

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015931 (3-Benzenesulfonylmethyl-7-methoxy-5,5,8-trioxo-5la...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase (HLE) | J Med Chem 33: 2529-35 (1990) BindingDB Entry DOI: 10.7270/Q2FQ9VK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015926 (7-Methoxy-3-methoxymethyl-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase (HLE) | J Med Chem 33: 2529-35 (1990) BindingDB Entry DOI: 10.7270/Q2FQ9VK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50283470 (2-(4-Chloro-phenylamino)-5H-[1,3]thiazino[5,4-b]in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leukocyte Elastase at 23 microM concentration from human polymorphonuclear leukocytes | Bioorg Med Chem Lett 4: 2399-2404 (1994) Article DOI: 10.1016/S0960-894X(01)80398-X BindingDB Entry DOI: 10.7270/Q2BZ65Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50283469 (2-Phenylamino-5H-[1,3]thiazino[5,4-b]indol-4-one |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leukocyte Elastase at 64 microM concentration from human polymorphonuclear leukocytes | Bioorg Med Chem Lett 4: 2399-2404 (1994) Article DOI: 10.1016/S0960-894X(01)80398-X BindingDB Entry DOI: 10.7270/Q2BZ65Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM85462 (Pyrazolooxadiazinone, 2c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70E+4 | -23.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Università di Ferrara | Assay Description The enzyme were assayed spectrophotometrically at 410nm with p-nitroanilide substrates at 25 C. | J Enzym Inhib 16: 15-34 (2001) Article DOI: 10.1080/14756360109162352 BindingDB Entry DOI: 10.7270/Q2XP73FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM85460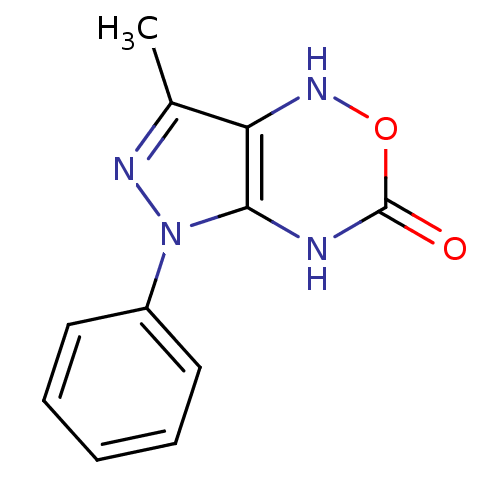 (Pyrazolooxadiazinone, 2a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.40E+4 | -23.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Università di Ferrara | Assay Description The enzyme were assayed spectrophotometrically at 410nm with p-nitroanilide substrates at 25 C. | J Enzym Inhib 16: 15-34 (2001) Article DOI: 10.1080/14756360109162352 BindingDB Entry DOI: 10.7270/Q2XP73FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM85466 (Pyrazolooxadiazinone, 2l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.13E+5 | -22.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Università di Ferrara | Assay Description The enzyme were assayed spectrophotometrically at 410nm with p-nitroanilide substrates at 25 C. | J Enzym Inhib 16: 15-34 (2001) Article DOI: 10.1080/14756360109162352 BindingDB Entry DOI: 10.7270/Q2XP73FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM85465 (Pyrazolooxadiazinone, 2j) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.99E+5 | -21.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Università di Ferrara | Assay Description The enzyme were assayed spectrophotometrically at 410nm with p-nitroanilide substrates at 25 C. | J Enzym Inhib 16: 15-34 (2001) Article DOI: 10.1080/14756360109162352 BindingDB Entry DOI: 10.7270/Q2XP73FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM85461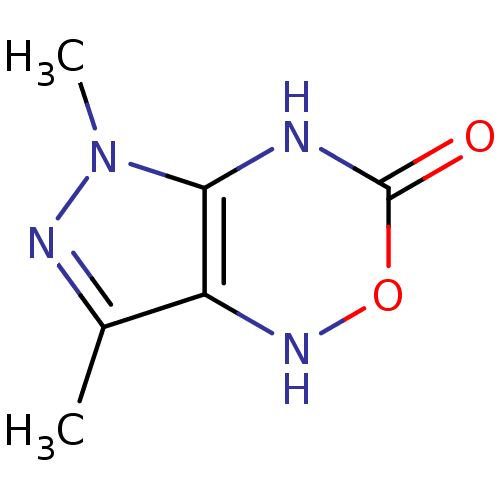 (Pyrazolooxadiazinone, 2b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.07E+5 | -21.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Università di Ferrara | Assay Description The enzyme were assayed spectrophotometrically at 410nm with p-nitroanilide substrates at 25 C. | J Enzym Inhib 16: 15-34 (2001) Article DOI: 10.1080/14756360109162352 BindingDB Entry DOI: 10.7270/Q2XP73FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM85464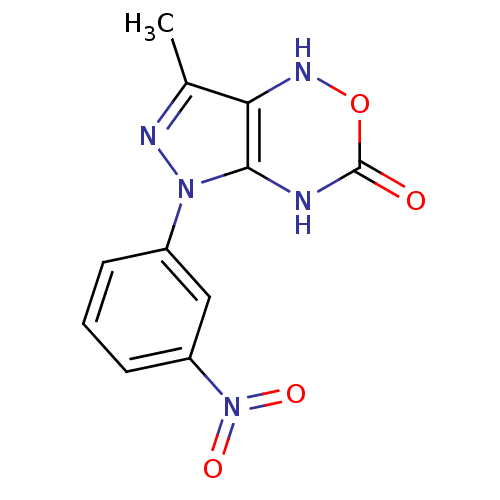 (Pyrazolooxadiazinone, 2h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >8.40E+6 | >-11.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Università di Ferrara | Assay Description The enzyme were assayed spectrophotometrically at 410nm with p-nitroanilide substrates at 25 C. | J Enzym Inhib 16: 15-34 (2001) Article DOI: 10.1080/14756360109162352 BindingDB Entry DOI: 10.7270/Q2XP73FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM85463 (Pyrazolooxadiazinone, 2g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >8.40E+6 | >-11.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Università di Ferrara | Assay Description The enzyme were assayed spectrophotometrically at 410nm with p-nitroanilide substrates at 25 C. | J Enzym Inhib 16: 15-34 (2001) Article DOI: 10.1080/14756360109162352 BindingDB Entry DOI: 10.7270/Q2XP73FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5459 (6-alkoxy-4-anilinoquinazoline 8e | N-{3-chloro-4-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5456 (6-alkoxy-4-anilinoquinazoline 8b | N-(3-ethynylphe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5460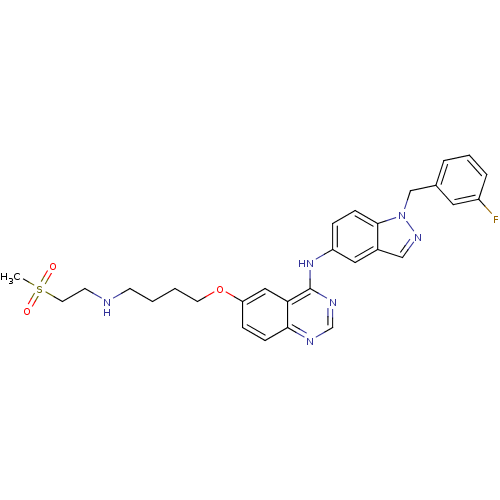 (6-alkoxy-4-anilinoquinazoline 8f | N-{1-[(3-fluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5457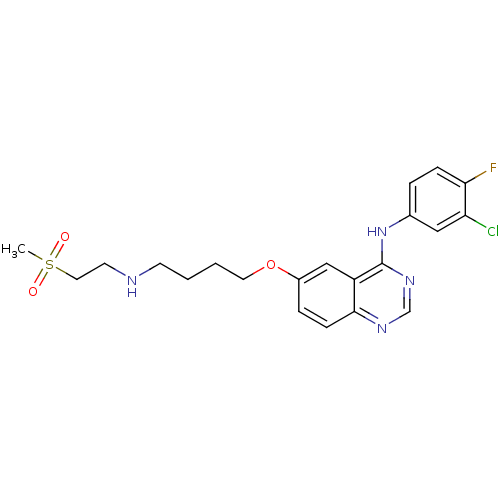 (6-alkoxy-4-anilinoquinazoline 8c | N-(3-chloro-4-f...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5460 (6-alkoxy-4-anilinoquinazoline 8f | N-{1-[(3-fluoro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5459 (6-alkoxy-4-anilinoquinazoline 8e | N-{3-chloro-4-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015924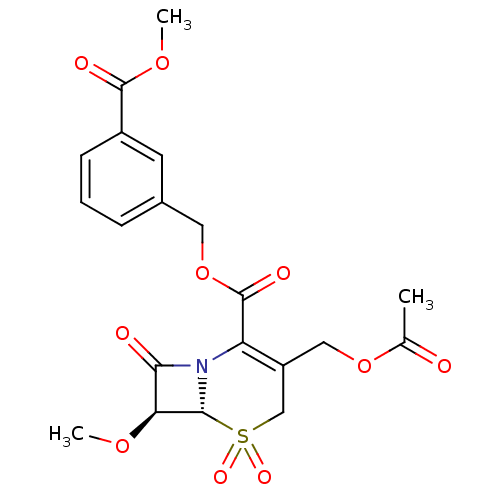 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5451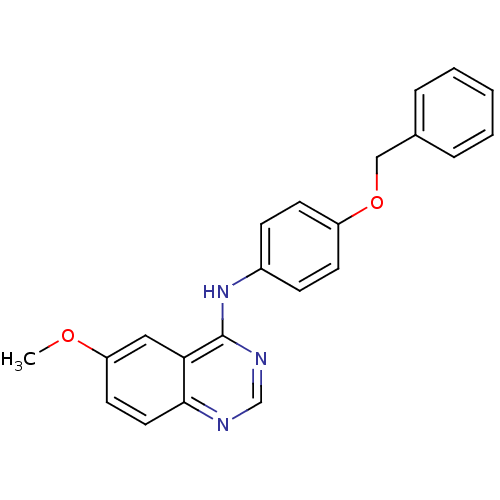 (6-alkoxy-4-anilinoquinazoline 9a | N-[4-(benzyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015916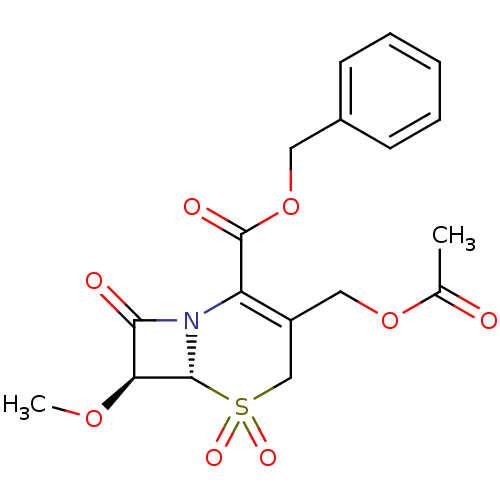 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5450 (6-alkoxy-4-anilinoquinazoline 8a | N-[4-(benzyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5455 (6-alkoxy-4-anilinoquinazoline 14a | N-{4-[(4-{[4-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5452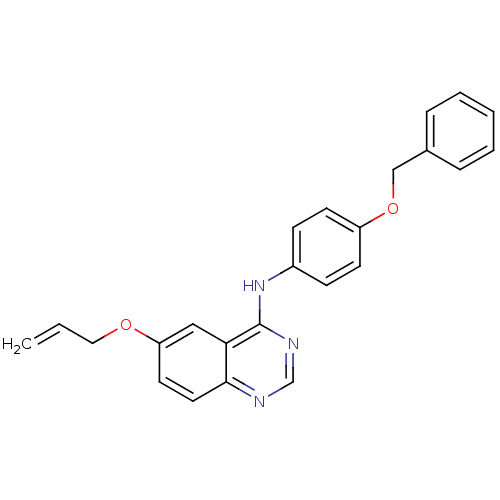 (6-alkoxy-4-anilinoquinazoline 10a | N-[4-(benzylox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5458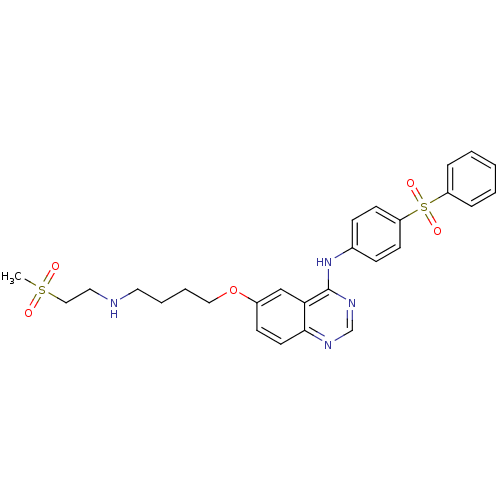 (6-alkoxy-4-anilinoquinazoline 8d | N-[4-(benzenesu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5450 (6-alkoxy-4-anilinoquinazoline 8a | N-[4-(benzyloxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5448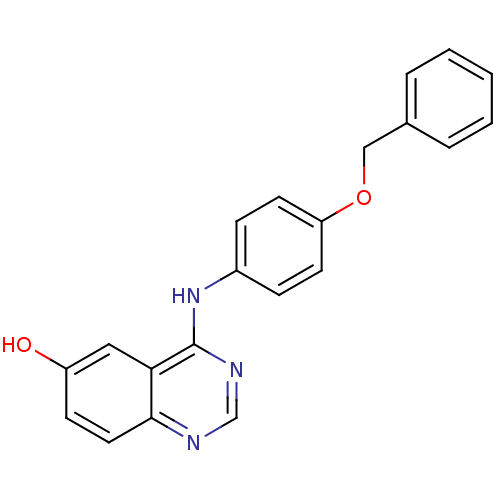 (4-anilinoquinazoline 4a | 4-{[4-(benzyloxy)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015922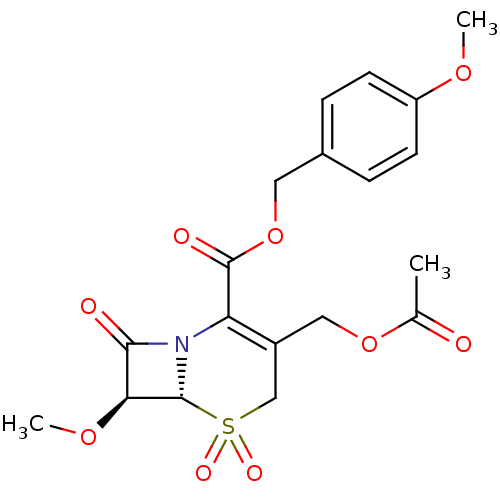 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015913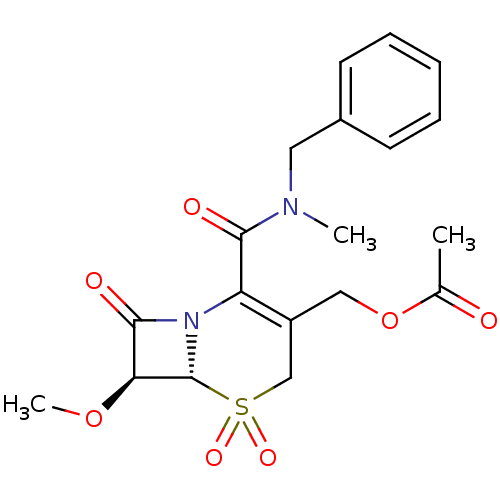 (Acetic acid 2-(benzyl-methyl-carbamoyl)-7-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015912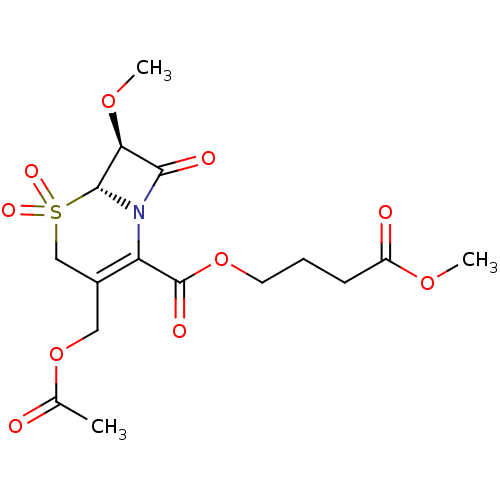 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015904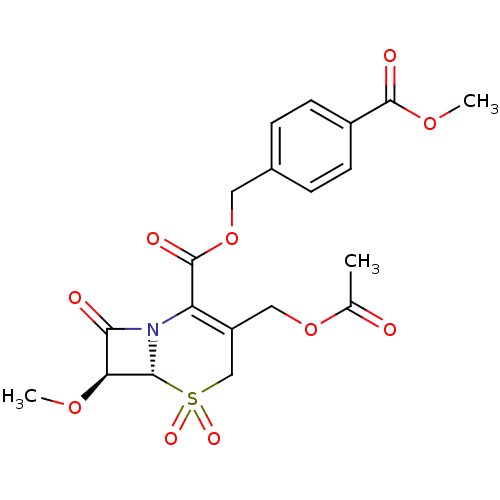 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015900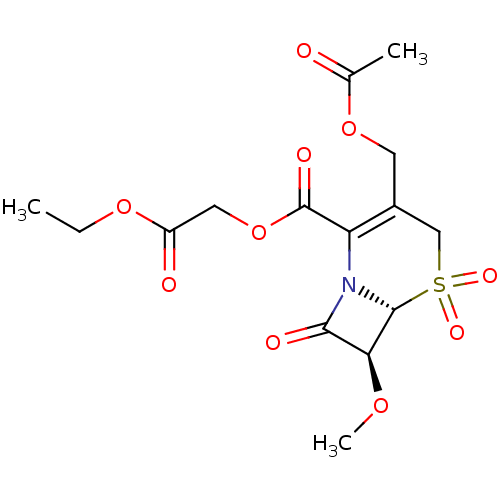 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5453 (6-alkoxy-4-anilinoquinazoline 12a | N-[4-(benzylox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5455 (6-alkoxy-4-anilinoquinazoline 14a | N-{4-[(4-{[4-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015935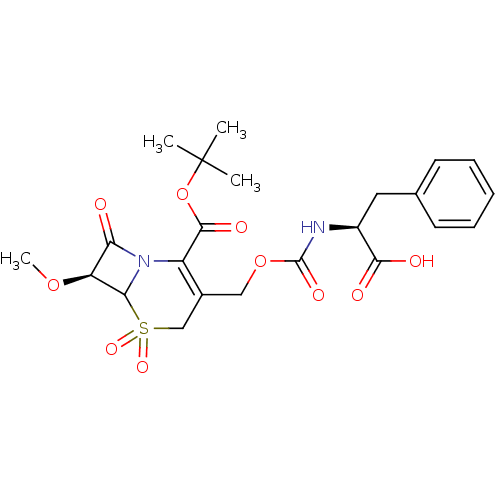 (3-(1-Carboxy-2-phenyl-ethylcarbamoyloxymethyl)-7-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase (HLE) | J Med Chem 33: 2529-35 (1990) BindingDB Entry DOI: 10.7270/Q2FQ9VK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5458 (6-alkoxy-4-anilinoquinazoline 8d | N-[4-(benzenesu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015909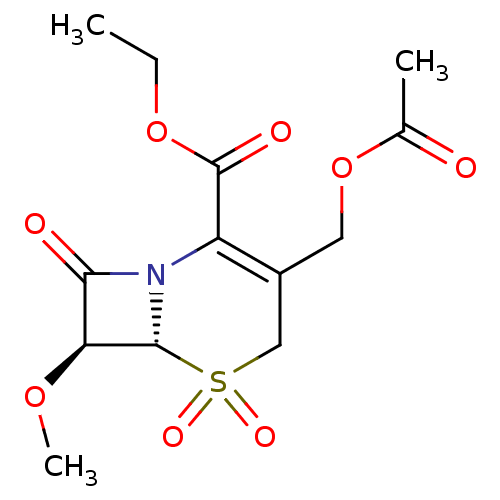 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015907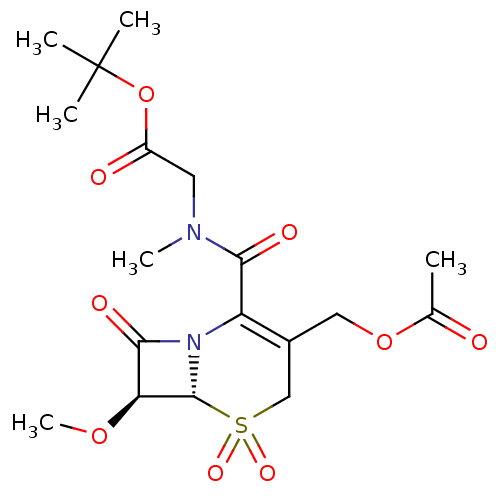 (CHEMBL432765 | [(3-Acetoxymethyl-7-methoxy-5,5,8-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015920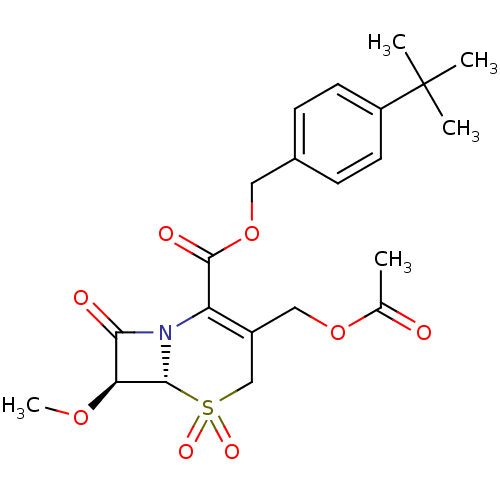 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015921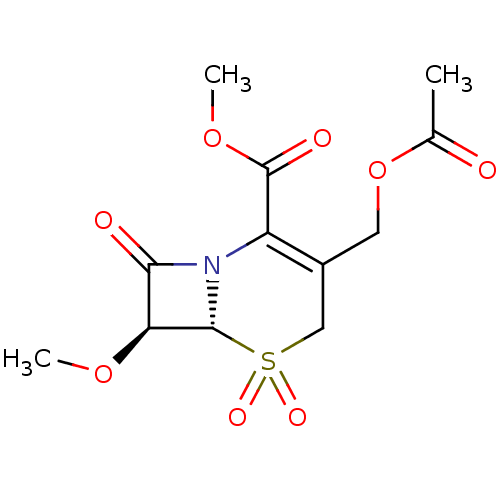 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015919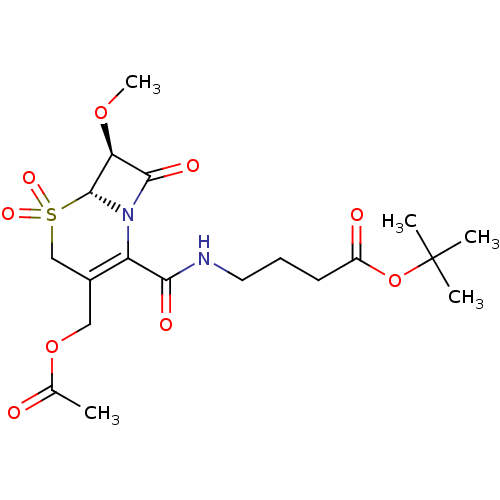 (4-[(3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5457 (6-alkoxy-4-anilinoquinazoline 8c | N-(3-chloro-4-f...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5453 (6-alkoxy-4-anilinoquinazoline 12a | N-[4-(benzylox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5456 (6-alkoxy-4-anilinoquinazoline 8b | N-(3-ethynylphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5449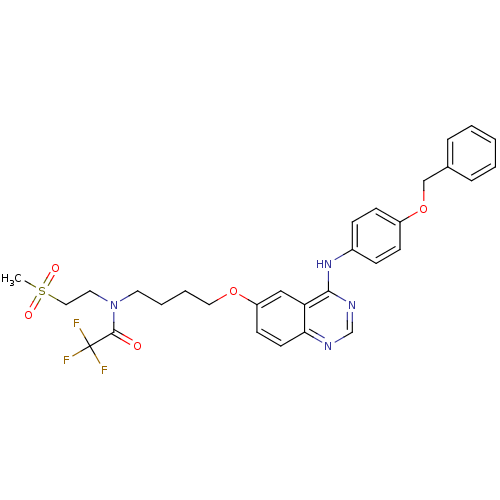 (6-alkoxy-4-anilinoquinazoline 7a | N-{4-[(4-{[4-(b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5448 (4-anilinoquinazoline 4a | 4-{[4-(benzyloxy)phenyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5451 (6-alkoxy-4-anilinoquinazoline 9a | N-[4-(benzyloxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 309 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5452 (6-alkoxy-4-anilinoquinazoline 10a | N-[4-(benzylox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015901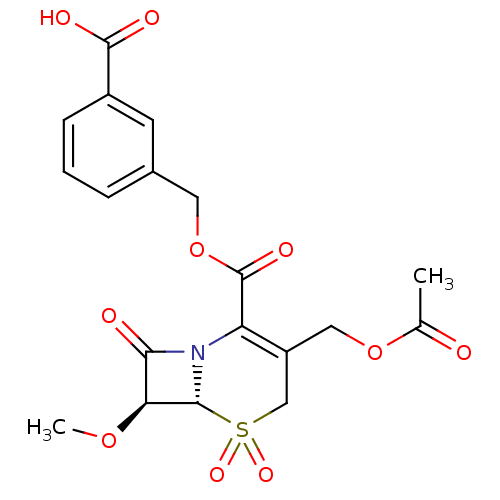 (3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015914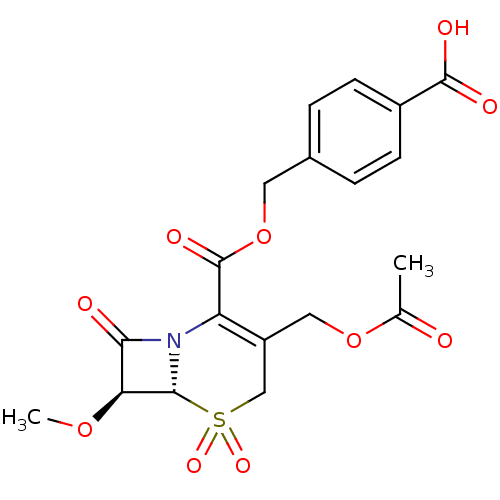 ((6R,7S)-3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5la...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015915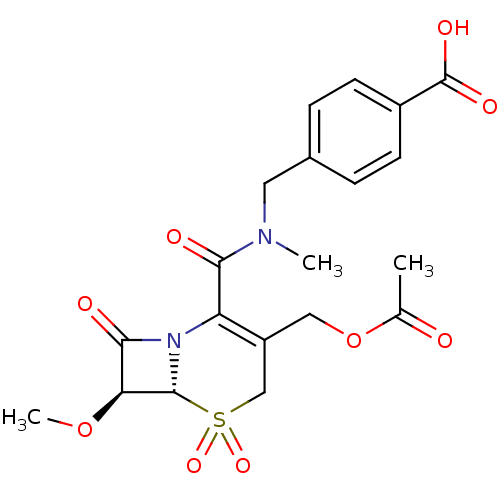 (4-{[((6R,7S)-3-Acetoxymethyl-7-methoxy-5,5,8-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Human Leukocyte Elastase (HLE) | J Med Chem 33: 2522-8 (1990) BindingDB Entry DOI: 10.7270/Q2KH0M9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |