

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021819 (CHEMBL3298828) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50202519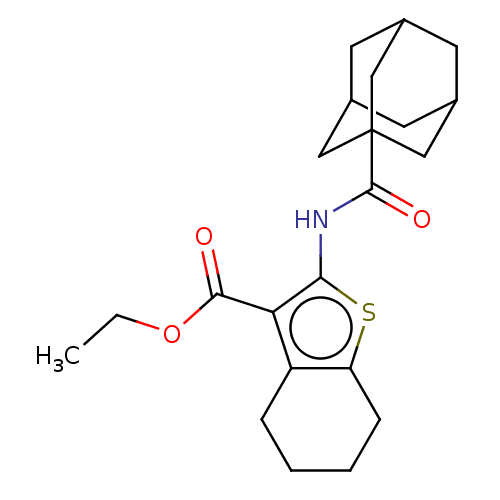 (CHEMBL3909238) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021818 (CHEMBL3298827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50202521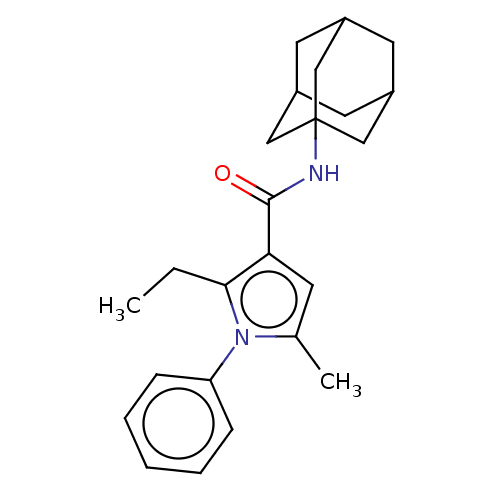 (CHEMBL3962080) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021817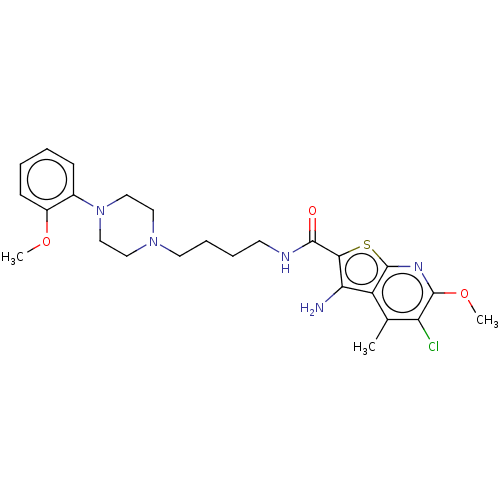 (CHEMBL3298826) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50202511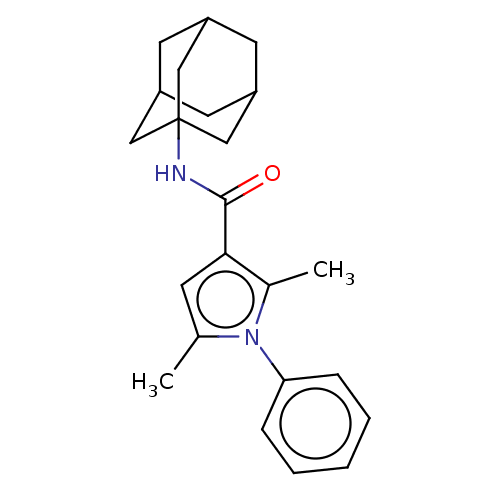 (CHEMBL3982062) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021771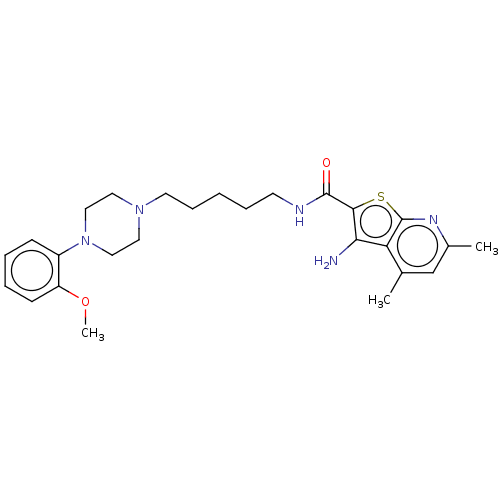 (CHEMBL3298896) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB1 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50037936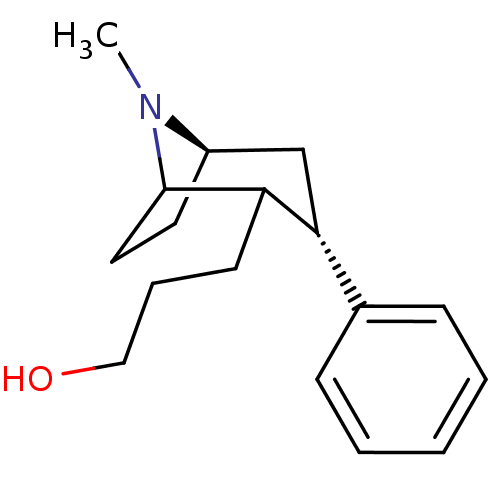 (3-((3S,5R)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity was determined against cocaine binding site by measuring the ability of compound to displace bound [3H]dopamine from rat caudate-put... | J Med Chem 37: 3875-7 (1994) BindingDB Entry DOI: 10.7270/Q2FB53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440293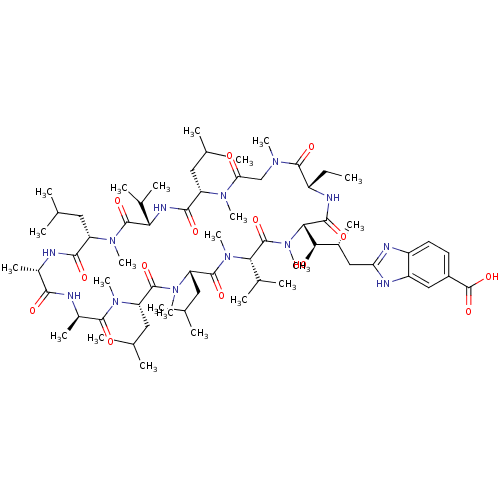 (CHEMBL2424822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50110741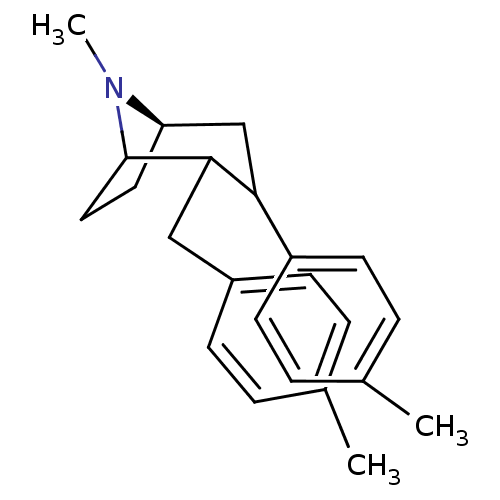 (8-Methyl-2-(4-methyl-benzyl)-3-p-tolyl-8-aza-bicyc...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Affinity for dopamine transporter was assessed by the ability to displace [3H]WIN-35428 from rat caudate-putamen tissue | J Med Chem 45: 1203-10 (2002) BindingDB Entry DOI: 10.7270/Q21C1XMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021770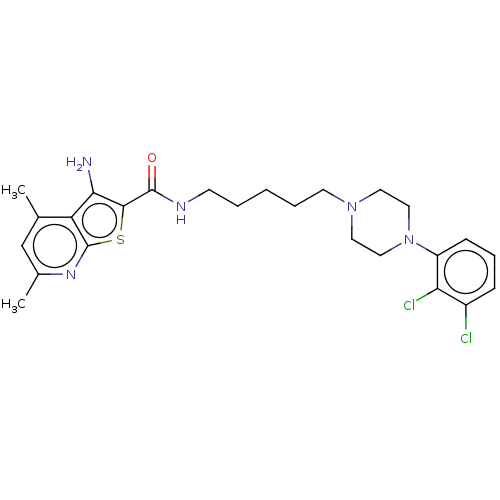 (CHEMBL3298895) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440299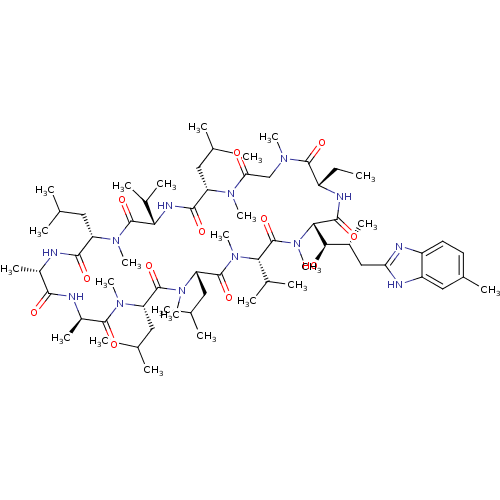 (CHEMBL2424823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021802 (CHEMBL3299096) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165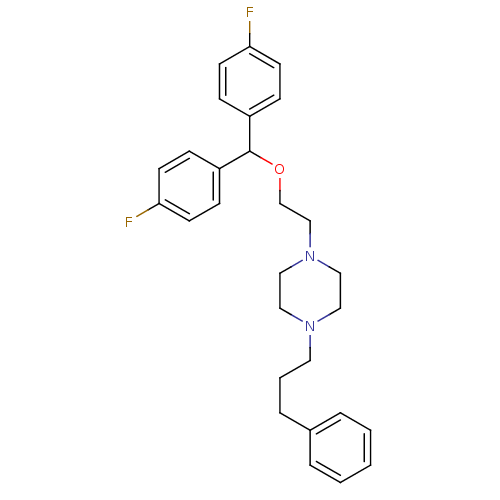 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from dopamine transporter of rat caudate putamen tissue | Bioorg Med Chem Lett 12: 2387-90 (2002) BindingDB Entry DOI: 10.7270/Q2X067KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440297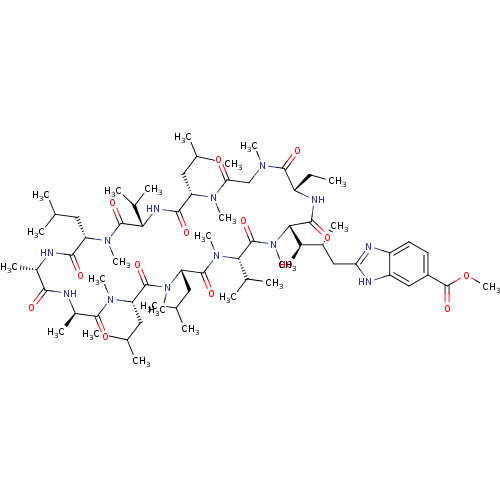 (CHEMBL2424817) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021769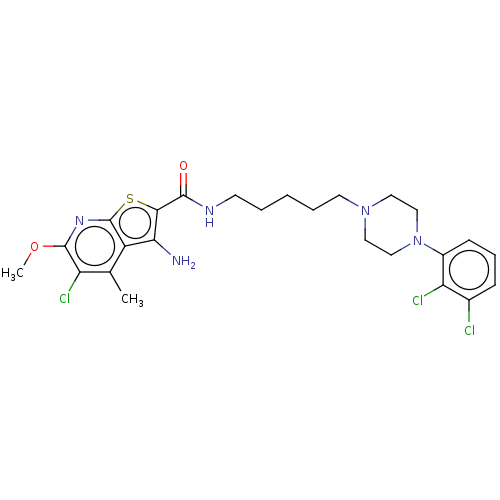 (CHEMBL3298830) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021796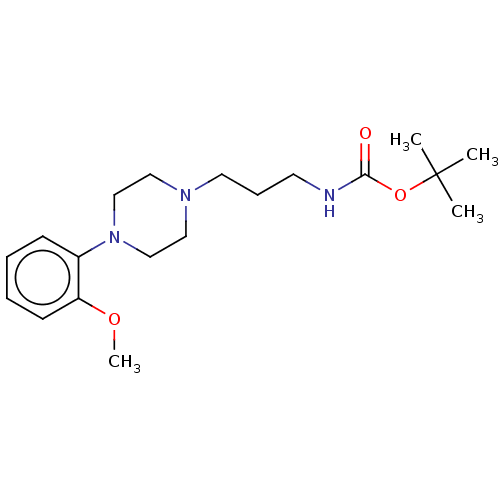 (CHEMBL3299094) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50110742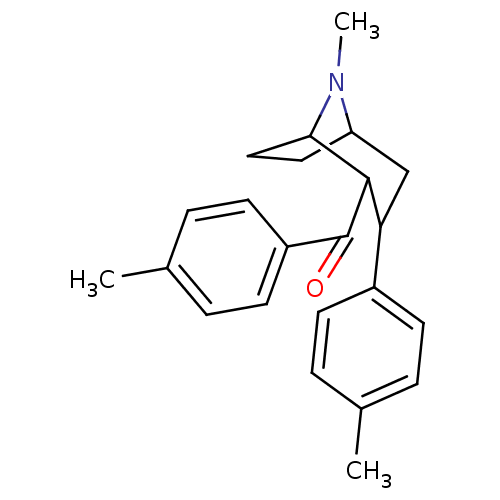 ((8-Methyl-3-p-tolyl-8-aza-bicyclo[3.2.1]oct-2-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Affinity for dopamine transporter was assessed by the ability to displace [3H]WIN-35428 from rat caudate-putamen tissue | J Med Chem 45: 1203-10 (2002) BindingDB Entry DOI: 10.7270/Q21C1XMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50202514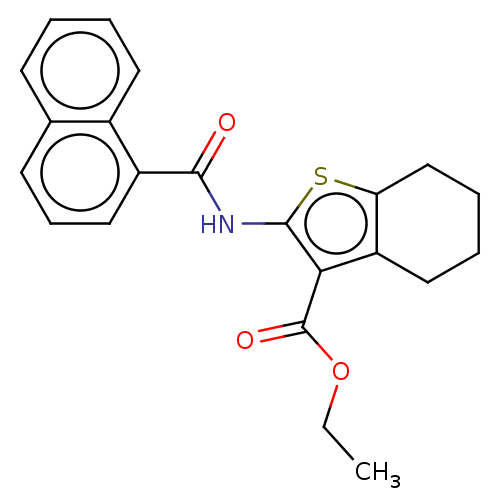 (CHEMBL3930225) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021800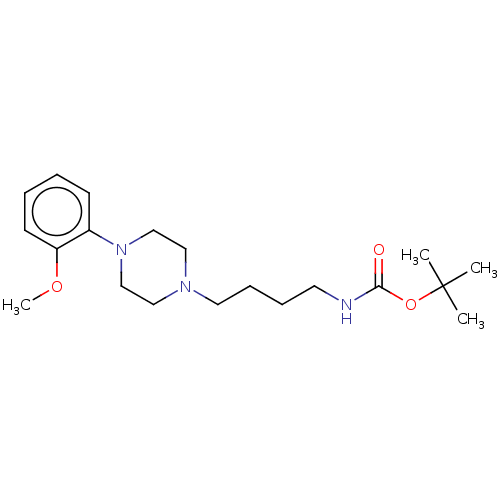 (CHEMBL3299095) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50037935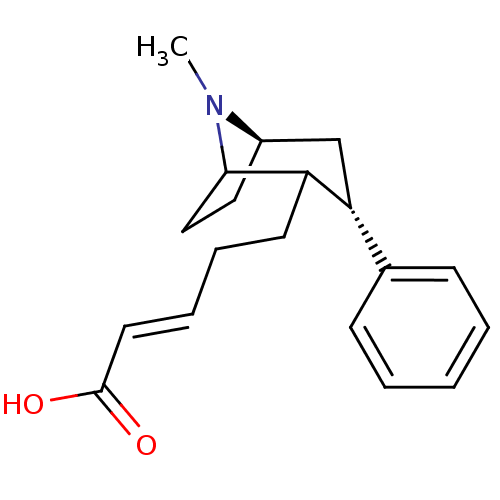 ((E)-5-((3S,5R)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity was determined against cocaine binding site by measuring the ability of compound to displace bound [3H]dopamine from rat caudate-put... | J Med Chem 37: 3875-7 (1994) BindingDB Entry DOI: 10.7270/Q2FB53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50202523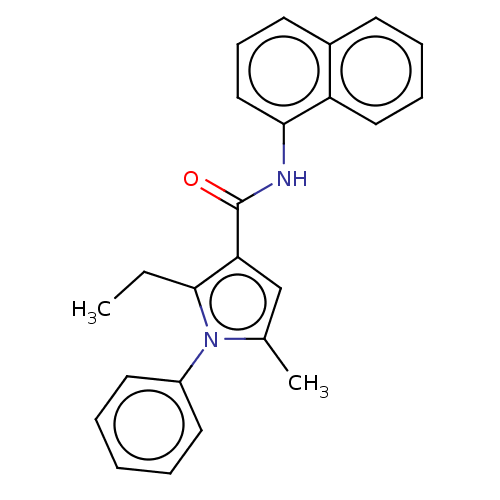 (CHEMBL3981080) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021816 (CHEMBL3298759) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50037938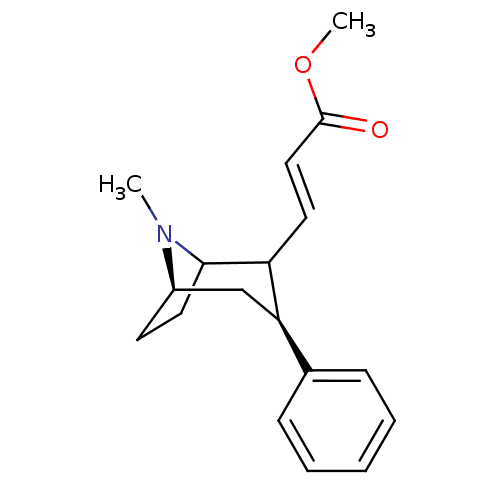 ((E)-3-((3S,5R)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity was determined against cocaine binding site by measuring the ability of compound to displace bound [3H]dopamine from rat caudate-put... | J Med Chem 37: 3875-7 (1994) BindingDB Entry DOI: 10.7270/Q2FB53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440300 (CHEMBL2424821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50037939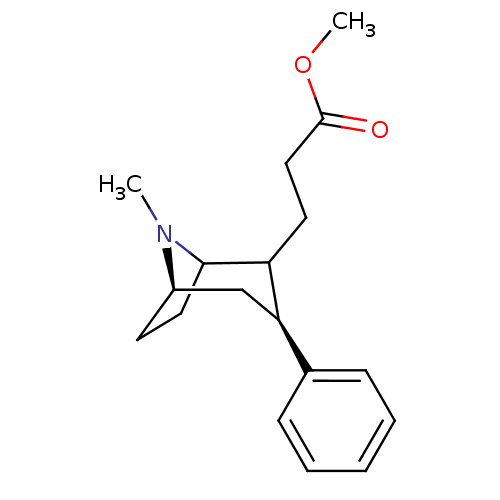 (3-((3S,5R)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity was determined against cocaine binding site by measuring the ability of compound to displace bound [3H]dopamine from rat caudate-put... | J Med Chem 37: 3875-7 (1994) BindingDB Entry DOI: 10.7270/Q2FB53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50049363 (4-{2-[Bis-(4-fluoro-phenyl)-methoxy]-ethyl}-1-(3-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from dopamine transporter of rat caudate putamen tissue | Bioorg Med Chem Lett 12: 2387-90 (2002) BindingDB Entry DOI: 10.7270/Q2X067KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50037937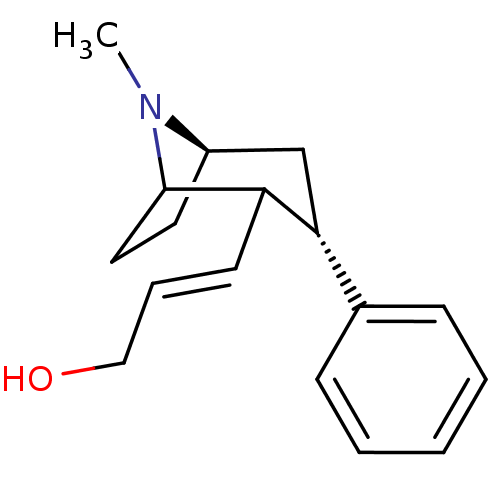 ((E)-3-((3S,5R)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity was determined against cocaine binding site by measuring the ability of compound to displace bound [3H]dopamine from rat caudate-put... | J Med Chem 37: 3875-7 (1994) BindingDB Entry DOI: 10.7270/Q2FB53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50084717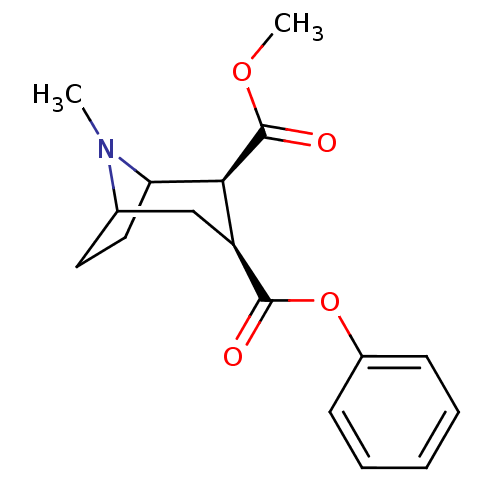 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity was determined against cocaine receptor by measuring the ability of compound to displace bound [3H]-3 from rat caudate-putamen tissu... | J Med Chem 37: 3875-7 (1994) BindingDB Entry DOI: 10.7270/Q2FB53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50084717 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Affinity for dopamine transporter was assessed by the ability to displace [3H]WIN-35428 from rat caudate-putamen tissue | J Med Chem 45: 1203-10 (2002) BindingDB Entry DOI: 10.7270/Q21C1XMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50084717 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity was determined against cocaine receptor by measuring the ability of compound to displace bound [3H]-3 from rat caudate-putamen tissu... | J Med Chem 37: 3875-7 (1994) BindingDB Entry DOI: 10.7270/Q2FB53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50084717 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity against dopamine transporter in rat caudate putamen tissue using [3H]WIN-35428 radioligand. (from other reference) | J Med Chem 39: 4744-9 (1997) Article DOI: 10.1021/jm960507d BindingDB Entry DOI: 10.7270/Q2GF0V51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50084717 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from dopamine transporter in rat caudate putamen tissue | J Med Chem 40: 4406-14 (1998) Article DOI: 10.1021/jm970549h BindingDB Entry DOI: 10.7270/Q22R3SBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50366564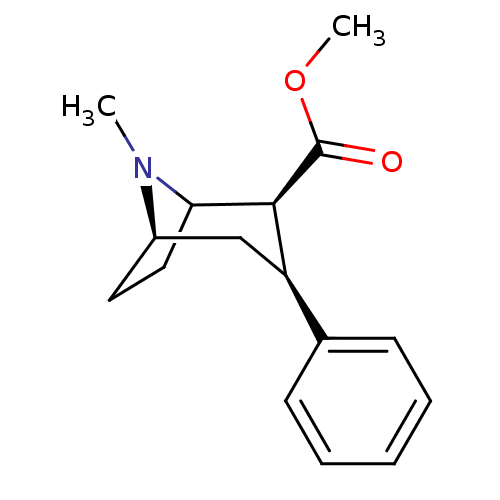 (CHEMBL1790051 | CHEMBL83729 | WIN-35065-2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity against dopamine transporter in rat caudate putamen tissue using [3H]WIN-35428 radioligand. (from other reference) | J Med Chem 39: 4744-9 (1997) Article DOI: 10.1021/jm960507d BindingDB Entry DOI: 10.7270/Q2GF0V51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50366564 (CHEMBL1790051 | CHEMBL83729 | WIN-35065-2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from dopamine transporter in rat caudate putamen tissue | J Med Chem 40: 4406-14 (1998) Article DOI: 10.1021/jm970549h BindingDB Entry DOI: 10.7270/Q22R3SBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50061954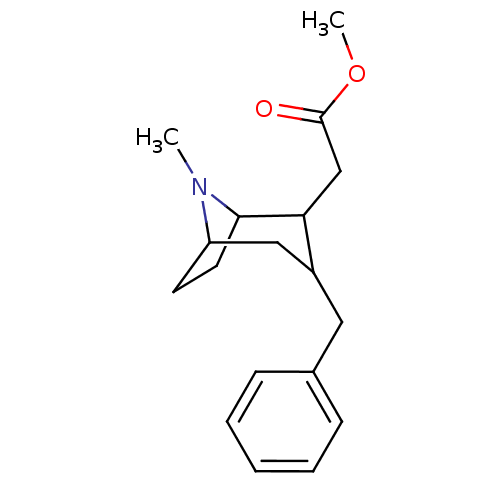 ((3-Benzyl-8-methyl-8-aza-bicyclo[3.2.1]oct-2-yl)-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from dopamine transporter in rat caudate putamen tissue | J Med Chem 40: 4406-14 (1998) Article DOI: 10.1021/jm970549h BindingDB Entry DOI: 10.7270/Q22R3SBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50366564 (CHEMBL1790051 | CHEMBL83729 | WIN-35065-2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity against dopamine transporter in rat caudate putamen tissue using [3H]WIN-35428 radioligand. (from other reference) | J Med Chem 39: 4744-9 (1997) Article DOI: 10.1021/jm960507d BindingDB Entry DOI: 10.7270/Q2GF0V51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50366564 (CHEMBL1790051 | CHEMBL83729 | WIN-35065-2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Binding affinity was determined against cocaine binding site by measuring the ability of compound to displace bound [3H]dopamine from rat caudate-put... | J Med Chem 37: 3875-7 (1994) BindingDB Entry DOI: 10.7270/Q2FB53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50440293 (CHEMBL2424822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypB PPIase activity (unknown origin) | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440296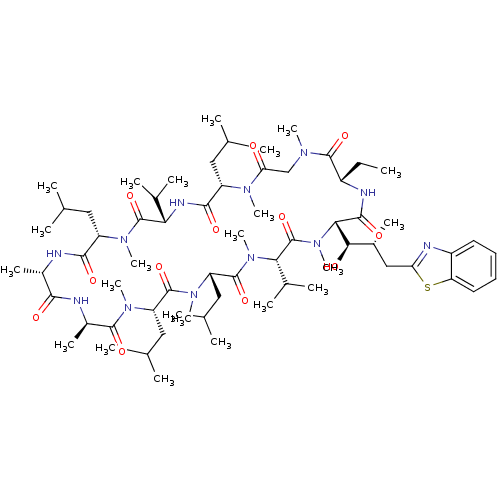 (CHEMBL2424818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021768 (CHEMBL3298829) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440298 (CHEMBL2424824) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50021815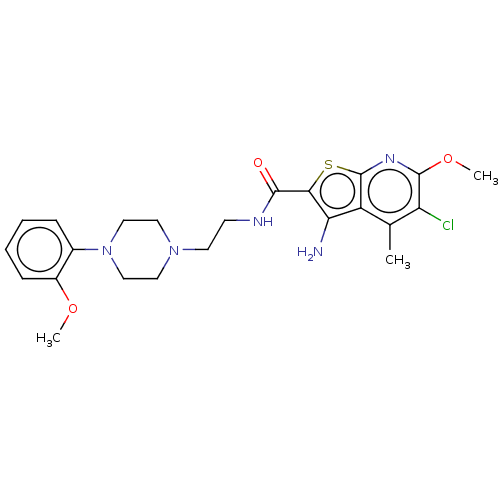 (CHEMBL3298758) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2L receptor expressed in FlpIn CHO cell membrane after 3 hrs by liquid scintillation counting analysis | J Med Chem 57: 4924-39 (2014) Article DOI: 10.1021/jm500457x BindingDB Entry DOI: 10.7270/Q29Z96FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50202539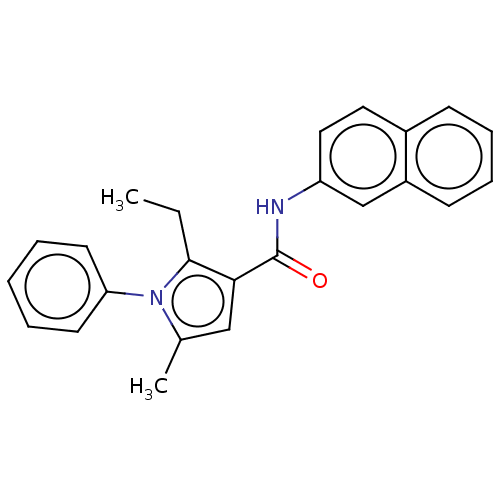 (CHEMBL3953330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50061956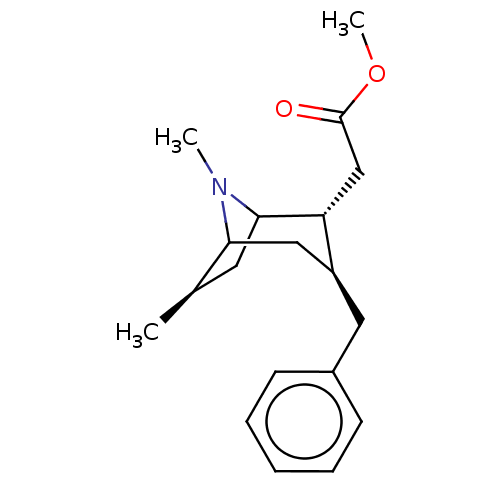 ((3-Benzyl-6,8-dimethyl-8-aza-bicyclo[3.2.1]oct-2-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from dopamine transporter in rat caudate putamen tissue | J Med Chem 40: 4406-14 (1998) Article DOI: 10.1021/jm970549h BindingDB Entry DOI: 10.7270/Q22R3SBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50202515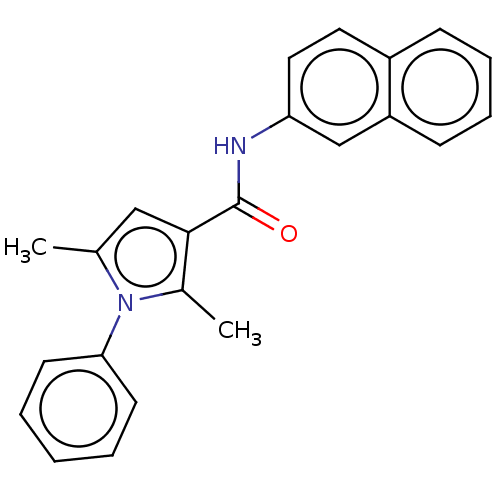 (CHEMBL3921132) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay | Eur J Med Chem 122: 619-634 (2016) Article DOI: 10.1016/j.ejmech.2016.07.012 BindingDB Entry DOI: 10.7270/Q2057HX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1817 total ) | Next | Last >> |