 Found 371 hits with Last Name = 'kett' and Initial = 'n'
Found 371 hits with Last Name = 'kett' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50234418
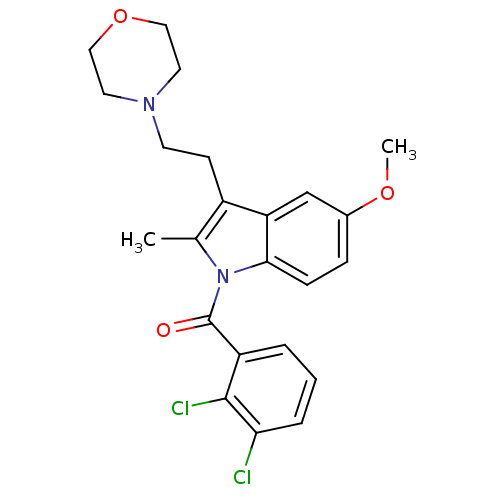
((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...)Show SMILES COc1ccc2n(C(=O)c3cccc(Cl)c3Cl)c(C)c(CCN3CCOCC3)c2c1 Show InChI InChI=1S/C23H24Cl2N2O3/c1-15-17(8-9-26-10-12-30-13-11-26)19-14-16(29-2)6-7-21(19)27(15)23(28)18-4-3-5-20(24)22(18)25/h3-7,14H,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50234418

((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...)Show SMILES COc1ccc2n(C(=O)c3cccc(Cl)c3Cl)c(C)c(CCN3CCOCC3)c2c1 Show InChI InChI=1S/C23H24Cl2N2O3/c1-15-17(8-9-26-10-12-30-13-11-26)19-14-16(29-2)6-7-21(19)27(15)23(28)18-4-3-5-20(24)22(18)25/h3-7,14H,8-13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340312
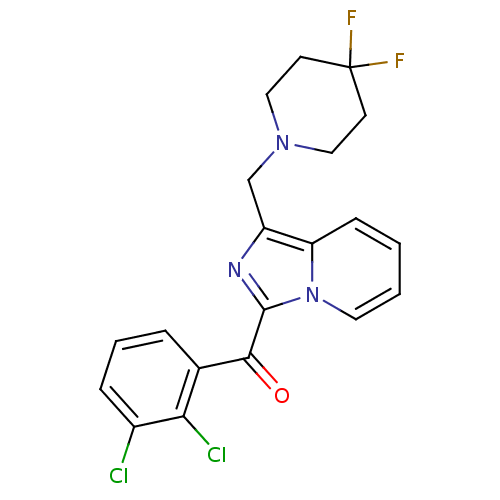
((2,3-dichlorophenyl)(1-((4,4-difluoropiperidin-1-y...)Show SMILES FC1(F)CCN(Cc2nc(C(=O)c3cccc(Cl)c3Cl)n3ccccc23)CC1 Show InChI InChI=1S/C20H17Cl2F2N3O/c21-14-5-3-4-13(17(14)22)18(28)19-25-15(16-6-1-2-9-27(16)19)12-26-10-7-20(23,24)8-11-26/h1-6,9H,7-8,10-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340311
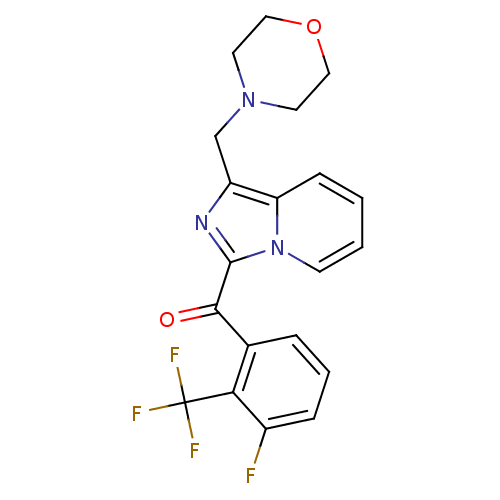
((3-fluoro-2-(trifluoromethyl)phenyl)(1-(morpholino...)Show SMILES Fc1cccc(C(=O)c2nc(CN3CCOCC3)c3ccccn23)c1C(F)(F)F Show InChI InChI=1S/C20H17F4N3O2/c21-14-5-3-4-13(17(14)20(22,23)24)18(28)19-25-15(12-26-8-10-29-11-9-26)16-6-1-2-7-27(16)19/h1-7H,8-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50507325

(CHEMBL4453586)Show SMILES C[C@@H](CN1CCC2(CC1)[C@@H](CNC2=O)c1ccc(F)cc1)NC(=O)c1cc2ccccc2[nH]1 |r| Show InChI InChI=1S/C26H29FN4O2/c1-17(29-24(32)23-14-19-4-2-3-5-22(19)30-23)16-31-12-10-26(11-13-31)21(15-28-25(26)33)18-6-8-20(27)9-7-18/h2-9,14,17,21,30H,10-13,15-16H2,1H3,(H,28,33)(H,29,32)/t17-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 in human Calu1 cells |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340313

(1-((4,4-difluoropiperidin-1-yl)methyl)-N-(6-(trifl...)Show SMILES FC(F)(F)c1cccc(NC(=O)c2nc(CN3CCC(F)(F)CC3)c3ccccn23)n1 Show InChI InChI=1S/C20H18F5N5O/c21-19(22)7-10-29(11-8-19)12-13-14-4-1-2-9-30(14)17(26-13)18(31)28-16-6-3-5-15(27-16)20(23,24)25/h1-6,9H,7-8,10-12H2,(H,27,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Phospholipase D2
(Homo sapiens (Human)) | BDBM50507332

(CHEMBL4475943)Show SMILES Fc1ccc(cc1)[C@@H]1CNC(=O)C11CCN(CCNC(=O)c2cc3ccccc3[nH]2)CC1 |r| Show InChI InChI=1S/C25H27FN4O2/c26-19-7-5-17(6-8-19)20-16-28-24(32)25(20)9-12-30(13-10-25)14-11-27-23(31)22-15-18-3-1-2-4-21(18)29-22/h1-8,15,20,29H,9-14,16H2,(H,27,31)(H,28,32)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of GFP-fused PLD2 (unknown origin) expressed in HEK293 cells by cellular assay |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340315
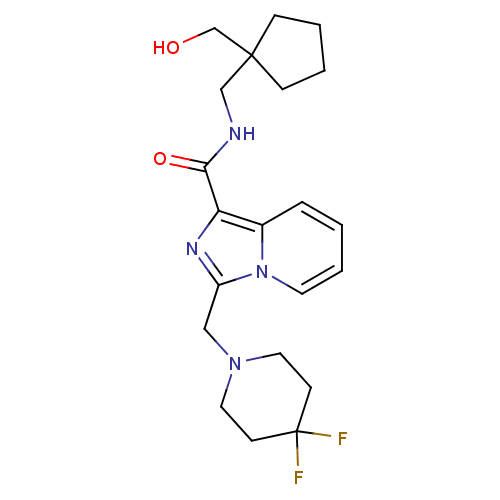
(3-((4,4-difluoropiperidin-1-yl)methyl)-N-((1-(hydr...)Show SMILES OCC1(CNC(=O)c2nc(CN3CCC(F)(F)CC3)n3ccccc23)CCCC1 Show InChI InChI=1S/C21H28F2N4O2/c22-21(23)8-11-26(12-9-21)13-17-25-18(16-5-1-4-10-27(16)17)19(29)24-14-20(15-28)6-2-3-7-20/h1,4-5,10,28H,2-3,6-9,11-15H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50257541
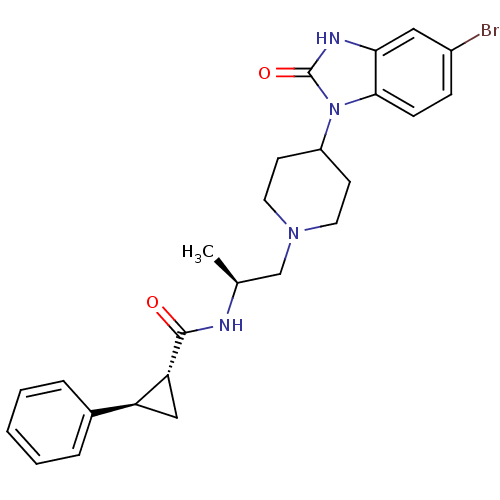
((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...)Show SMILES C[C@@H](CN1CCC(CC1)n1c2ccc(Br)cc2[nH]c1=O)NC(=O)[C@@H]1C[C@H]1c1ccccc1 |r| Show InChI InChI=1S/C25H29BrN4O2/c1-16(27-24(31)21-14-20(21)17-5-3-2-4-6-17)15-29-11-9-19(10-12-29)30-23-8-7-18(26)13-22(23)28-25(30)32/h2-8,13,16,19-21H,9-12,14-15H2,1H3,(H,27,31)(H,28,32)/t16-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 (unknown origin) |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340310
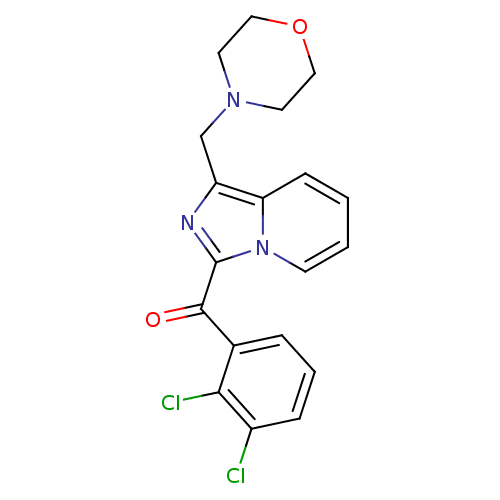
((2,3-dichlorophenyl)(1-(morpholinomethyl)imidazo[1...)Show SMILES Clc1cccc(C(=O)c2nc(CN3CCOCC3)c3ccccn23)c1Cl Show InChI InChI=1S/C19H17Cl2N3O2/c20-14-5-3-4-13(17(14)21)18(25)19-22-15(12-23-8-10-26-11-9-23)16-6-1-2-7-24(16)19/h1-7H,8-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM154517

(ML299 (5))Show SMILES C[C@@H](CN1CCC2(CC1)N(CNC2=O)c1cccc(F)c1)NC(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C23H26BrFN4O2/c1-16(27-21(30)17-5-7-18(24)8-6-17)14-28-11-9-23(10-12-28)22(31)26-15-29(23)20-4-2-3-19(25)13-20/h2-8,13,16H,9-12,14-15H2,1H3,(H,26,31)(H,27,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 (unknown origin) |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Neuropeptide S receptor
(Homo sapiens (Human)) | BDBM50322836

(CHEMBL1210244 | rac-N-(3-methyl-1-(piperidin-1-yl)...)Show SMILES CCC(C)(CCN1CCCCC1)N(Cc1cc2ccccc2n(C)c1=O)C(=O)C1CCCCC1 Show InChI InChI=1S/C29H43N3O2/c1-4-29(2,17-20-31-18-11-6-12-19-31)32(28(34)23-13-7-5-8-14-23)22-25-21-24-15-9-10-16-26(24)30(3)27(25)33/h9-10,15-16,21,23H,4-8,11-14,17-20,22H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... |
Bioorg Med Chem Lett 20: 4700-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.143
BindingDB Entry DOI: 10.7270/Q2PR7W6H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340316
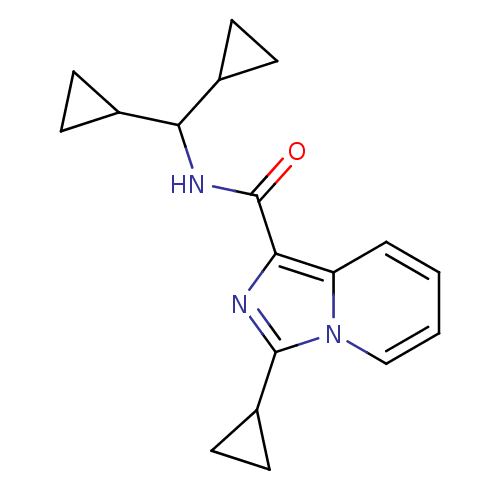
(3-cyclopropyl-N-(dicyclopropylmethyl)imidazo[1,5-a...)Show InChI InChI=1S/C18H21N3O/c22-18(20-15(11-4-5-11)12-6-7-12)16-14-3-1-2-10-21(14)17(19-16)13-8-9-13/h1-3,10-13,15H,4-9H2,(H,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235240
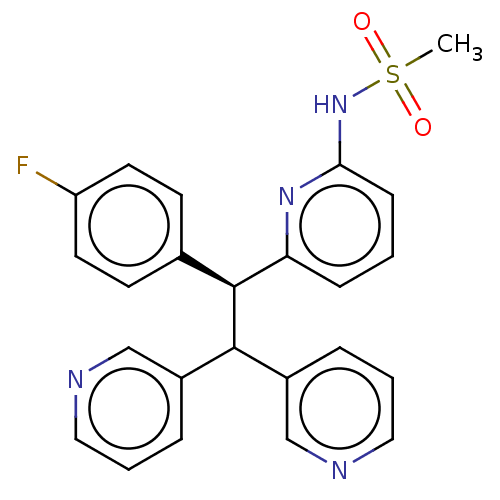
(CHEMBL4065169)Show SMILES CS(=O)(=O)Nc1cccc(n1)[C@@H](C(c1cccnc1)c1cccnc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN4O2S/c1-32(30,31)29-22-8-2-7-21(28-22)24(17-9-11-20(25)12-10-17)23(18-5-3-13-26-15-18)19-6-4-14-27-16-19/h2-16,23-24H,1H3,(H,28,29)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity towards LTB4 receptor was evaluated by inhibition of binding of [3H]LTB4 to human neutrophils |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Neuropeptide S receptor
(Homo sapiens (Human)) | BDBM50322839
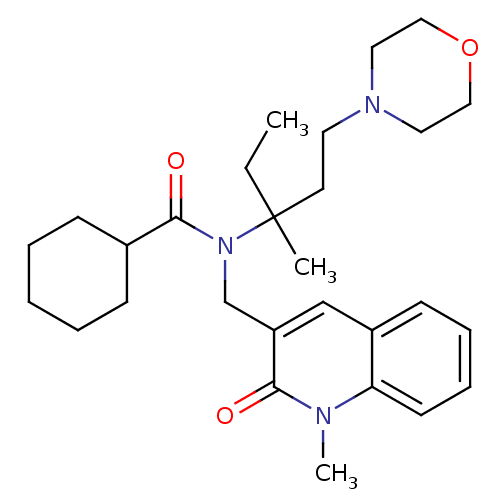
(CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...)Show SMILES CCC(C)(CCN1CCOCC1)N(Cc1cc2ccccc2n(C)c1=O)C(=O)C1CCCCC1 Show InChI InChI=1S/C28H41N3O3/c1-4-28(2,14-15-30-16-18-34-19-17-30)31(27(33)22-10-6-5-7-11-22)21-24-20-23-12-8-9-13-25(23)29(3)26(24)32/h8-9,12-13,20,22H,4-7,10-11,14-19,21H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... |
Bioorg Med Chem Lett 20: 4700-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.143
BindingDB Entry DOI: 10.7270/Q2PR7W6H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50340310

((2,3-dichlorophenyl)(1-(morpholinomethyl)imidazo[1...)Show SMILES Clc1cccc(C(=O)c2nc(CN3CCOCC3)c3ccccn23)c1Cl Show InChI InChI=1S/C19H17Cl2N3O2/c20-14-5-3-4-13(17(14)21)18(25)19-22-15(12-23-8-10-26-11-9-23)16-6-1-2-7-24(16)19/h1-7H,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at rat CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235250

(CHEMBL4104525)Show SMILES O[C@H](C(c1cccnc1)c1cccnc1)c1cccnc1-c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H17Cl2N3O/c24-18-10-17(11-19(25)12-18)22-20(6-3-9-28-22)23(29)21(15-4-1-7-26-13-15)16-5-2-8-27-14-16/h1-14,21,23,29H/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 mediated ultra-rapid delayed rectifier current Ikur in human atrial myocytes by voltage-patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235240

(CHEMBL4065169)Show SMILES CS(=O)(=O)Nc1cccc(n1)[C@@H](C(c1cccnc1)c1cccnc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN4O2S/c1-32(30,31)29-22-8-2-7-21(28-22)24(17-9-11-20(25)12-10-17)23(18-5-3-13-26-15-18)19-6-4-14-27-16-19/h2-16,23-24H,1H3,(H,28,29)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 mediated ultra-rapid delayed rectifier current Ikur in human atrial myocytes by voltage-patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Phospholipase D2
(Homo sapiens (Human)) | BDBM50507325

(CHEMBL4453586)Show SMILES C[C@@H](CN1CCC2(CC1)[C@@H](CNC2=O)c1ccc(F)cc1)NC(=O)c1cc2ccccc2[nH]1 |r| Show InChI InChI=1S/C26H29FN4O2/c1-17(29-24(32)23-14-19-4-2-3-5-22(19)30-23)16-31-12-10-26(11-13-31)21(15-28-25(26)33)18-6-8-20(27)9-7-18/h2-9,14,17,21,30H,10-13,15-16H2,1H3,(H,28,33)(H,29,32)/t17-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of GFP-fused PLD2 (unknown origin) expressed in HEK293 cells by cellular assay |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Neuropeptide S receptor
(Homo sapiens (Human)) | BDBM50322837
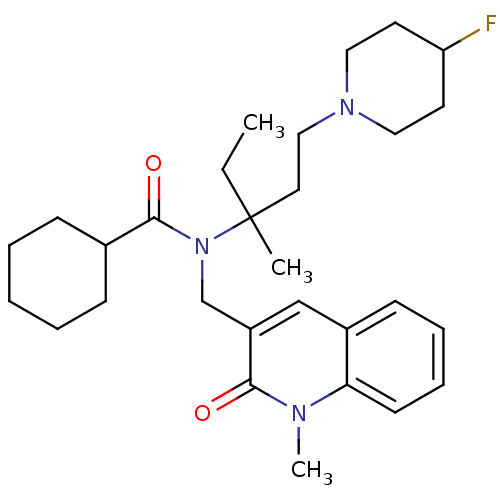
(CHEMBL1210311 | rac-N-(1-(4-fluoropiperidin-1-yl)-...)Show SMILES CCC(C)(CCN1CCC(F)CC1)N(Cc1cc2ccccc2n(C)c1=O)C(=O)C1CCCCC1 Show InChI InChI=1S/C29H42FN3O2/c1-4-29(2,16-19-32-17-14-25(30)15-18-32)33(28(35)22-10-6-5-7-11-22)21-24-20-23-12-8-9-13-26(23)31(3)27(24)34/h8-9,12-13,20,22,25H,4-7,10-11,14-19,21H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... |
Bioorg Med Chem Lett 20: 4700-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.143
BindingDB Entry DOI: 10.7270/Q2PR7W6H |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50507322
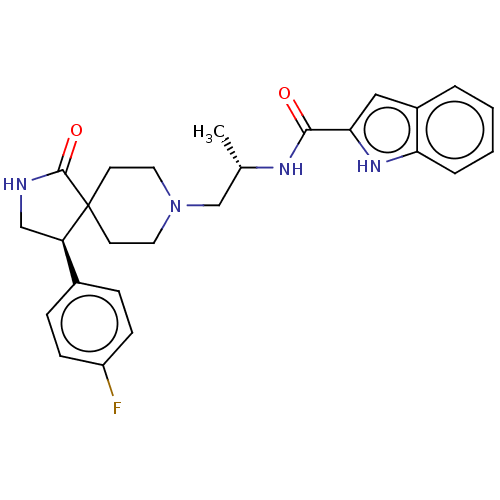
(CHEMBL4448918)Show SMILES C[C@@H](CN1CCC2(CC1)[C@H](CNC2=O)c1ccc(F)cc1)NC(=O)c1cc2ccccc2[nH]1 |r| Show InChI InChI=1S/C26H29FN4O2/c1-17(29-24(32)23-14-19-4-2-3-5-22(19)30-23)16-31-12-10-26(11-13-31)21(15-28-25(26)33)18-6-8-20(27)9-7-18/h2-9,14,17,21,30H,10-13,15-16H2,1H3,(H,28,33)(H,29,32)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 in human Calu1 cells |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Catechol O-methyltransferase
(Rattus norvegicus (Rat)) | BDBM50086213
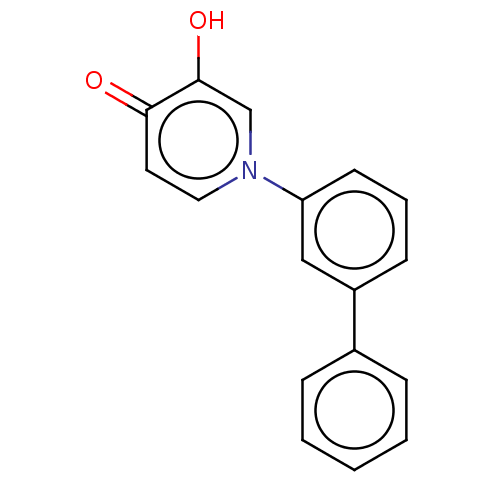
(CHEMBL3425734)Show InChI InChI=1S/C17H13NO2/c19-16-9-10-18(12-17(16)20)15-8-4-7-14(11-15)13-5-2-1-3-6-13/h1-12,20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat membrane bound COMT expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL using dopamine/SAM as substrate/cofactor pre... |
ACS Med Chem Lett 6: 318-23 (2015)
Article DOI: 10.1021/ml500502d
BindingDB Entry DOI: 10.7270/Q2K35WDZ |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50507327

(CHEMBL4514153)Show SMILES C[C@@H](CN1CCC2(CC1)[C@@H](CNC2=O)c1ccc(F)cc1)NC(=O)C#Cc1ccccc1 |r| Show InChI InChI=1S/C26H28FN3O2/c1-19(29-24(31)12-7-20-5-3-2-4-6-20)18-30-15-13-26(14-16-30)23(17-28-25(26)32)21-8-10-22(27)11-9-21/h2-6,8-11,19,23H,13-18H2,1H3,(H,28,32)(H,29,31)/t19-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 in human Calu1 cells |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50395966
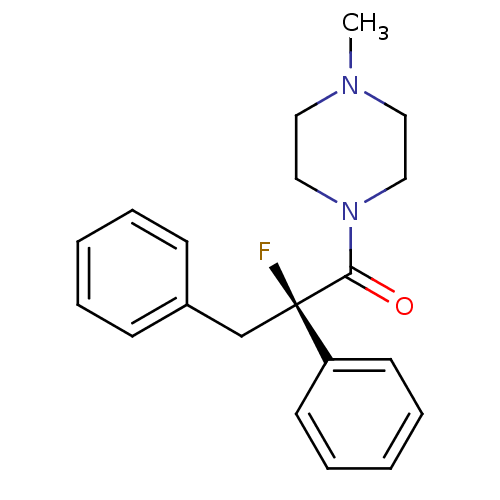
(CHEMBL2164905)Show SMILES CN1CCN(CC1)C(=O)[C@@](F)(Cc1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C20H23FN2O/c1-22-12-14-23(15-13-22)19(24)20(21,18-10-6-3-7-11-18)16-17-8-4-2-5-9-17/h2-11H,12-16H2,1H3/t20-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M1 receptor |
Bioorg Med Chem Lett 22: 6923-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.011
BindingDB Entry DOI: 10.7270/Q2348MH4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50395966

(CHEMBL2164905)Show SMILES CN1CCN(CC1)C(=O)[C@@](F)(Cc1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C20H23FN2O/c1-22-12-14-23(15-13-22)19(24)20(21,18-10-6-3-7-11-18)16-17-8-4-2-5-9-17/h2-11H,12-16H2,1H3/t20-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M1 receptor |
Bioorg Med Chem Lett 22: 6923-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.011
BindingDB Entry DOI: 10.7270/Q2348MH4 |
More data for this
Ligand-Target Pair | |
Neuropeptide S receptor
(Homo sapiens (Human)) | BDBM50322842
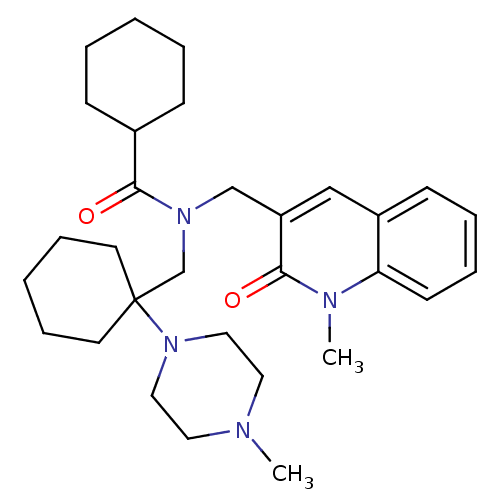
(CHEMBL1210177 | N-((1-methyl-2-oxo-1,2-dihydroquin...)Show SMILES CN1CCN(CC1)C1(CN(Cc2cc3ccccc3n(C)c2=O)C(=O)C2CCCCC2)CCCCC1 Show InChI InChI=1S/C30H44N4O2/c1-31-17-19-34(20-18-31)30(15-9-4-10-16-30)23-33(29(36)24-11-5-3-6-12-24)22-26-21-25-13-7-8-14-27(25)32(2)28(26)35/h7-8,13-14,21,24H,3-6,9-12,15-20,22-23H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... |
Bioorg Med Chem Lett 20: 4700-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.143
BindingDB Entry DOI: 10.7270/Q2PR7W6H |
More data for this
Ligand-Target Pair | |
Catechol O-methyltransferase
(Rattus norvegicus (Rat)) | BDBM50086214

(CHEMBL3425744)Show InChI InChI=1S/C17H14BNO4/c20-16-11-19(10-15(17(16)21)18(22)23)14-8-4-7-13(9-14)12-5-2-1-3-6-12/h1-11,20,22-23H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat membrane bound COMT expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL using dopamine/SAM as substrate/cofactor pre... |
ACS Med Chem Lett 6: 318-23 (2015)
Article DOI: 10.1021/ml500502d
BindingDB Entry DOI: 10.7270/Q2K35WDZ |
More data for this
Ligand-Target Pair | |
Catechol O-methyltransferase
(Rattus norvegicus (Rat)) | BDBM50086210
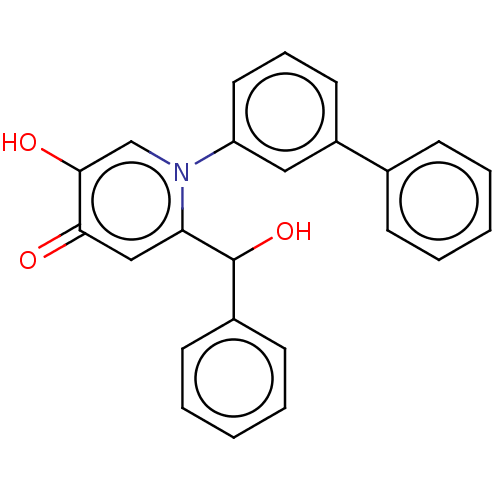
(CHEMBL3425739)Show SMILES OC(c1ccccc1)c1cc(=O)c(O)cn1-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C24H19NO3/c26-22-15-21(24(28)18-10-5-2-6-11-18)25(16-23(22)27)20-13-7-12-19(14-20)17-8-3-1-4-9-17/h1-16,24,27-28H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat membrane bound COMT expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL using dopamine/SAM as substrate/cofactor pre... |
ACS Med Chem Lett 6: 318-23 (2015)
Article DOI: 10.1021/ml500502d
BindingDB Entry DOI: 10.7270/Q2K35WDZ |
More data for this
Ligand-Target Pair | |
Phospholipase D2
(Homo sapiens (Human)) | BDBM87120

(CHEMBL1254577 | N-[2-[1-(3-fluorophenyl)-4-keto-1,...)Show SMILES Fc1cccc(c1)N1CNC(=O)C11CCN(CCNC(=O)c2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C26H27FN4O2/c27-22-6-3-7-23(17-22)31-18-29-25(33)26(31)10-13-30(14-11-26)15-12-28-24(32)21-9-8-19-4-1-2-5-20(19)16-21/h1-9,16-17H,10-15,18H2,(H,28,32)(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD2 (unknown origin) |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Phospholipase D2
(Homo sapiens (Human)) | BDBM154517

(ML299 (5))Show SMILES C[C@@H](CN1CCC2(CC1)N(CNC2=O)c1cccc(F)c1)NC(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C23H26BrFN4O2/c1-16(27-21(30)17-5-7-18(24)8-6-17)14-28-11-9-23(10-12-28)22(31)26-15-29(23)20-4-2-3-19(25)13-20/h2-8,13,16H,9-12,14-15H2,1H3,(H,26,31)(H,27,30)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD2 (unknown origin) |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340317
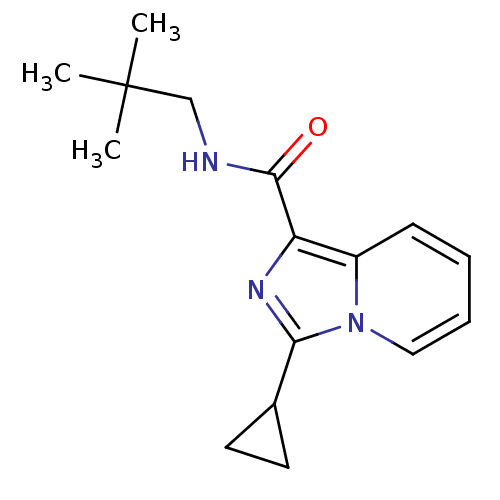
(3-cyclopropyl-N-neopentylimidazo[1,5-a]pyridine-1-...)Show InChI InChI=1S/C16H21N3O/c1-16(2,3)10-17-15(20)13-12-6-4-5-9-19(12)14(18-13)11-7-8-11/h4-6,9,11H,7-8,10H2,1-3H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50206160
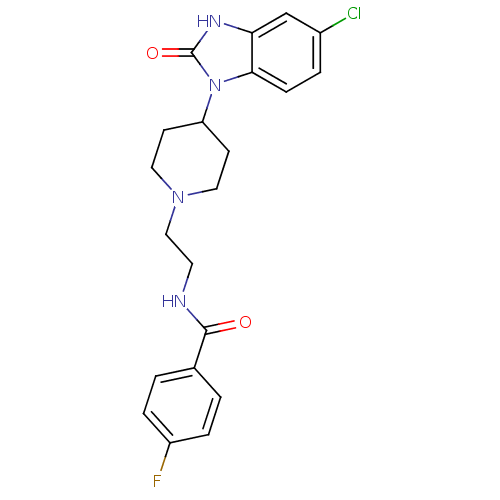
(CHEMBL245621 | Halopemide | Halopemide, 8 | N-(2-(...)Show SMILES Fc1ccc(cc1)C(=O)NCCN1CCC(CC1)n1c2ccc(Cl)cc2[nH]c1=O Show InChI InChI=1S/C21H22ClFN4O2/c22-15-3-6-19-18(13-15)25-21(29)27(19)17-7-10-26(11-8-17)12-9-24-20(28)14-1-4-16(23)5-2-14/h1-6,13,17H,7-12H2,(H,24,28)(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 (unknown origin) |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50507332

(CHEMBL4475943)Show SMILES Fc1ccc(cc1)[C@@H]1CNC(=O)C11CCN(CCNC(=O)c2cc3ccccc3[nH]2)CC1 |r| Show InChI InChI=1S/C25H27FN4O2/c26-19-7-5-17(6-8-19)20-16-28-24(32)25(20)9-12-30(13-10-25)14-11-27-23(31)22-15-18-3-1-2-4-21(18)29-22/h1-8,15,20,29H,9-14,16H2,(H,27,31)(H,28,32)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 in human Calu1 cells |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340318

((R)-3-morpholino-N-(2,2,2-trifluoro-1-(pyridin-2-y...)Show SMILES FC(F)(F)[C@H](NC(=O)c1nc(N2CCOCC2)n2ccccc12)c1ccccn1 |r| Show InChI InChI=1S/C19H18F3N5O2/c20-19(21,22)16(13-5-1-3-7-23-13)25-17(28)15-14-6-2-4-8-27(14)18(24-15)26-9-11-29-12-10-26/h1-8,16H,9-12H2,(H,25,28)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Neuropeptide S receptor
(Homo sapiens (Human)) | BDBM50322838
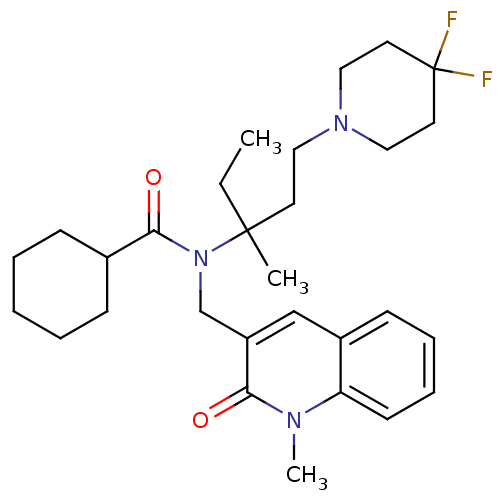
(CHEMBL1210312 | rac-N-(1-(4,4-difluoropiperidin-1-...)Show SMILES CCC(C)(CCN1CCC(F)(F)CC1)N(Cc1cc2ccccc2n(C)c1=O)C(=O)C1CCCCC1 Show InChI InChI=1S/C29H41F2N3O2/c1-4-28(2,14-17-33-18-15-29(30,31)16-19-33)34(27(36)22-10-6-5-7-11-22)21-24-20-23-12-8-9-13-25(23)32(3)26(24)35/h8-9,12-13,20,22H,4-7,10-11,14-19,21H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... |
Bioorg Med Chem Lett 20: 4700-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.143
BindingDB Entry DOI: 10.7270/Q2PR7W6H |
More data for this
Ligand-Target Pair | |
Neuropeptide S receptor
(Homo sapiens (Human)) | BDBM50322839

(CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...)Show SMILES CCC(C)(CCN1CCOCC1)N(Cc1cc2ccccc2n(C)c1=O)C(=O)C1CCCCC1 Show InChI InChI=1S/C28H41N3O3/c1-4-28(2,14-15-30-16-18-34-19-17-30)31(27(33)22-10-6-5-7-11-22)21-24-20-23-12-8-9-13-25(23)29(3)26(24)32/h8-9,12-13,20,22H,4-7,10-11,14-19,21H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... |
Bioorg Med Chem Lett 20: 4700-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.143
BindingDB Entry DOI: 10.7270/Q2PR7W6H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235255

(CHEMBL4085436)Show InChI InChI=1S/C23H18ClN3/c24-22-10-2-1-9-20(22)23-17(6-5-13-27-23)14-21(18-7-3-11-25-15-18)19-8-4-12-26-16-19/h1-13,15-16,21H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Neuropeptide S receptor
(Homo sapiens (Human)) | BDBM50322839

(CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...)Show SMILES CCC(C)(CCN1CCOCC1)N(Cc1cc2ccccc2n(C)c1=O)C(=O)C1CCCCC1 Show InChI InChI=1S/C28H41N3O3/c1-4-28(2,14-15-30-16-18-34-19-17-30)31(27(33)22-10-6-5-7-11-22)21-24-20-23-12-8-9-13-25(23)29(3)26(24)32/h8-9,12-13,20,22H,4-7,10-11,14-19,21H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... |
Bioorg Med Chem Lett 20: 4700-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.143
BindingDB Entry DOI: 10.7270/Q2PR7W6H |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50507324
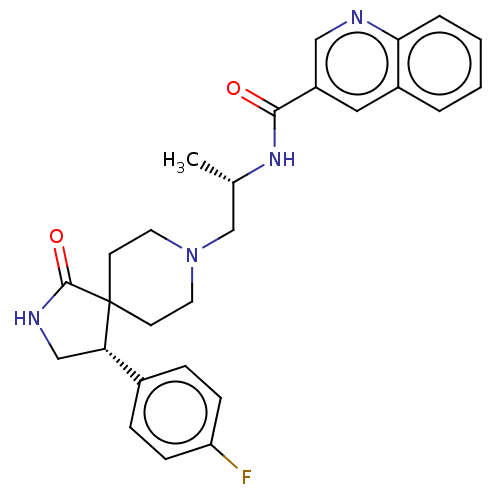
(CHEMBL4570440)Show SMILES C[C@@H](CN1CCC2(CC1)[C@@H](CNC2=O)c1ccc(F)cc1)NC(=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C27H29FN4O2/c1-18(31-25(33)21-14-20-4-2-3-5-24(20)29-15-21)17-32-12-10-27(11-13-32)23(16-30-26(27)34)19-6-8-22(28)9-7-19/h2-9,14-15,18,23H,10-13,16-17H2,1H3,(H,30,34)(H,31,33)/t18-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 in human Calu1 cells |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Phospholipase D2
(Homo sapiens (Human)) | BDBM50507321
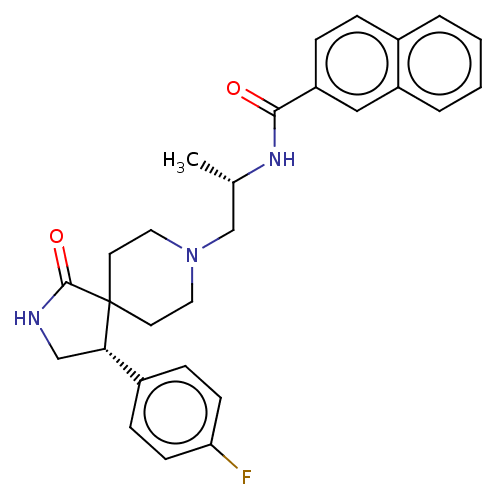
(CHEMBL4594070)Show SMILES C[C@@H](CN1CCC2(CC1)[C@@H](CNC2=O)c1ccc(F)cc1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H30FN3O2/c1-19(31-26(33)23-7-6-20-4-2-3-5-22(20)16-23)18-32-14-12-28(13-15-32)25(17-30-27(28)34)21-8-10-24(29)11-9-21/h2-11,16,19,25H,12-15,17-18H2,1H3,(H,30,34)(H,31,33)/t19-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of GFP-fused PLD2 (unknown origin) expressed in HEK293 cells by cellular assay |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235259
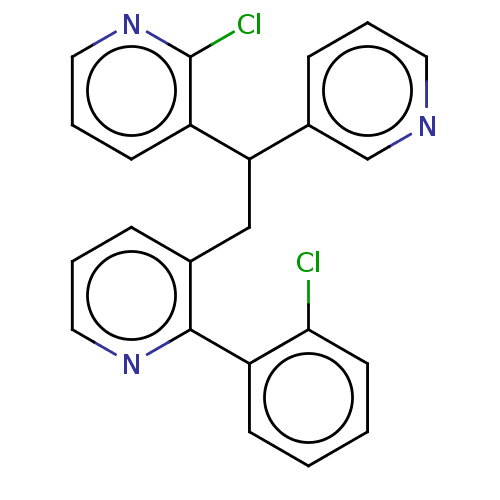
(CHEMBL4105245)Show InChI InChI=1S/C23H17Cl2N3/c24-21-10-2-1-8-19(21)22-16(6-4-12-27-22)14-20(17-7-3-11-26-15-17)18-9-5-13-28-23(18)25/h1-13,15,20H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Sorbitol dehydrogenase
(Homo sapiens (Human)) | BDBM50102724
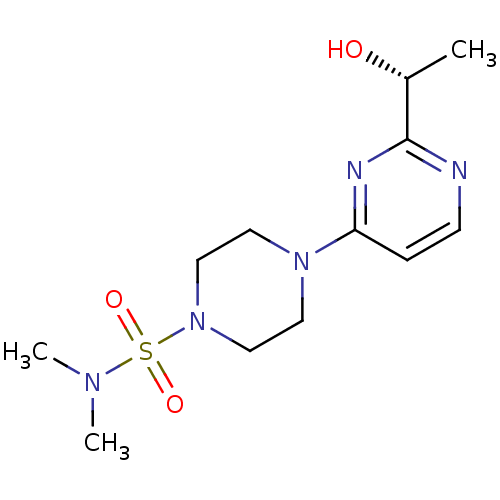
(4-[2-(1-Hydroxy-ethyl)-pyrimidin-4-yl]-piperazine-...)Show SMILES C[C@@H](O)c1nccc(n1)N1CCN(CC1)S(=O)(=O)N(C)C Show InChI InChI=1S/C12H21N5O3S/c1-10(18)12-13-5-4-11(14-12)16-6-8-17(9-7-16)21(19,20)15(2)3/h4-5,10,18H,6-9H2,1-3H3/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human SDH |
J Med Chem 44: 2695-700 (2001)
BindingDB Entry DOI: 10.7270/Q21G0KJZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235250

(CHEMBL4104525)Show SMILES O[C@H](C(c1cccnc1)c1cccnc1)c1cccnc1-c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H17Cl2N3O/c24-18-10-17(11-19(25)12-18)22-20(6-3-9-28-22)23(29)21(15-4-1-7-26-13-15)16-5-2-8-27-14-16/h1-14,21,23,29H/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50395965

(CHEMBL2164910)Show InChI InChI=1S/C20H23FN2O/c1-22-12-14-23(15-13-22)19(24)20(21,18-10-6-3-7-11-18)16-17-8-4-2-5-9-17/h2-11H,12-16H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M1 receptor |
Bioorg Med Chem Lett 22: 6923-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.011
BindingDB Entry DOI: 10.7270/Q2348MH4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235275
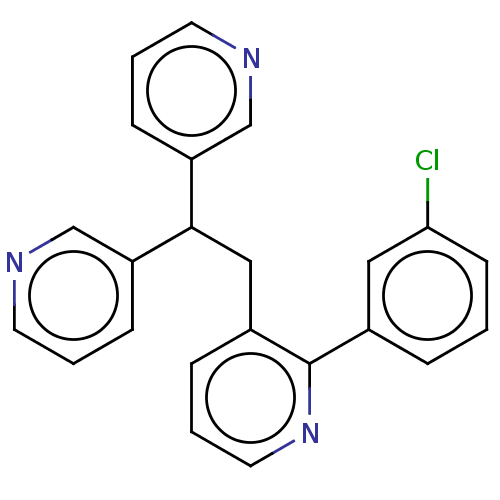
(CHEMBL4062996)Show InChI InChI=1S/C23H18ClN3/c24-21-9-1-5-17(13-21)23-18(6-4-12-27-23)14-22(19-7-2-10-25-15-19)20-8-3-11-26-16-20/h1-13,15-16,22H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Neuropeptide S receptor
(Homo sapiens (Human)) | BDBM50322834

(CHEMBL1210242 | N-((1-methyl-2-oxo-1,2-dihydroquin...)Show SMILES Cn1c2ccccc2cc(CN(C(=O)C2CCCCC2)C(C)(C)CCN2CCCCC2)c1=O Show InChI InChI=1S/C28H41N3O2/c1-28(2,16-19-30-17-10-5-11-18-30)31(27(33)22-12-6-4-7-13-22)21-24-20-23-14-8-9-15-25(23)29(3)26(24)32/h8-9,14-15,20,22H,4-7,10-13,16-19,21H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... |
Bioorg Med Chem Lett 20: 4700-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.143
BindingDB Entry DOI: 10.7270/Q2PR7W6H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340319

(CHEMBL1760939 | N-((1-(hydroxymethyl)cyclopentyl)m...)Show InChI InChI=1S/C19H26N4O3/c24-14-19(6-2-3-7-19)13-20-17(25)16-15-5-1-4-8-23(15)18(21-16)22-9-11-26-12-10-22/h1,4-5,8,24H,2-3,6-7,9-14H2,(H,20,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235253
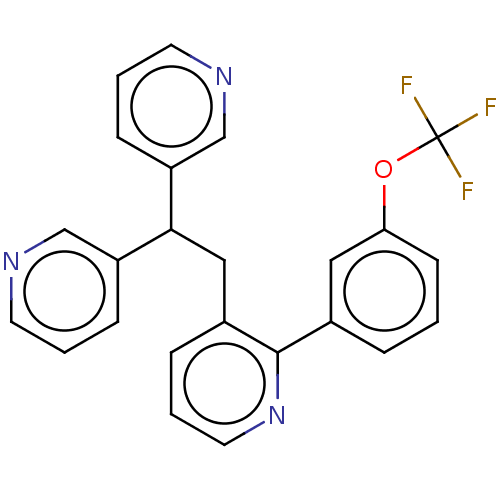
(CHEMBL4075394)Show SMILES FC(F)(F)Oc1cccc(c1)-c1ncccc1CC(c1cccnc1)c1cccnc1 Show InChI InChI=1S/C24H18F3N3O/c25-24(26,27)31-21-9-1-5-17(13-21)23-18(6-4-12-30-23)14-22(19-7-2-10-28-15-19)20-8-3-11-29-16-20/h1-13,15-16,22H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50396000

(CHEMBL2164906)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C20H24N2O/c1-21-12-14-22(15-13-21)20(23)19(18-10-6-3-7-11-18)16-17-8-4-2-5-9-17/h2-11,19H,12-16H2,1H3/t19-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M1 receptor |
Bioorg Med Chem Lett 22: 6923-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.011
BindingDB Entry DOI: 10.7270/Q2348MH4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340321
((R)-1-(4,4-difluoropiperidin-1-yl)-N-(3,3-dimethyl...)Show SMILES C[C@@H](NC(=O)c1nc(N2CCC(F)(F)CC2)c2cnccn12)C(C)(C)C |r| Show InChI InChI=1S/C18H25F2N5O/c1-12(17(2,3)4)22-16(26)15-23-14(13-11-21-7-10-25(13)15)24-8-5-18(19,20)6-9-24/h7,10-12H,5-6,8-9H2,1-4H3,(H,22,26)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data