17)13-5-3-6-13/h1-2,4,7-10,13H,3,5-6,11H2,(H,18,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155234
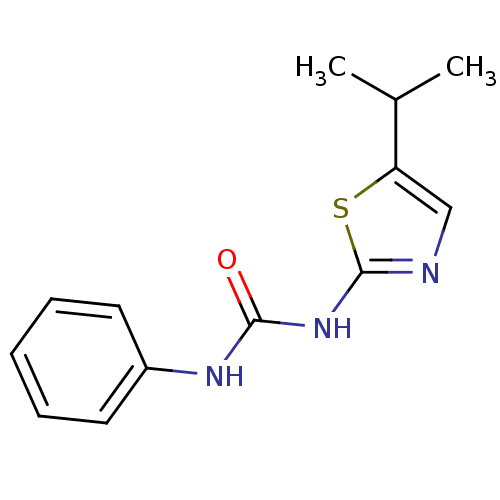
(1-(5-Isopropyl-thiazol-2-yl)-3-phenyl-urea | CHEMB...)Show InChI InChI=1S/C13H15N3OS/c1-9(2)11-8-14-13(18-11)16-12(17)15-10-6-4-3-5-7-10/h3-9H,1-2H3,(H2,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155216

(CHEMBL185734 | N-(5-Methylsulfanyl-thiazol-2-yl)-2...)Show InChI InChI=1S/C12H12N2OS2/c1-16-11-8-13-12(17-11)14-10(15)7-9-5-3-2-4-6-9/h2-6,8H,7H2,1H3,(H,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155232

(1-(3H-Benzotriazol-4-yl)-3-(5-cyclobutyl-thiazol-2...)Show InChI InChI=1S/C14H14N6OS/c21-13(16-9-5-2-6-10-12(9)19-20-18-10)17-14-15-7-11(22-14)8-3-1-4-8/h2,5-8H,1,3-4H2,(H,18,19,20)(H2,15,16,17,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415041

(CHEMBL572188)Show SMILES CN([C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1)C(C)=O |r,wU:4.6,2.1,(-10.8,-41.68,;-9.46,-42.44,;-8.14,-41.65,;-7.45,-40.28,;-6.06,-40.96,;-6.76,-42.34,;-4.6,-40.47,;-4.14,-39,;-2.6,-38.98,;-2.11,-40.44,;-.78,-41.22,;-.79,-42.76,;-2.12,-43.52,;.54,-43.53,;.54,-45.07,;1.87,-45.84,;1.87,-47.38,;.53,-48.15,;-.81,-47.37,;-2.13,-48.14,;-3.45,-47.37,;-3.45,-45.83,;-2.12,-45.07,;-.8,-45.84,;-3.35,-41.36,;-9.45,-43.98,;-10.77,-44.76,;-8.11,-44.73,)| Show InChI InChI=1S/C22H24N4O2/c1-15(27)25(2)18-11-19(12-18)26-13-21(23-14-26)24-22(28)10-17-8-5-7-16-6-3-4-9-20(16)17/h3-9,13-14,18-19H,10-12H2,1-2H3,(H,24,28)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415025

(CHEMBL578621)Show InChI InChI=1S/C16H19N3O2/c1-21-14-7-5-12(6-8-14)9-16(20)18-15-10-19(11-17-15)13-3-2-4-13/h5-8,10-11,13H,2-4,9H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415035
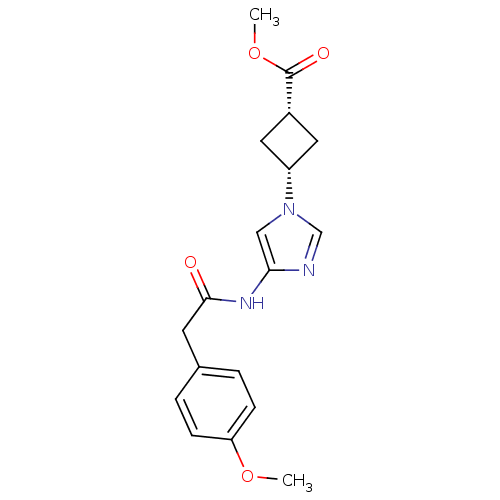
(CHEMBL576126)Show SMILES COC(=O)[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2ccc(OC)cc2)c1 |r,wU:6.8,4.3,(-9.08,-15.29,;-7.72,-14.56,;-7.67,-13.02,;-8.98,-12.21,;-6.32,-12.29,;-5.63,-10.91,;-4.24,-11.59,;-4.94,-12.98,;-2.78,-11.1,;-2.32,-9.63,;-.78,-9.62,;-.29,-11.08,;1.04,-11.85,;1.04,-13.39,;-.3,-14.16,;2.37,-14.17,;2.36,-15.71,;1.02,-16.47,;1.02,-18.01,;2.35,-18.79,;2.34,-20.33,;1.01,-21.09,;3.69,-18.01,;3.69,-16.48,;-1.53,-12,)| Show InChI InChI=1S/C18H21N3O4/c1-24-15-5-3-12(4-6-15)7-17(22)20-16-10-21(11-19-16)14-8-13(9-14)18(23)25-2/h3-6,10-11,13-14H,7-9H2,1-2H3,(H,20,22)/t13-,14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data