

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105466 ((S)-6-Amino-2-[(R)-2-[(4-benzenesulfonyl-piperazin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105472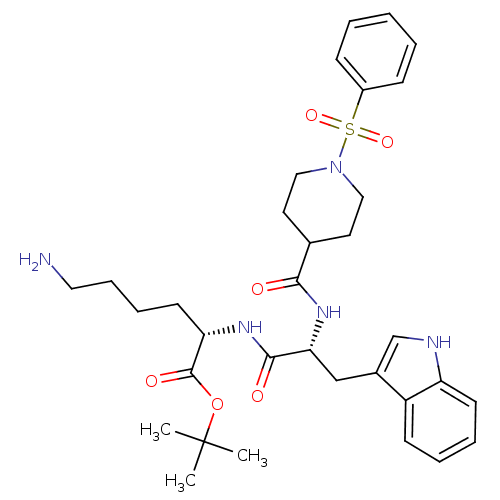 ((S)-6-Amino-2-[(R)-2-[(1-benzenesulfonyl-piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105465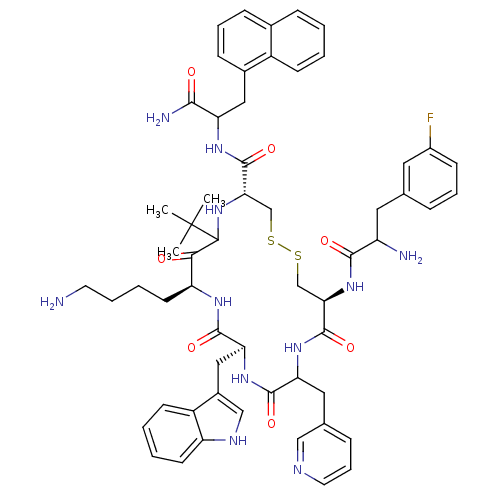 ((4R,8S,11S,17S)-8-(4-Amino-butyl)-17-(2-amino-3-na...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105470 ((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(4-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105469 ((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(2-oxo-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Antagonistic activity of the compound towards sst2 receptor in GH4C1 cells a concentration of 1-2 x 10e6/mL incubated for 20 minutes | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105469 ((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(2-oxo-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Antagonistic activity of the compound towards sst2 receptor in GH4C1 cells a concentration of 1-2 x 10e6/mL incubated for 20 minutes | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105468 ((S)-6-Amino-2-[(R)-2-[(4-benzoyl-piperazine-1-carb...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105468 ((S)-6-Amino-2-[(R)-2-[(4-benzoyl-piperazine-1-carb...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105471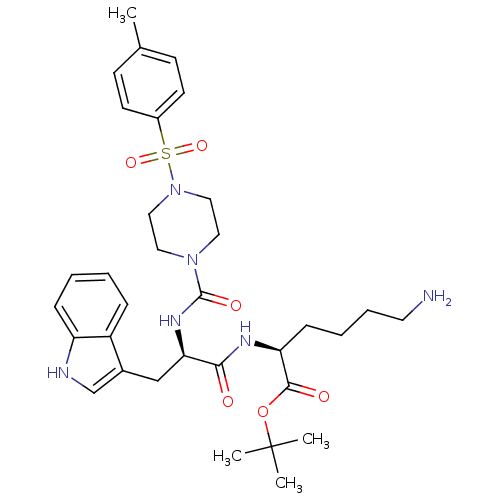 ((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(toluen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105466 ((S)-6-Amino-2-[(R)-2-[(4-benzenesulfonyl-piperazin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137438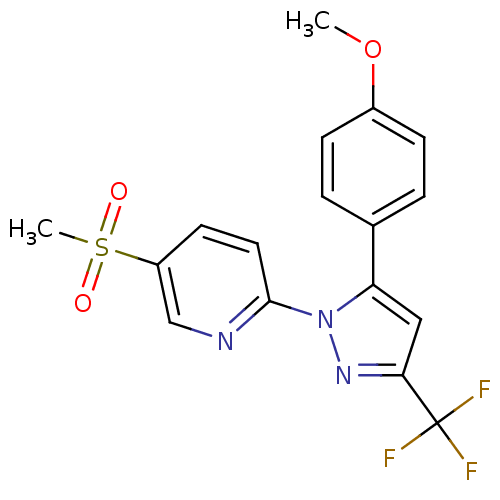 (5-Methanesulfonyl-2-[5-(4-methoxy-phenyl)-3-triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137423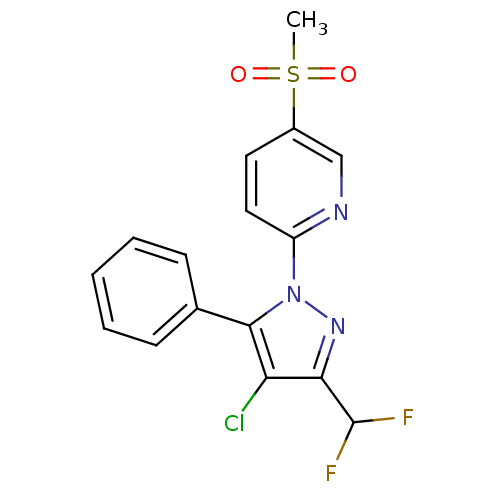 (2-(4-Chloro-3-difluoromethyl-5-phenyl-pyrazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Canis familiaris) | BDBM50137420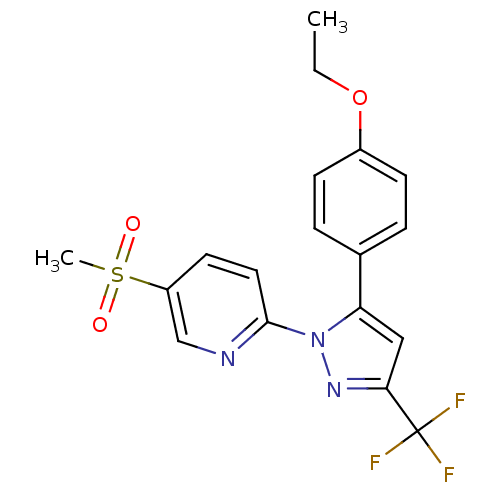 (2-[5-(4-Ethoxy-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137442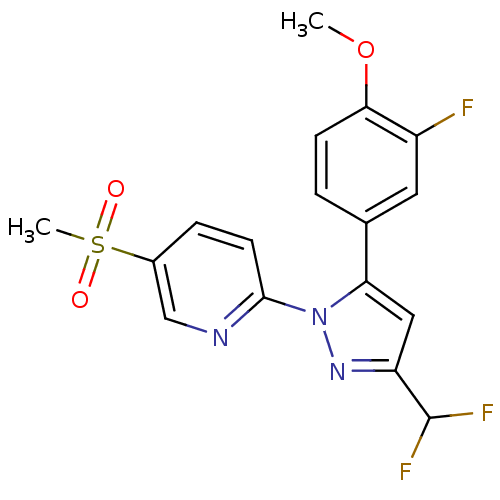 (2-[3-Difluoromethyl-5-(3-fluoro-4-methoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137439 (2-[5-(2-Fluoro-4-methoxy-phenyl)-3-trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137435 (2-(4-Chloro-5-phenyl-3-trifluoromethyl-pyrazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137424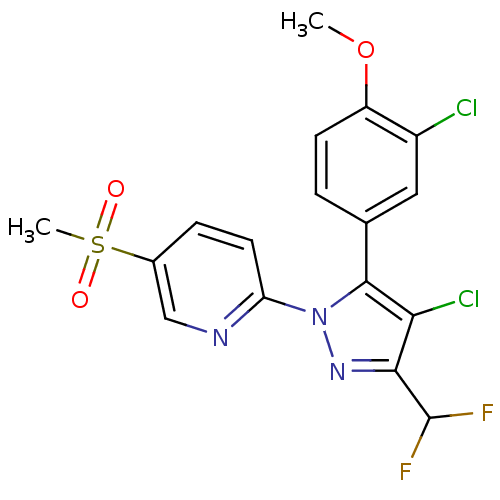 (2-[4-Chloro-5-(3-chloro-4-methoxy-phenyl)-3-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137411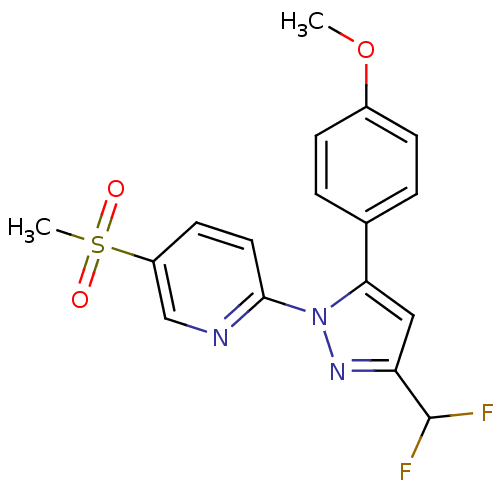 (2-[3-Difluoromethyl-5-(4-methoxy-phenyl)-pyrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105470 ((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(4-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Antagonistic activity of the compound towards sst2 receptor in GH4C1 cells a concentration of 1-2 x 10e6/mL incubated for 20 minutes | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137437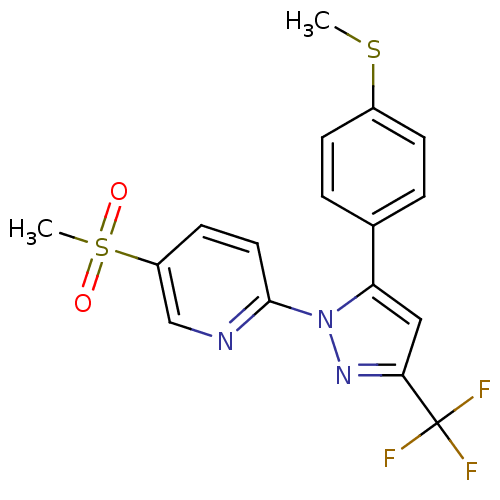 (5-Methanesulfonyl-2-[5-(4-methylsulfanyl-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137427 (2-(4-Bromo-3-difluoromethyl-5-phenyl-pyrazol-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137428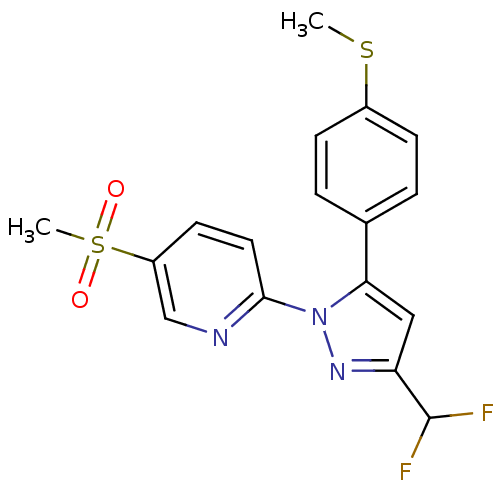 (2-[3-Difluoromethyl-5-(4-methylsulfanyl-phenyl)-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137414 (2-[5-(4-Chloro-phenyl)-3-difluoromethyl-pyrazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137440 (CHEMBL176934 | {4-[2-(5-Methanesulfonyl-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105472 ((S)-6-Amino-2-[(R)-2-[(1-benzenesulfonyl-piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Antagonistic activity of the compound towards sst2 receptor in GH4C1 cells a concentration of 1-2 x 10e6/mL incubated for 20 minutes | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137420 (2-[5-(4-Ethoxy-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137412 (5-Methanesulfonyl-2-(4-methyl-5-phenyl-3-trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137434 (2-[3-Difluoromethyl-5-(4-methoxy-3-methyl-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137418 (2-(3-Difluoromethyl-5-p-tolyl-pyrazol-1-yl)-5-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137432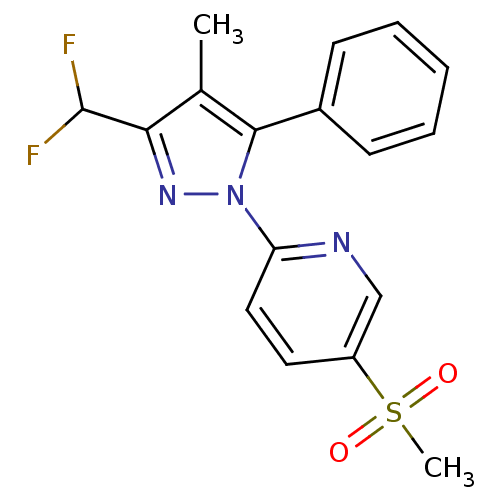 (2-(3-Difluoromethyl-4-methyl-5-phenyl-pyrazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Canis familiaris) | BDBM50137411 (2-[3-Difluoromethyl-5-(4-methoxy-phenyl)-pyrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137416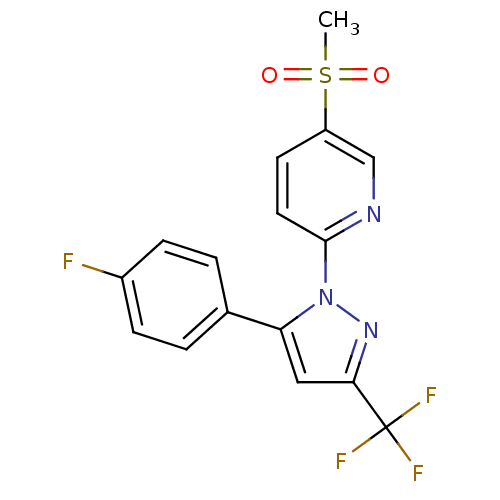 (2-(5-(4-fluorophenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137413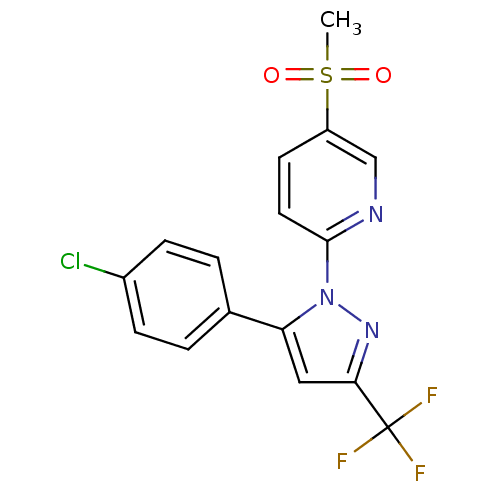 (2-[5-(4-Chloro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50105465 ((4R,8S,11S,17S)-8-(4-Amino-butyl)-17-(2-amino-3-na...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Antagonistic activity of the compound towards sst2 receptor in GH4C1 cells a concentration of 1-2 x 10e6/mL incubated for 20 minutes | Bioorg Med Chem Lett 11: 2731-4 (2001) BindingDB Entry DOI: 10.7270/Q2JW8D5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137415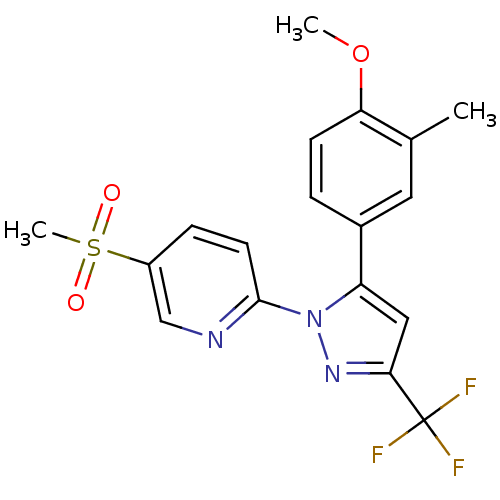 (5-Methanesulfonyl-2-[5-(4-methoxy-3-methyl-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137410 (2-[5-(4-Bromo-phenyl)-3-difluoromethyl-pyrazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137441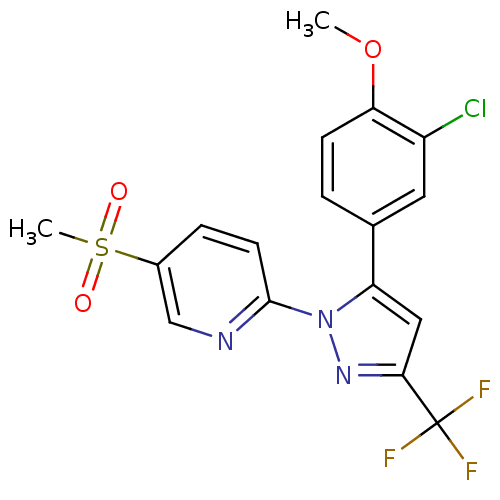 (2-[5-(3-Chloro-4-methoxy-phenyl)-3-trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137429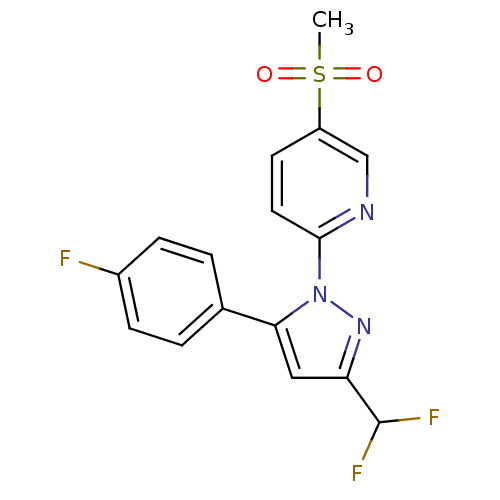 (2-[3-Difluoromethyl-5-(4-fluoro-phenyl)-pyrazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137421 (2-[5-(3-Fluoro-4-methoxy-phenyl)-3-trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137425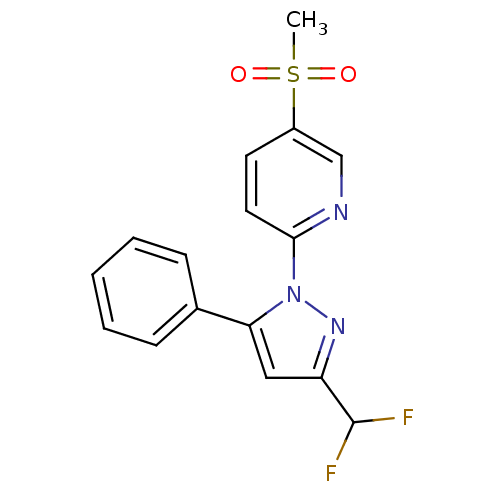 (2-(3-(difluoromethyl)-5-phenyl-1H-pyrazol-1-yl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Canis familiaris) | BDBM50137423 (2-(4-Chloro-3-difluoromethyl-5-phenyl-pyrazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137436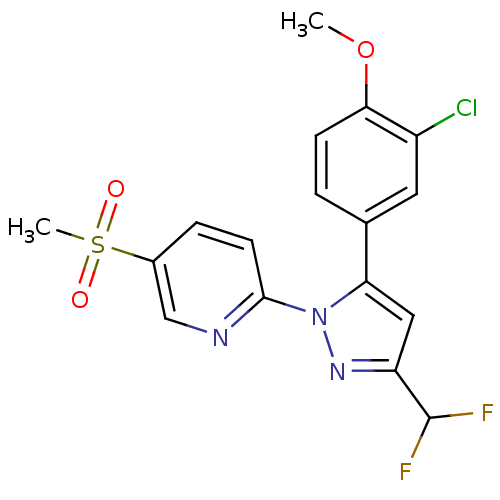 (2-[5-(3-Chloro-4-methoxy-phenyl)-3-difluoromethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137422 (2-[3-Difluoromethyl-5-(2-fluoro-4-methoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137417 (2-[3-Difluoromethyl-5-(4-ethyl-phenyl)-pyrazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137433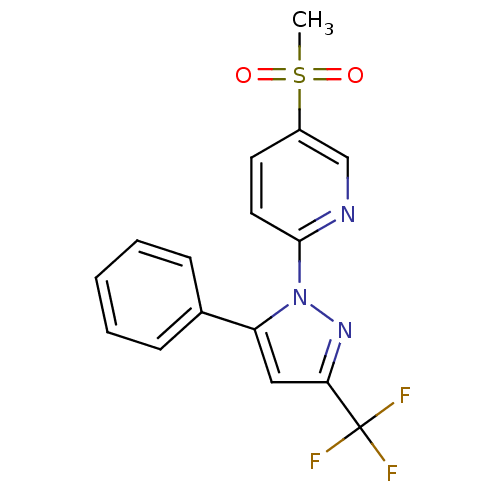 (5-(methylsulfonyl)-2-(5-phenyl-3-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137419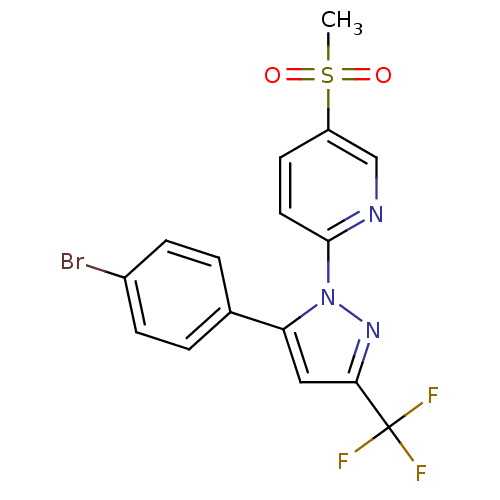 (2-[5-(4-Bromo-phenyl)-3-trifluoromethyl-pyrazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50057583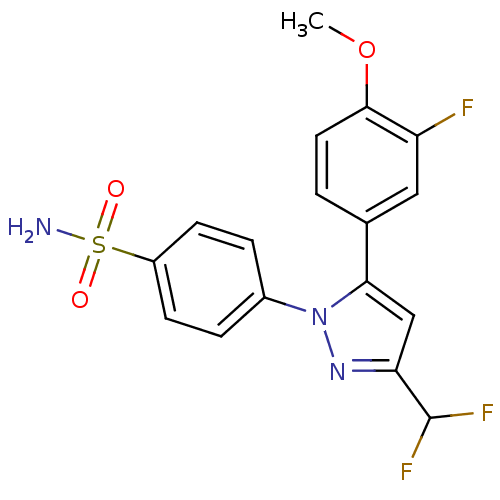 (4-[3-Difluoromethyl-5-(3-fluoro-4-methoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Canis familiaris) | BDBM50137438 (5-Methanesulfonyl-2-[5-(4-methoxy-phenyl)-3-triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Canis familiaris) | BDBM50137428 (2-[3-Difluoromethyl-5-(4-methylsulfanyl-phenyl)-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |