

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50456028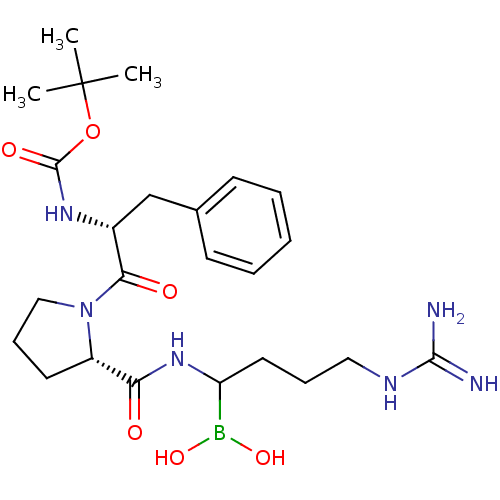 (CHEMBL2028993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition constant for binding with thrombin was determined | J Med Chem 36: 1831-8 (1993) BindingDB Entry DOI: 10.7270/Q21G0KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12693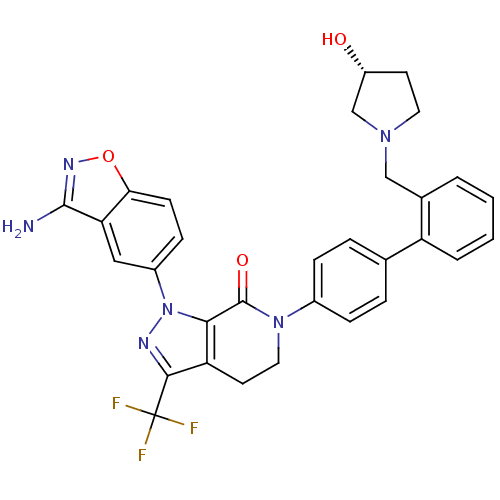 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069922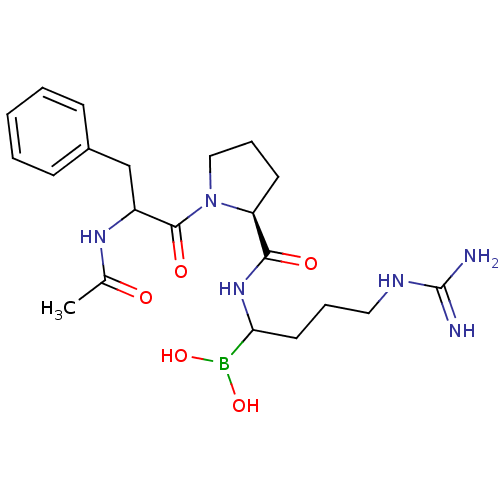 (Boropeptide analogue | CHEMBL102069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the binding affinity against thrombin | Bioorg Med Chem Lett 7: 79-84 (1997) Checked by Author Article DOI: 10.1016/S0960-894X(96)00584-7 BindingDB Entry DOI: 10.7270/Q2FN16P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12681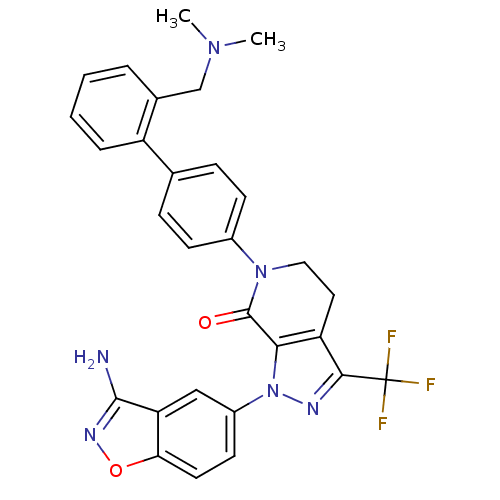 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288406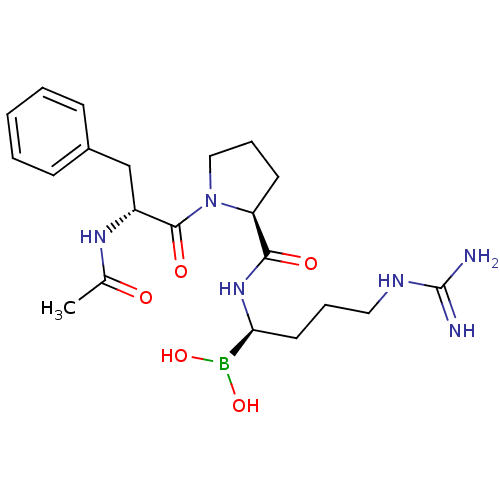 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 7: 1595-1600 (1997) Article DOI: 10.1016/S0960-894X(97)00254-0 BindingDB Entry DOI: 10.7270/Q2VQ32PJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288632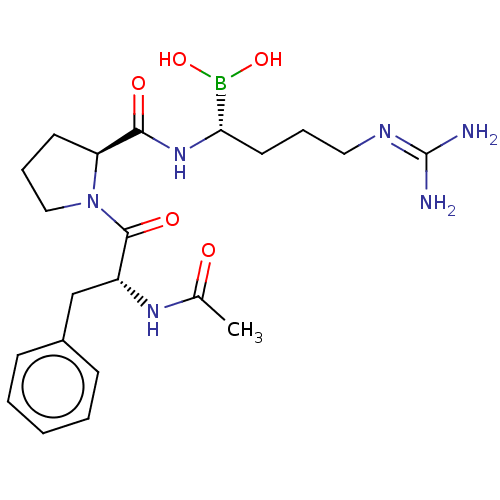 (Boropeptide | CHEMBL607008) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288632 (Boropeptide | CHEMBL607008) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity towards thrombin was tested. | Bioorg Med Chem Lett 8: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2542MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288406 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro for inhibition of thrombin. | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50451005 (CHEMBL290376 | DuP-714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition constant for binding with thrombin was determined | J Med Chem 36: 1831-8 (1993) BindingDB Entry DOI: 10.7270/Q21G0KB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50288406 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of trypsin | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50288406 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 7: 1595-1600 (1997) Article DOI: 10.1016/S0960-894X(97)00254-0 BindingDB Entry DOI: 10.7270/Q2VQ32PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50069922 (Boropeptide analogue | CHEMBL102069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for selectivity against trypsin | Bioorg Med Chem Lett 7: 79-84 (1997) Checked by Author Article DOI: 10.1016/S0960-894X(96)00584-7 BindingDB Entry DOI: 10.7270/Q2FN16P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069928 (Boropeptide analogue | CHEMBL102385) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity towards thrombin was tested. | Bioorg Med Chem Lett 8: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2542MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069926 (Boropeptide analogue | CHEMBL105137) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity towards thrombin was tested. | Bioorg Med Chem Lett 8: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2542MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288621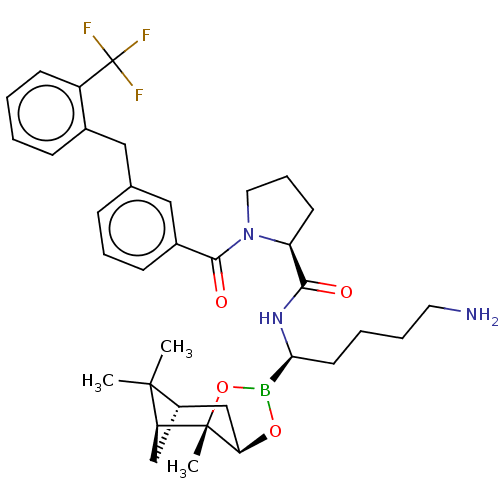 ((S)-1-[3-(2-Trifluoromethyl-benzyl)-benzoyl]-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288628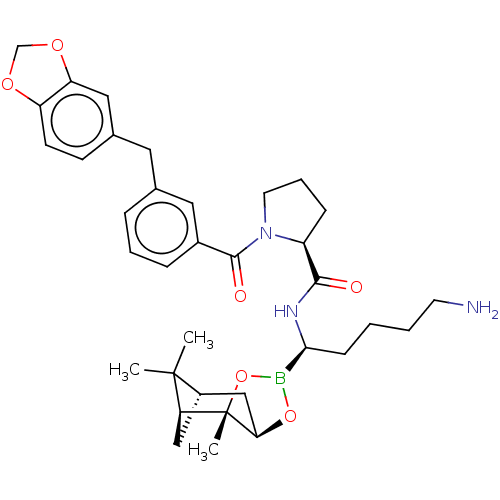 ((S)-1-(3-Benzo[1,3]dioxol-5-ylmethyl-benzoyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50000020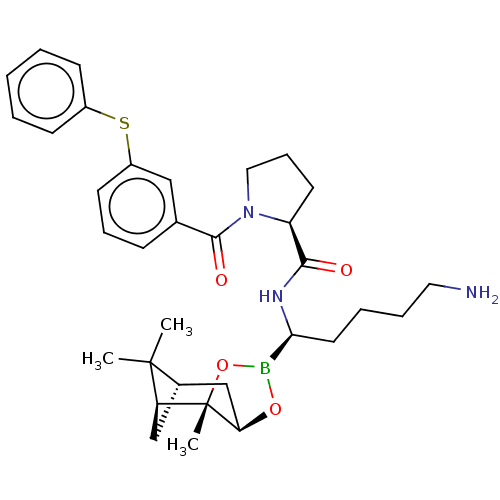 (CHEMBL2448361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12690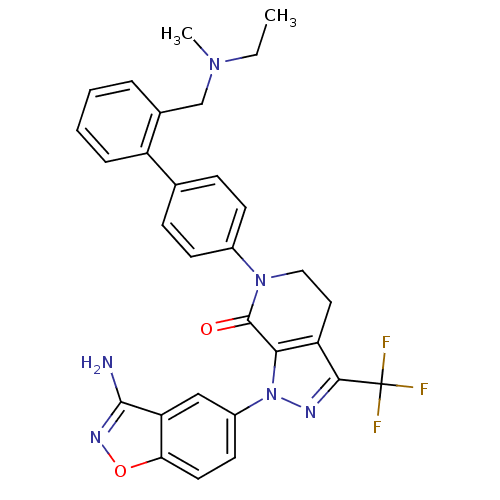 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[ethyl(me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.100 | <-56.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50289586 (3-Methyl-2'-sulfamoyl-biphenyl-4-carboxylic acid [...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 7: 1595-1600 (1997) Article DOI: 10.1016/S0960-894X(97)00254-0 BindingDB Entry DOI: 10.7270/Q2VQ32PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12689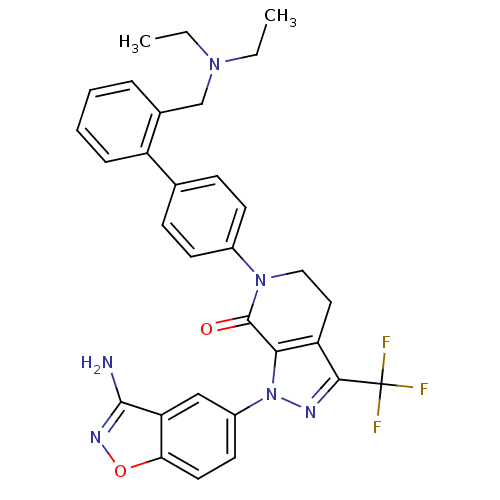 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(diethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12694 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3S)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12682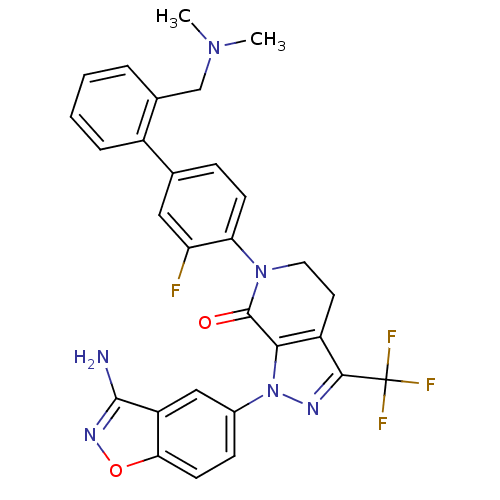 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12692 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288631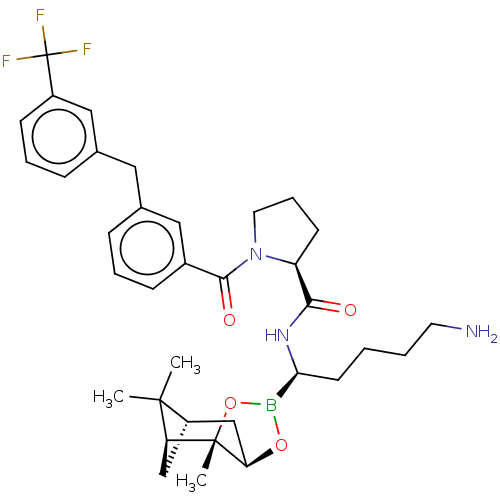 ((S)-1-[3-(3-Trifluoromethyl-benzyl)-benzoyl]-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288405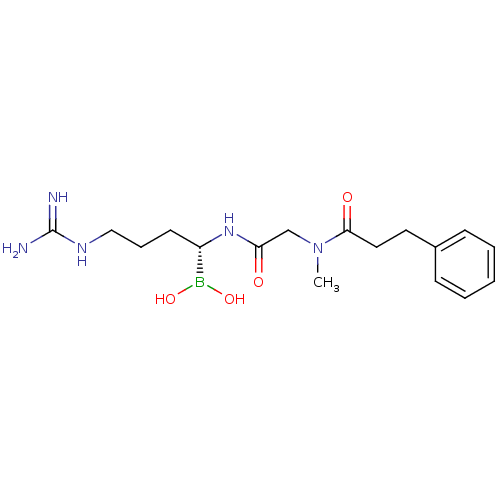 (CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro for inhibition of thrombin. | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12700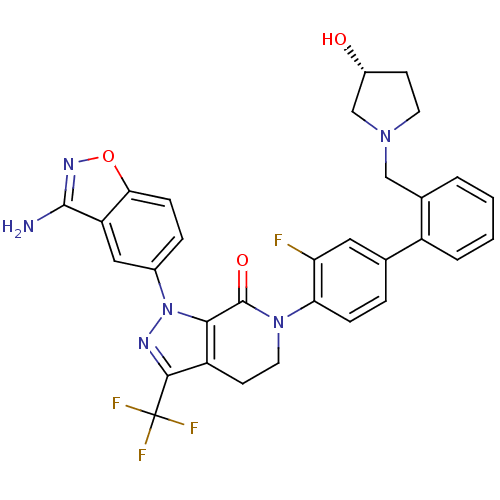 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288623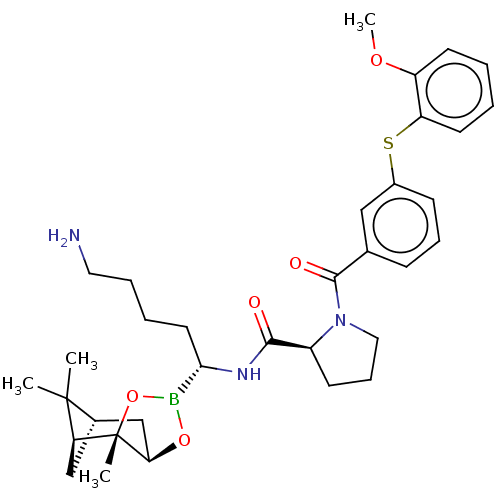 ((S)-1-[3-(2-Methoxy-phenylsulfanyl)-benzoyl]-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288634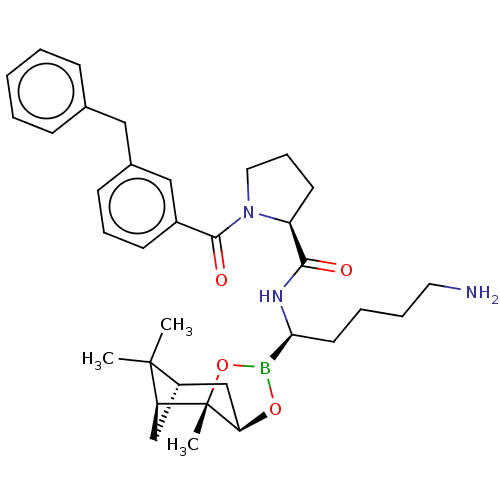 ((S)-1-(3-Benzyl-benzoyl)-pyrrolidine-2-carboxylic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50084682 (1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 7: 1595-1600 (1997) Article DOI: 10.1016/S0960-894X(97)00254-0 BindingDB Entry DOI: 10.7270/Q2VQ32PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12695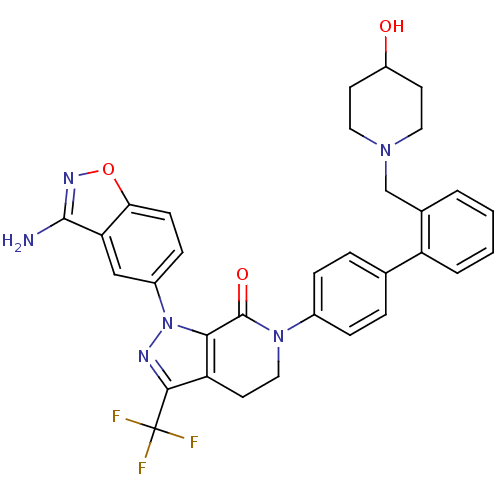 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -54.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288627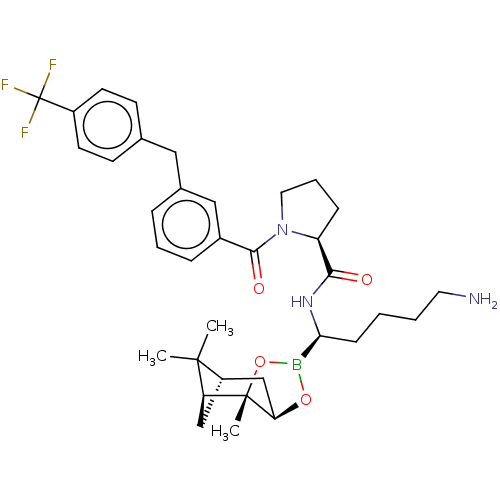 ((S)-1-[3-(4-Trifluoromethyl-benzyl)-benzoyl]-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288629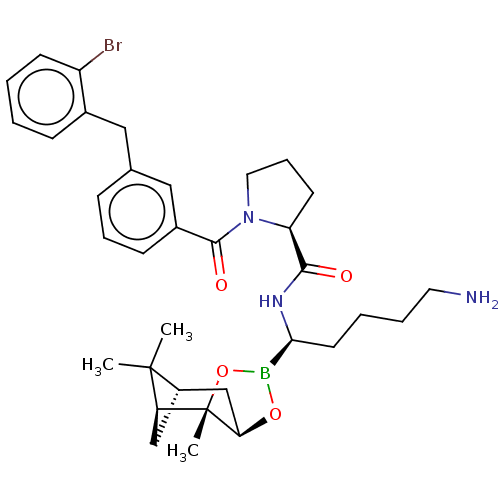 ((S)-1-[3-(2-Bromo-benzyl)-benzoyl]-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288620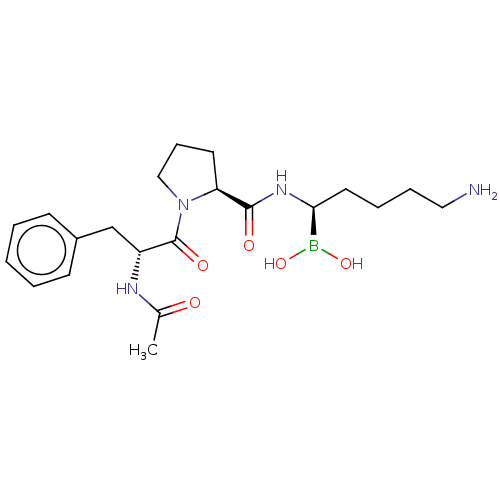 (Boropeptide | CHEMBL3038261) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12699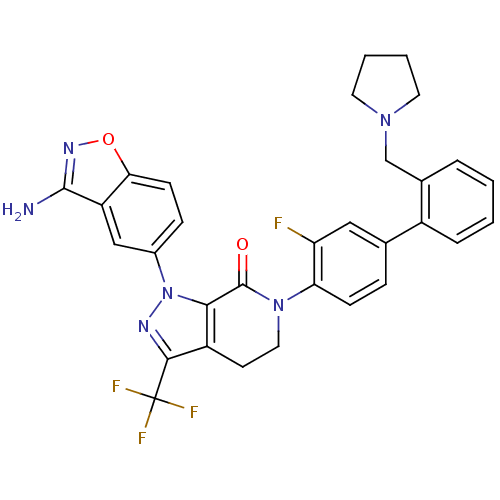 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{2-fluoro-4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288414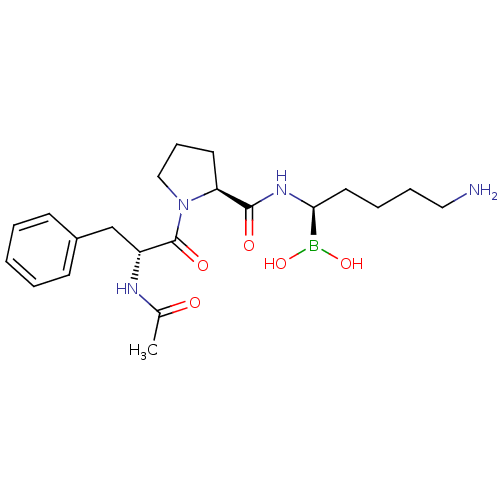 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro for inhibition of thrombin. | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12687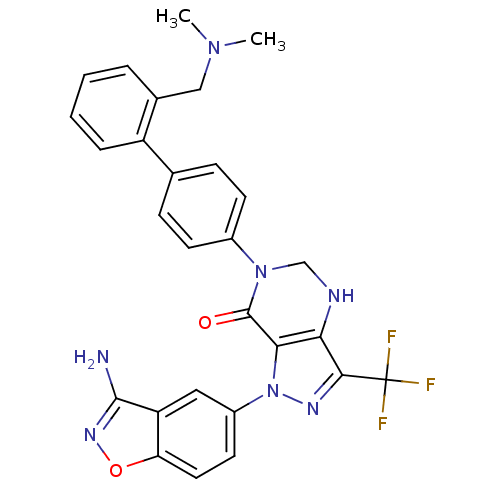 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | -54.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288637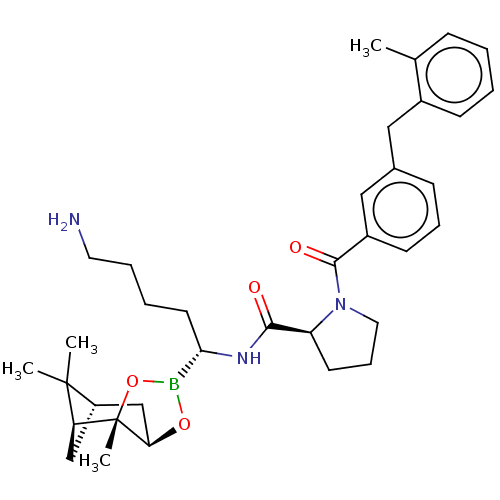 ((S)-1-[3-(2-Methyl-benzyl)-benzoyl]-pyrrolidine-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12688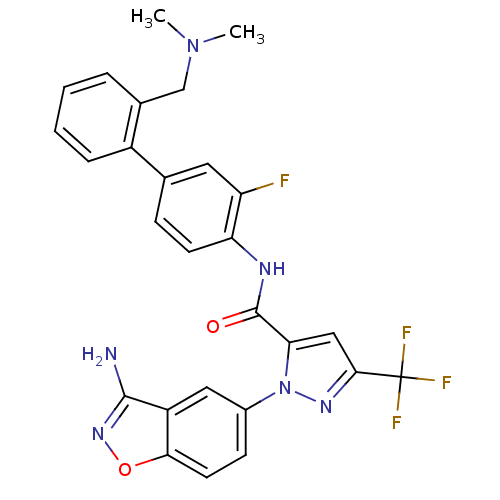 (1-(3-amino-1,2-benzoxazol-5-yl)-N-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288630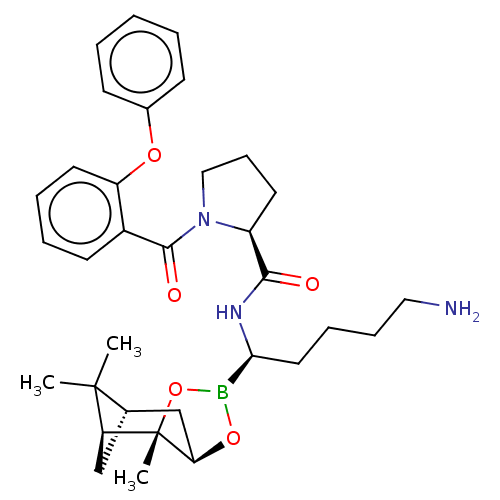 ((S)-1-(2-Phenoxy-benzoyl)-pyrrolidine-2-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12703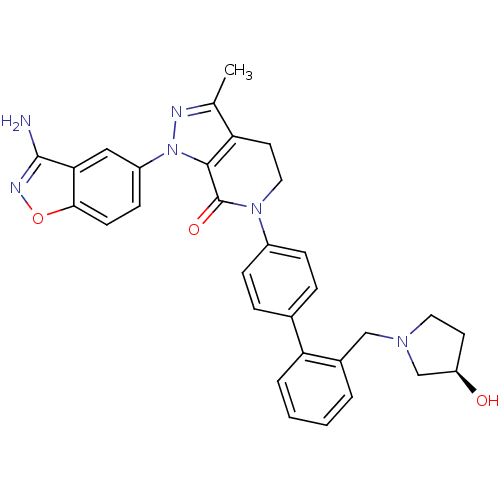 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069933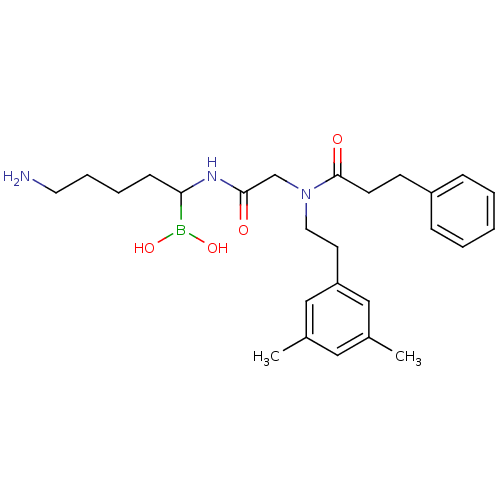 (Boropeptide analogue | CHEMBL102916) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity towards thrombin was tested. | Bioorg Med Chem Lett 8: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2542MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288636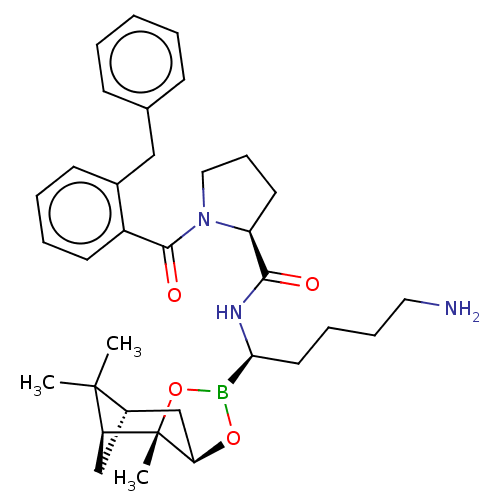 ((S)-1-(2-Benzyl-benzoyl)-pyrrolidine-2-carboxylic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069172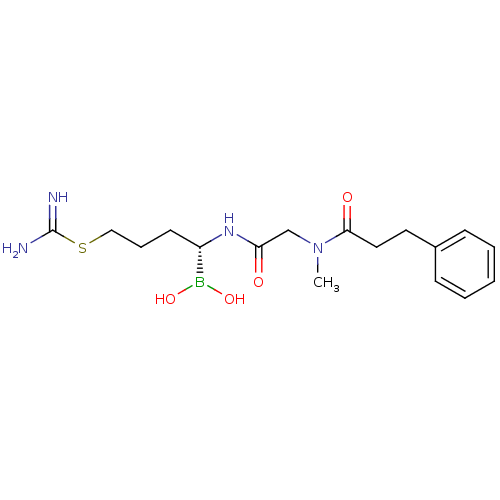 (Boronate Ester analogue | CHEMBL423259) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition against human thrombin | Bioorg Med Chem Lett 8: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2P84B1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069935 (Boropeptide analogue | CHEMBL320213) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity towards thrombin was tested. | Bioorg Med Chem Lett 8: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2542MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12702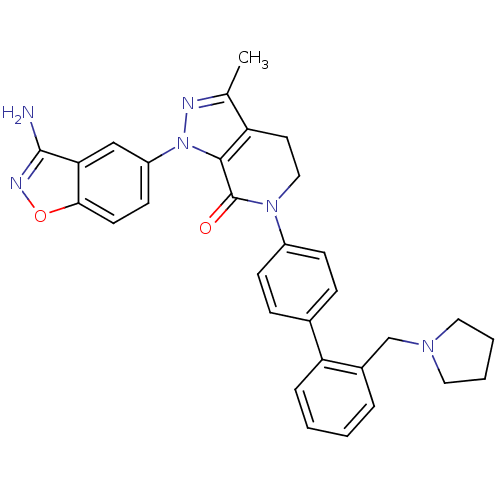 (1-(3-amino-1,2-benzoxazol-5-yl)-3-methyl-6-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288615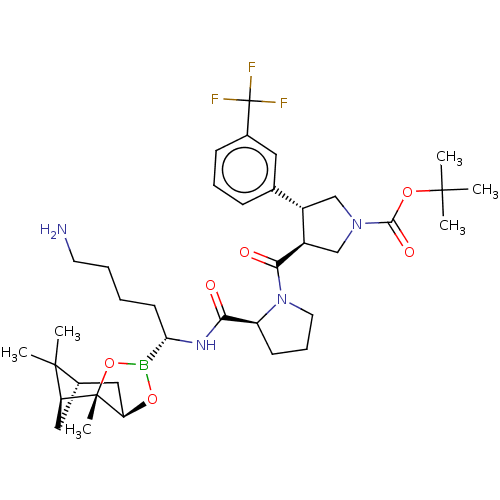 ((3S,4R)-3-{(S)-2-[(R)-5-Amino-1-((1S,2S,6R,8S)-2,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against thrombin. | Bioorg Med Chem Lett 6: 295-300 (1996) Article DOI: 10.1016/0960-894X(96)00015-7 BindingDB Entry DOI: 10.7270/Q2DZ089B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12691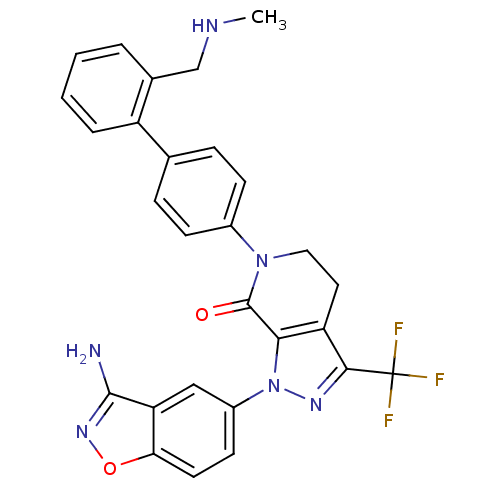 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(methylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12704 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12696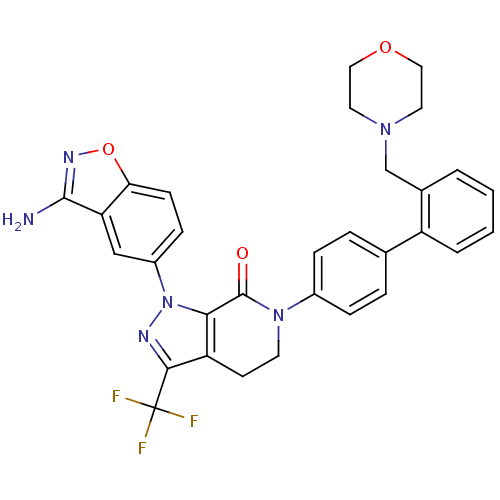 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(morpholin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 343 total ) | Next | Last >> |