

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor expressed in CHO cells | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266832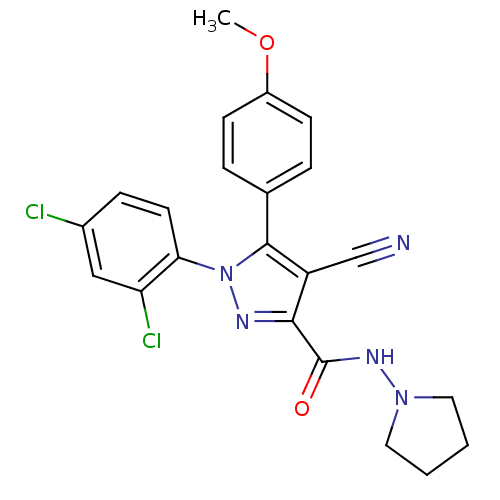 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-methoxyphenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267374 (1-(2-bromophenyl)-4-cyano-5-(4-methoxyphenyl)-N-(p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM25299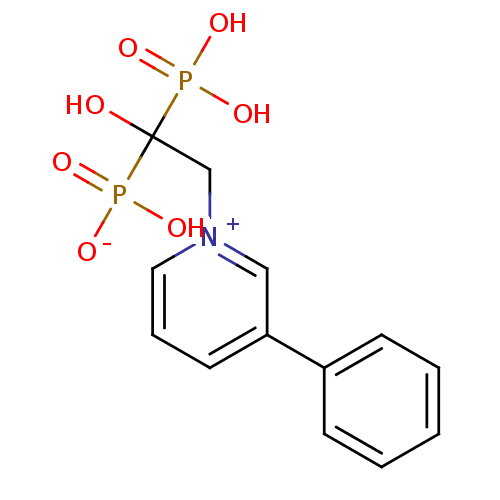 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266833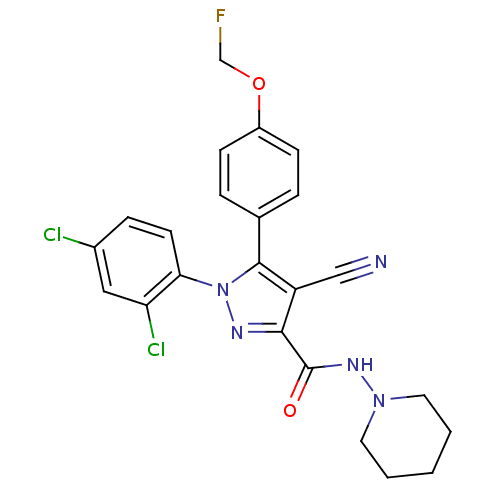 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-(fluoromethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM12578 (2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267373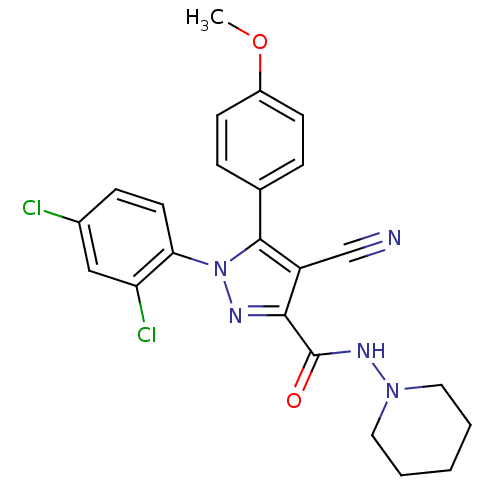 (4-cyano-1-(2,4-dichlorophenyl)-5-(4-methoxyphenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267375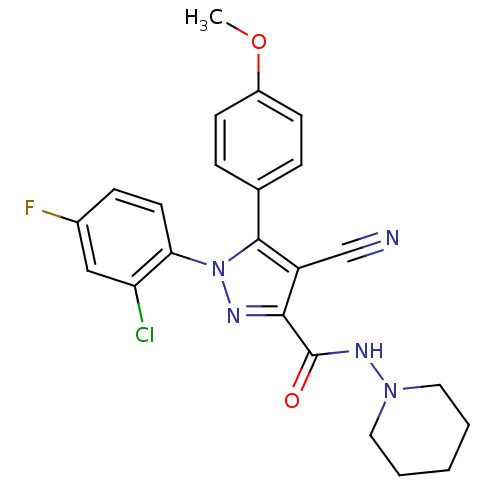 (1-(2-Chloro-4-fluorophenyl)-4-cyano-5-(4-methoxyph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266809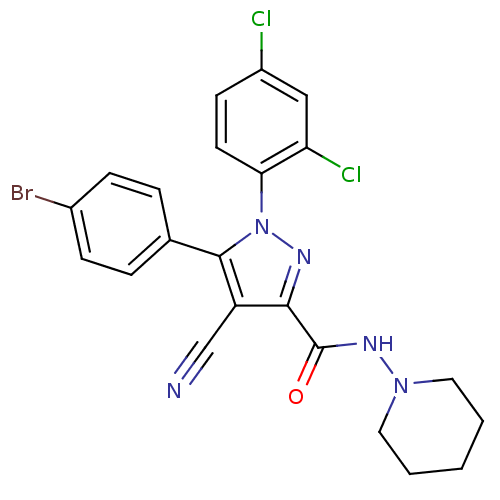 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-bromophenyl)-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493249 (CHEMBL2420249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM12576 (Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM25310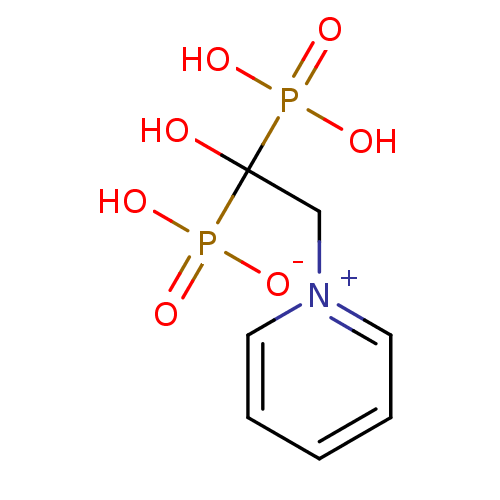 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165353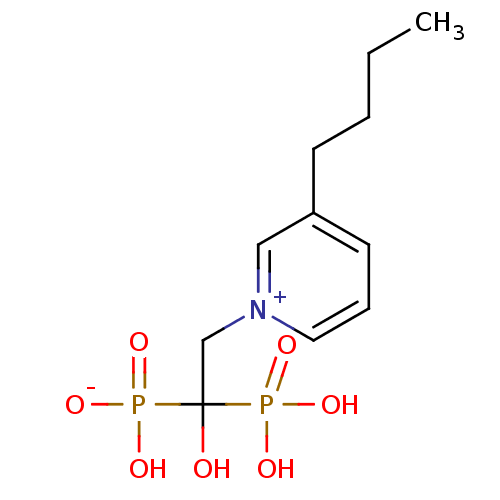 (CHEMBL373332 | hydrogen 2-(3-butylpyridinium-1-yl)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165352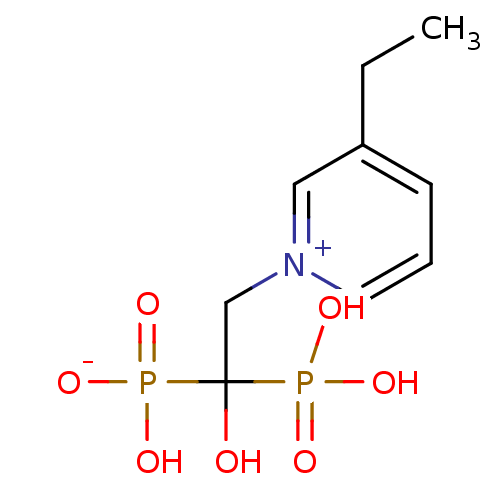 (CHEMBL192043 | hydrogen 2-(3-ethylpyridinium-1-yl)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266864 (5-(4-chlorophenyl)-4-cyano-1-(2,4-dichlorophenyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493245 (CHEMBL2420251) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165349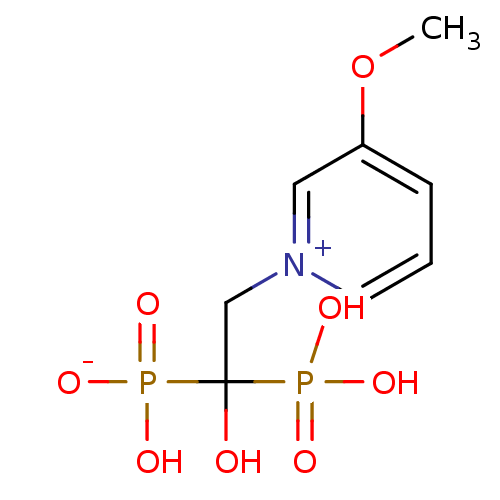 (CHEMBL363145 | hydrogen 1-hydroxy-2-(3-methoxypyri...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266807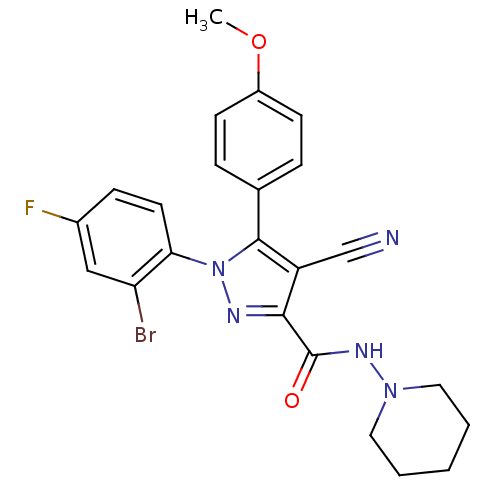 (1-(2-Bromo-4-fluorophenyl)-4-cyano-5-(4-methoxyphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266808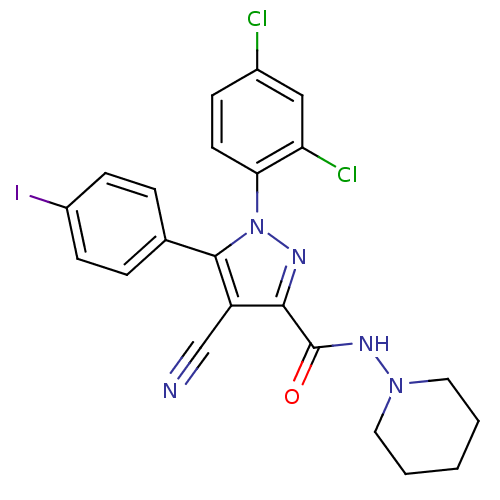 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-iodophenyl)-N-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165342 (CHEMBL193356 | hydrogen 1-hydroxy-2-(3-methylpyrid...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165340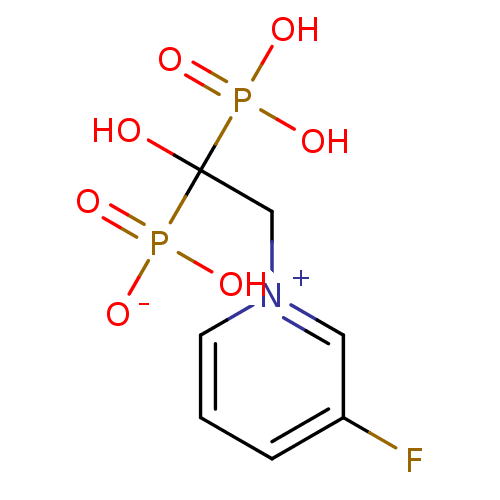 (BPH-461 | CHEMBL193722 | hydrogen 2-(3-fluoropyrid...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165350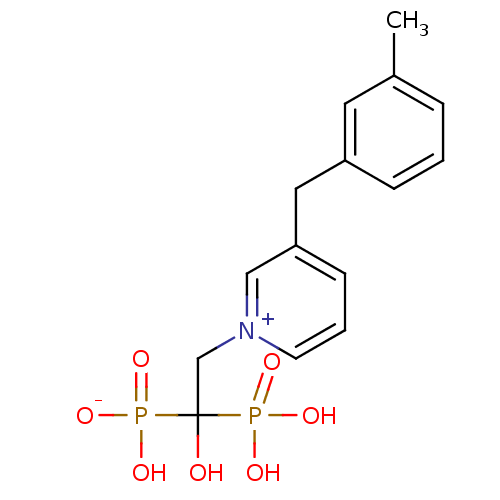 (CHEMBL192938 | hydrogen 1-hydroxy-2-[3-(3-methylbe...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165351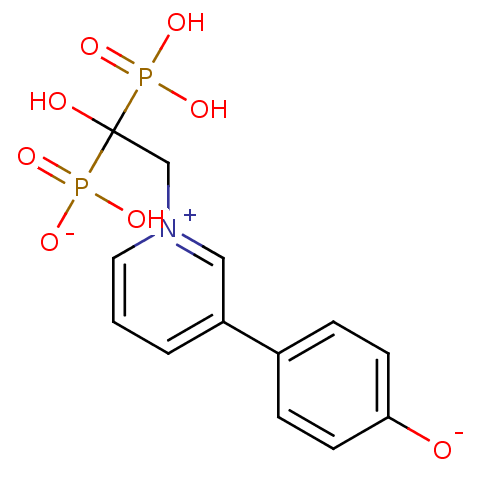 (CHEMBL193619 | sodium hydrogen 1-hydroxy-2-[3-(4-o...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165348 (CHEMBL425896 | hydrogen 1-hydroxy-2-isoquinolinium...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493248 (CHEMBL2420252) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM25313 ((4-amino-1-hydroxy-1-phosphonobutyl)phosphonic aci...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165338 (CHEMBL190258 | hydrogen 2-(4-benzylpyridinium-1-yl...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266830 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-iodophenyl)-N-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165339 (CHEMBL363434 | hydrogen 2-(3-benzylpyridinium-1-yl...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM12581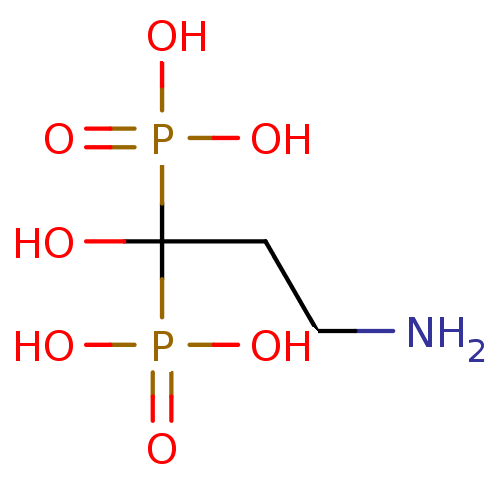 ((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165341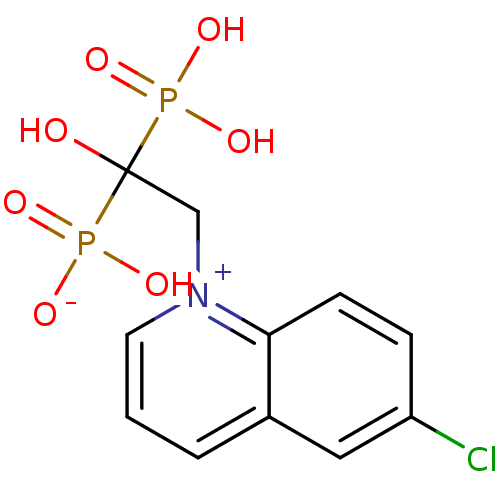 (CHEMBL193131 | hydrogen 2-(6-chloroquinolinium-1-y...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267372 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493247 (CHEMBL2420248) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 527 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM25297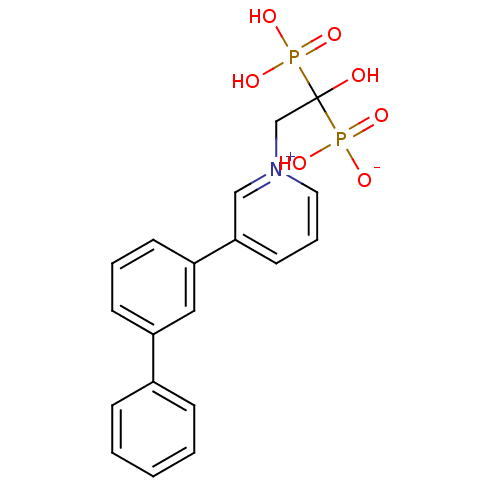 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266831 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-bromophenyl)-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493246 (CHEMBL2420250) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266810 (1-(2-Bromophenyl)-5-(4-methoxyphenyl)-N3-(piperidi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM12578 (2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50422472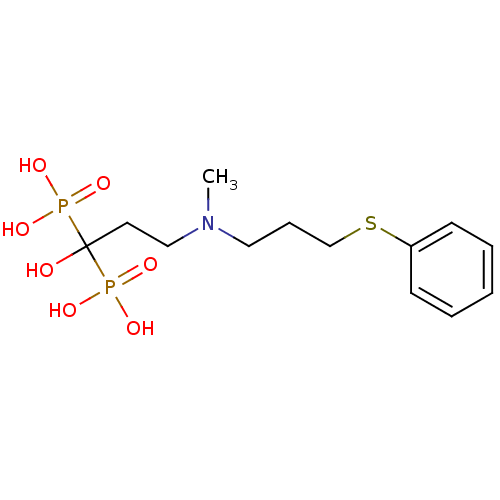 (CHEMBL100827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25290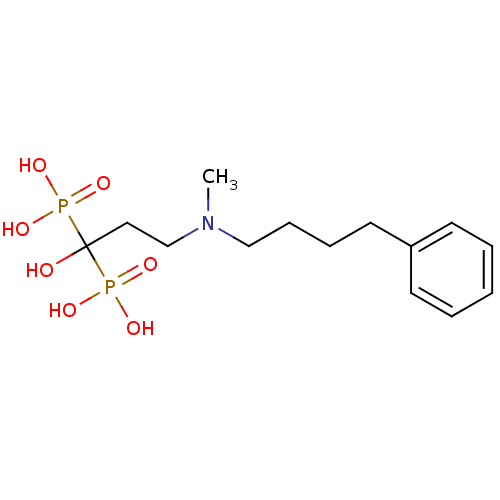 (CHEMBL56073 | bisphosphonate, 39 | {1-hydroxy-3-[m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50098389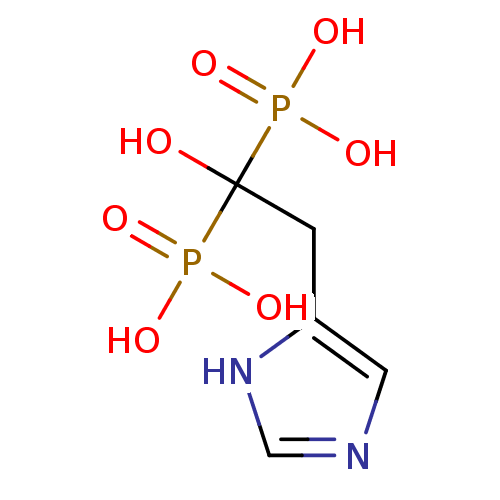 (1-hydroxy-2-(1H-imidazol-5-yl)ethane-1,1-diyldipho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50422449 (CHEMBL101886) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50422469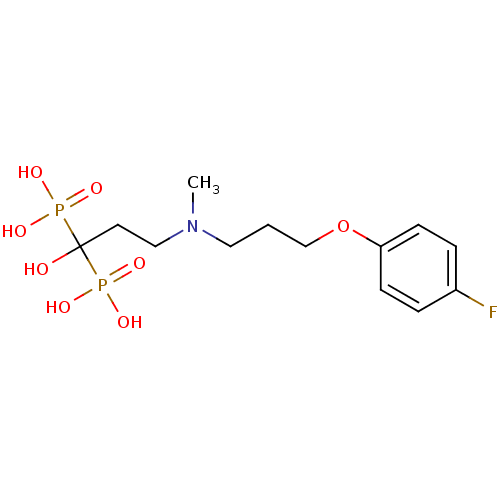 (CHEMBL101472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50422457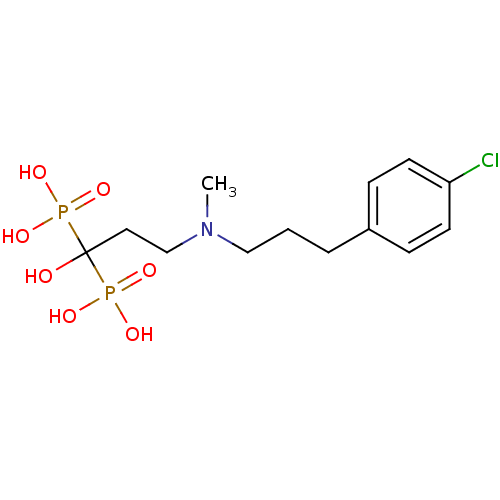 (CHEMBL101230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50422470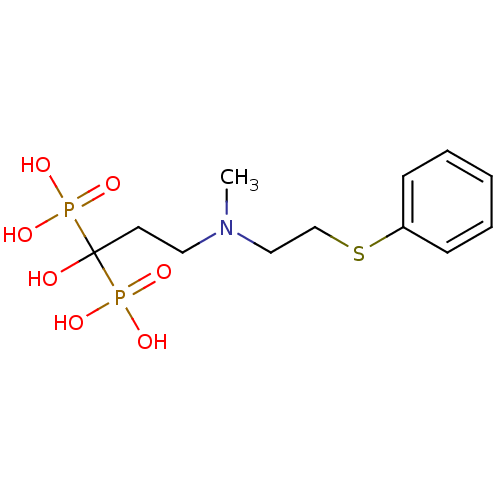 (CHEMBL100508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50422463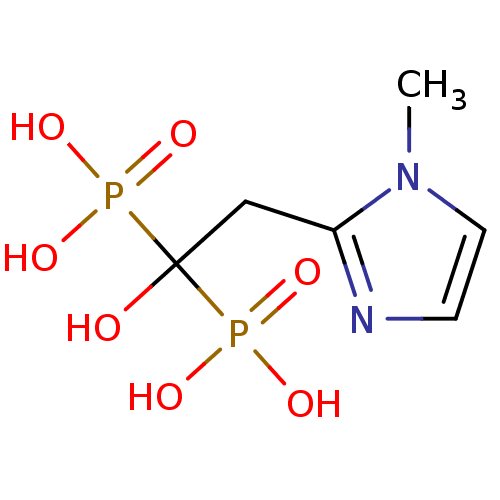 (CHEMBL101207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50117257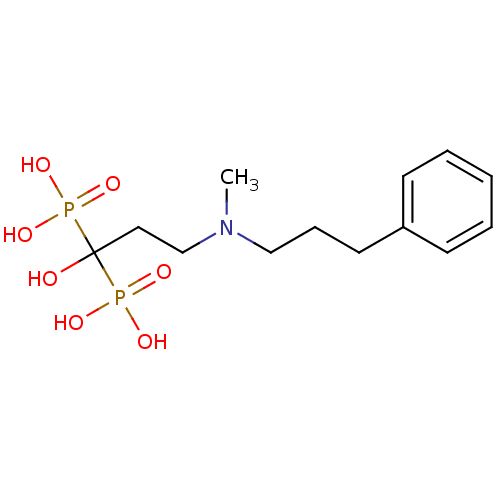 (1-hydroxy-3-(methyl(3-phenylpropyl)amino)propane-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Negative logarithm of inhibitory concentration against bone resorption | J Med Chem 46: 2932-44 (2003) Article DOI: 10.1021/jm030054u BindingDB Entry DOI: 10.7270/Q2R78GHD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 190 total ) | Next | Last >> |