

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582801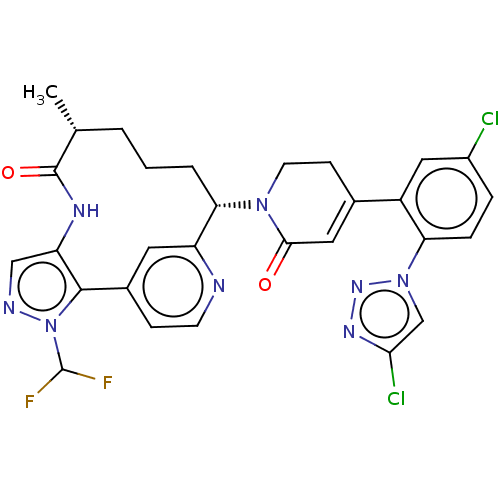 (CHEMBL5076656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247411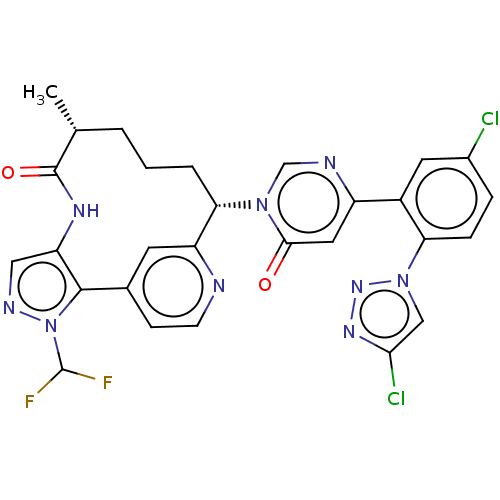 (US10336754, Example 353 | US11053247, Example 353 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582799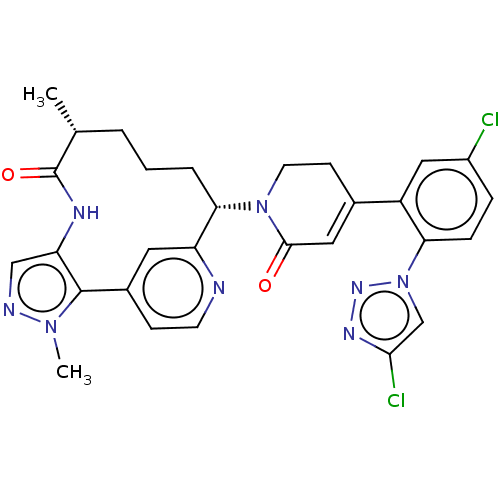 (CHEMBL5094166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50454871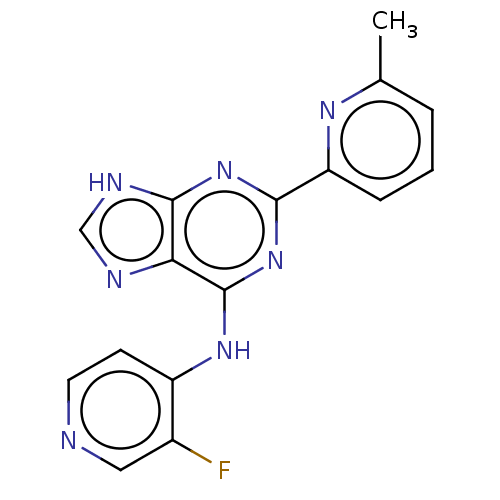 (CHEMBL4209835) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412734 (N-(3-fluoropyridin-4-yl)-2-(6-methylpyridin-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412755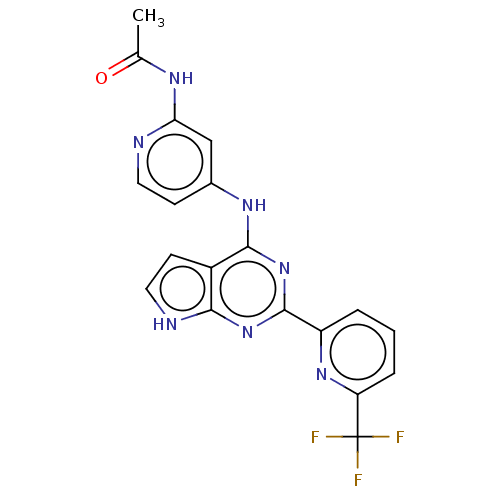 (N-(4-((2-(6-(trifluoromethyl)pyridin-2-yl)-7H-pyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582800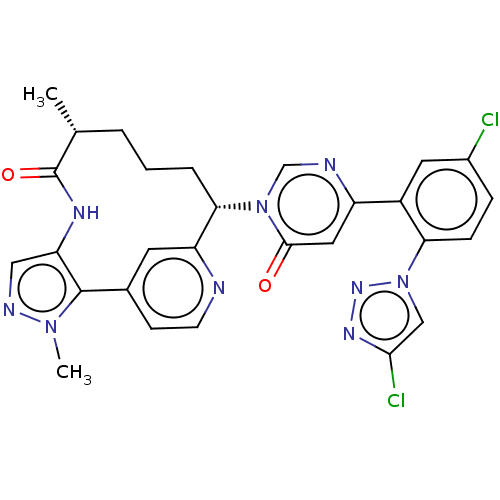 (CHEMBL5093567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27210 ((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27208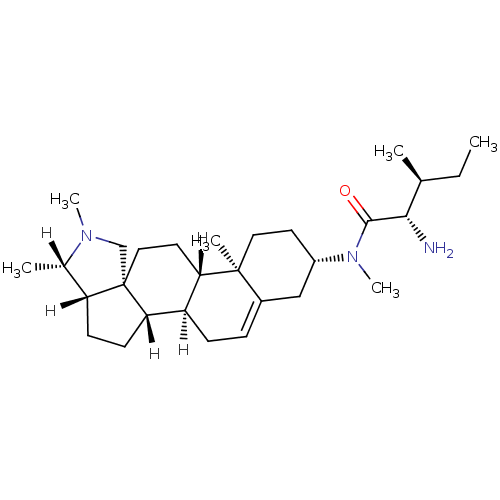 ((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412745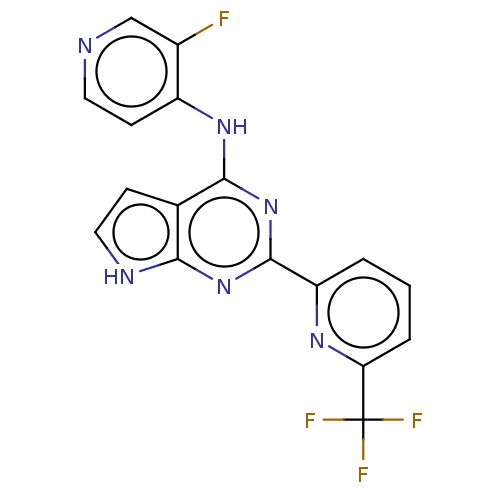 (N-(3-fluoropyridin-4-yl)-2-(6-(trifluoromethyl)pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PDB UniChem | PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541586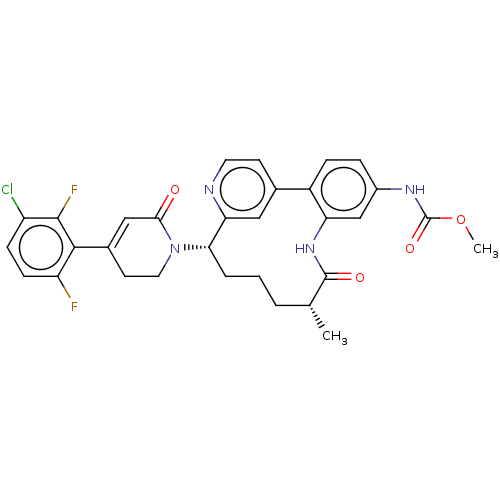 (CHEMBL4638245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120625 (CHEMBL3618330) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27209 ((2R,3R)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120625 (CHEMBL3618330) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120624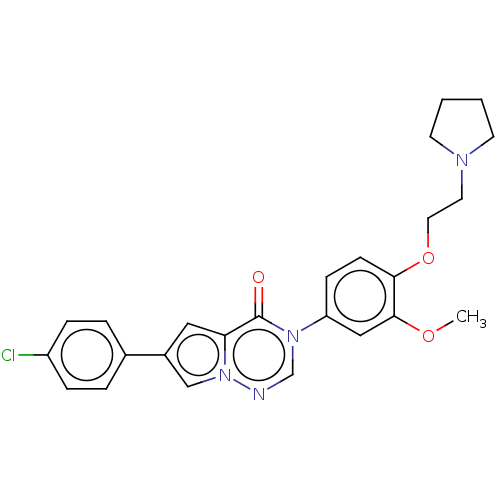 (CHEMBL3618324) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27208 ((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120635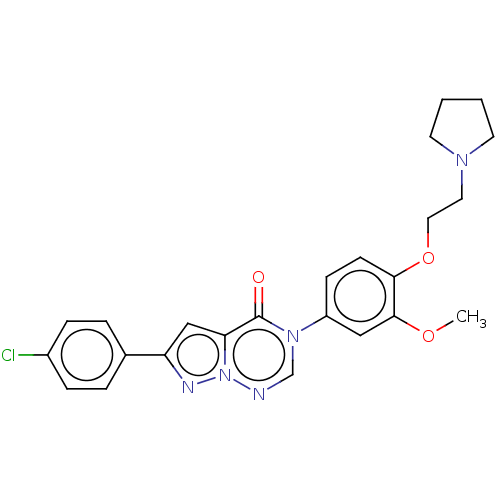 (CHEMBL3618325) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120624 (CHEMBL3618324) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120635 (CHEMBL3618325) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120628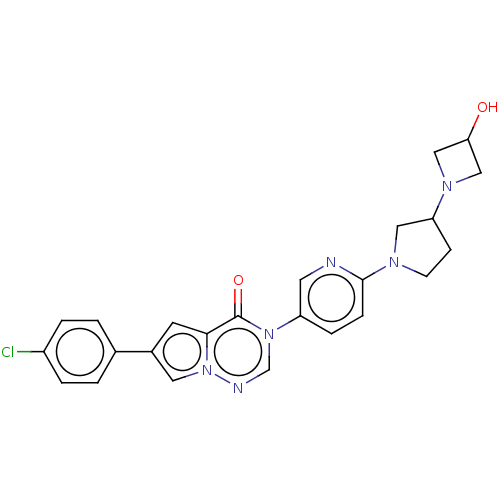 (CHEMBL3618334) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582798 (CHEMBL5082323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582795 (CHEMBL5081455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27212 ((2S)-2-acetamido-N-methyl-3-phenyl-N-[(1R,2S,5S,6S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.15 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582794 (CHEMBL5077709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120796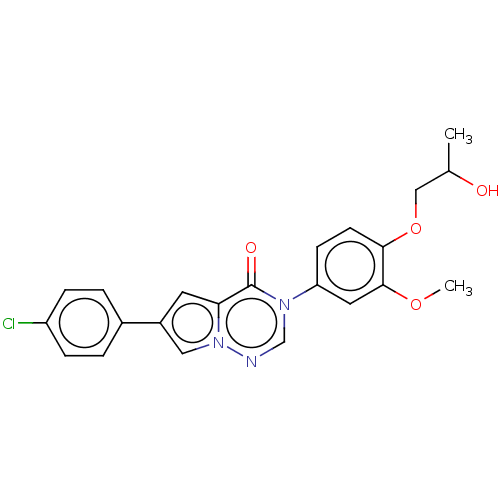 (CHEMBL3618363) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50119707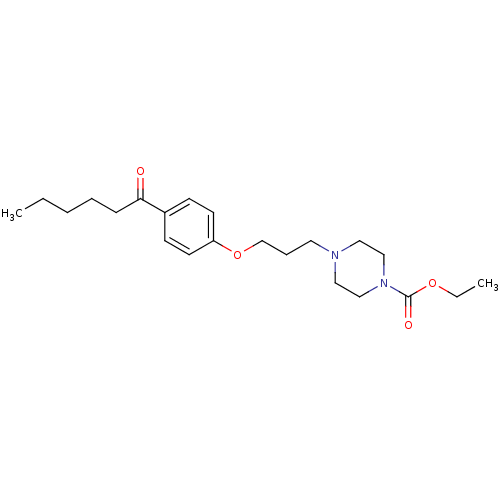 (4-[3-(4-Hexanoyl-phenoxy)-propyl]-piperazine-1-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity for the rat cortical Histamine H3 receptor | Bioorg Med Chem Lett 12: 2031-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6G21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120628 (CHEMBL3618334) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120676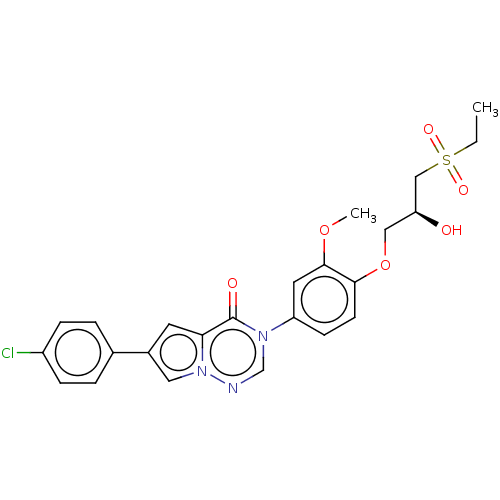 (CHEMBL3618340) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27211 ((2S)-2-acetamido-N,4-dimethyl-N-[(1R,2S,5S,6S,9R,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.62 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27201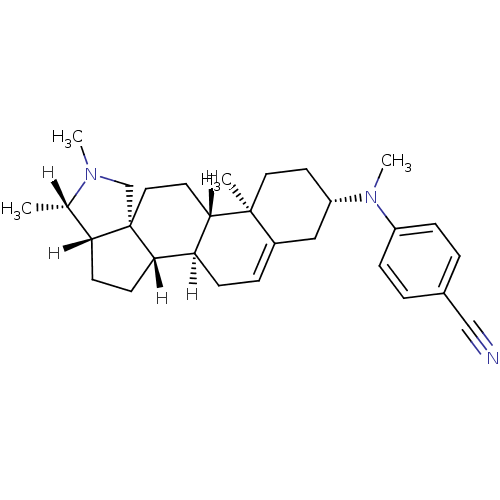 (4-{methyl[(1R,2S,5S,6S,9R,12S,13R,16S)-6,7,13-trim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.66 | -50.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582793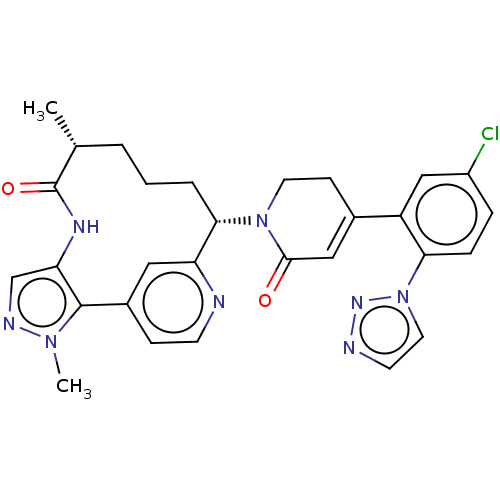 (CHEMBL5082956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120629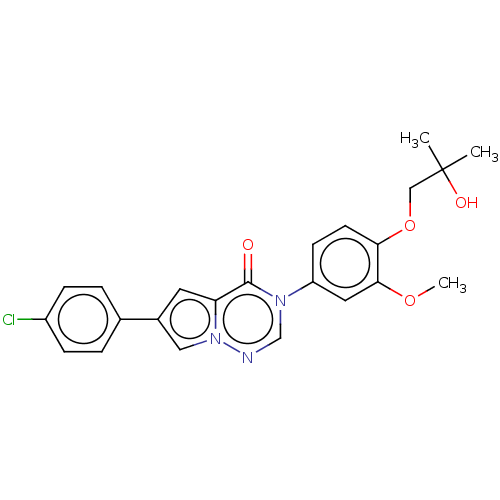 (CHEMBL3618336) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50220338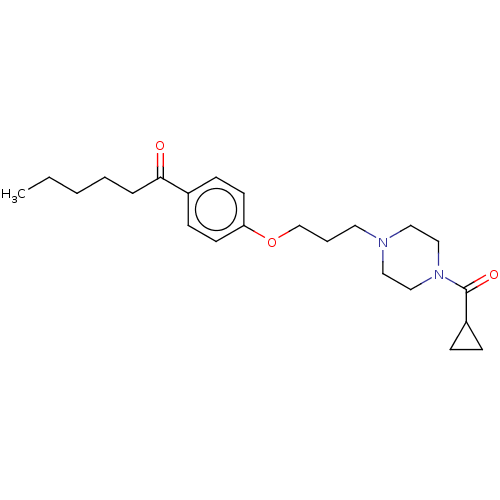 (CHEMBL302886) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity for the rat cortical Histamine H3 receptor | Bioorg Med Chem Lett 12: 2031-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6G21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27212 ((2S)-2-acetamido-N-methyl-3-phenyl-N-[(1R,2S,5S,6S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120629 (CHEMBL3618336) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120670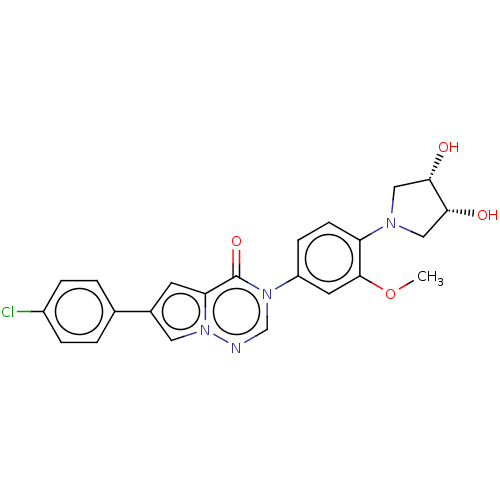 (CHEMBL3618338) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120676 (CHEMBL3618340) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27210 ((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120634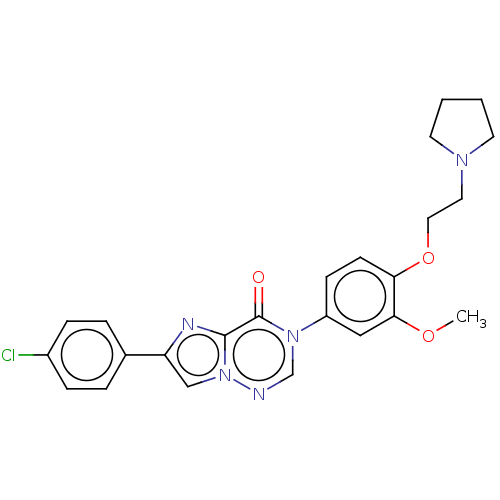 (CHEMBL3618326) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6571-80 (2008) Article DOI: 10.1021/jm8005959 BindingDB Entry DOI: 10.7270/Q24J0CD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582791 (CHEMBL5094070) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120633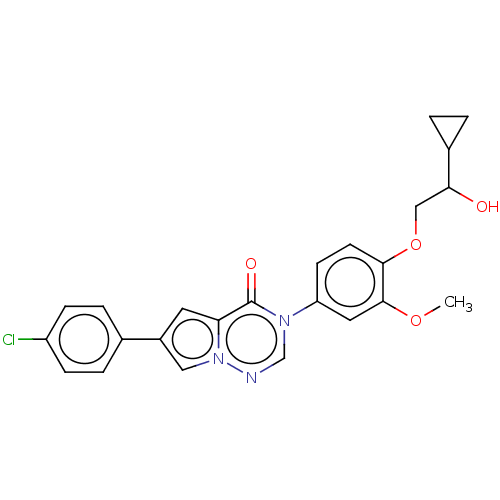 (CHEMBL3618327) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27209 ((2R,3R)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50220339 (CHEMBL64408) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity for the rat cortical Histamine H3 receptor | Bioorg Med Chem Lett 12: 2031-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6G21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120670 (CHEMBL3618338) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50220101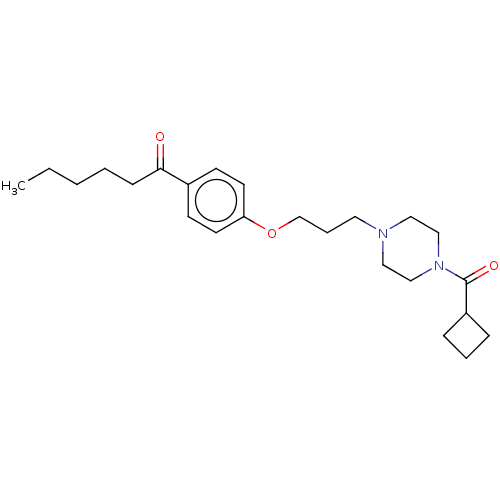 (CHEMBL64100) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity for the rat cortical Histamine H3 receptor | Bioorg Med Chem Lett 12: 2031-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6G21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50220401 (CHEMBL294502) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity for the rat cortical Histamine H3 receptor | Bioorg Med Chem Lett 12: 2031-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6G21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27200 (Conessine analogue, 12f | N-methyl-2-(thiophen-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 3.31 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120675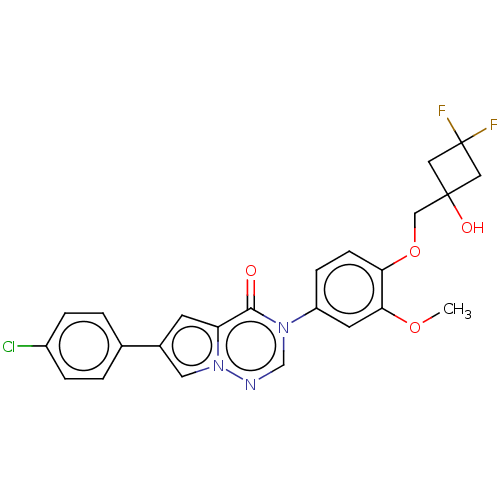 (CHEMBL3618339) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3040 total ) | Next | Last >> |