

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156461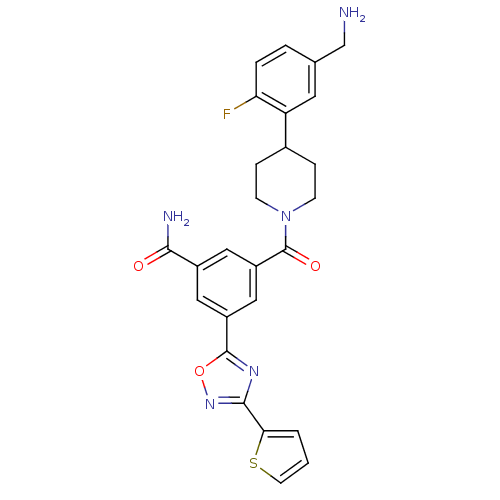 (3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156460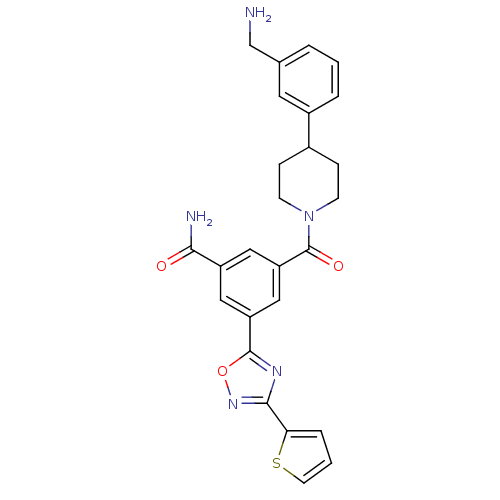 (3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbonyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156457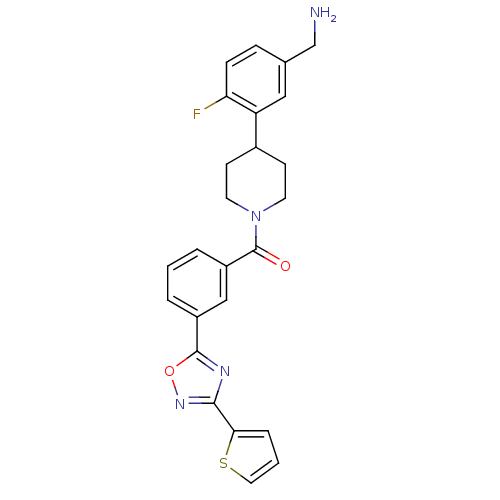 (CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50299277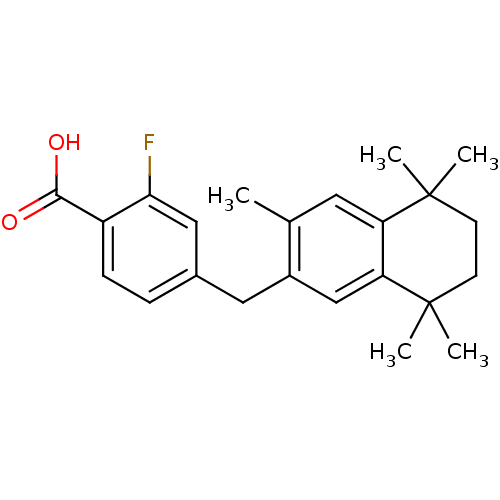 (2-Fluoro-4-(1-(1,2,3,4-tetrahydro-1,1,4,4,6-pentam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid form human RXRalpha expressed in human Caco2 cells after 16 hrs | J Med Chem 52: 5950-66 (2009) Article DOI: 10.1021/jm900496b BindingDB Entry DOI: 10.7270/Q2Q81D41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid form human RXRalpha expressed in human Caco2 cells after 16 hrs | J Med Chem 52: 5950-66 (2009) Article DOI: 10.1021/jm900496b BindingDB Entry DOI: 10.7270/Q2Q81D41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325662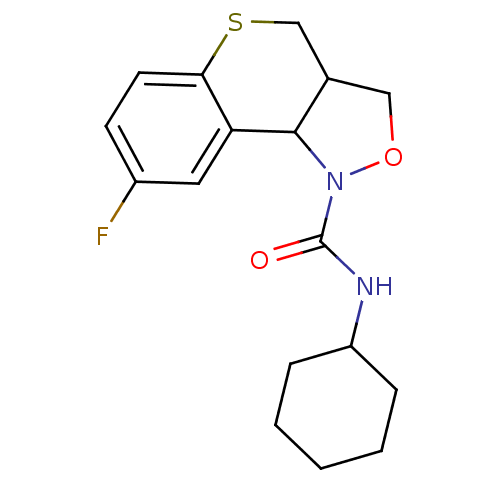 (CHEMBL1224034 | N-cyclohexyl-8-fluoro-3,3a,4,9b-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156459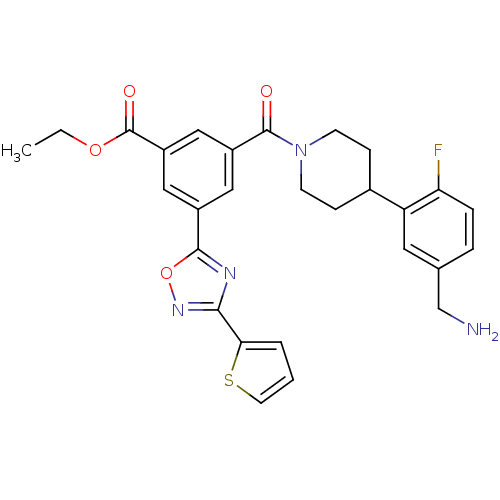 (3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50299279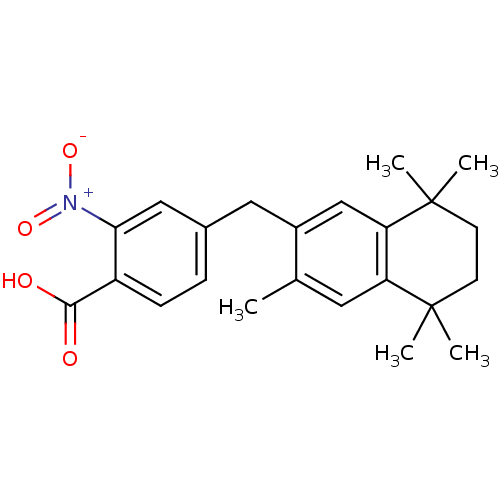 (4-(1-(1,2,3,4-Tetrahydro-1,1,4,4,6-pentamethylnaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid form human RXRalpha expressed in human Caco2 cells after 16 hrs | J Med Chem 52: 5950-66 (2009) Article DOI: 10.1021/jm900496b BindingDB Entry DOI: 10.7270/Q2Q81D41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156458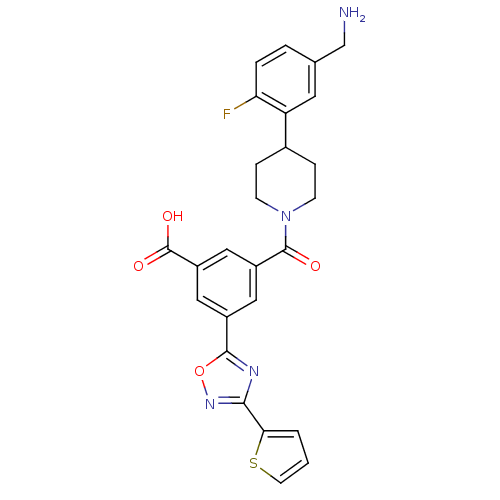 (3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50182491 (CHEBI:69015 | Malabaricone C) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60 mi... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50299278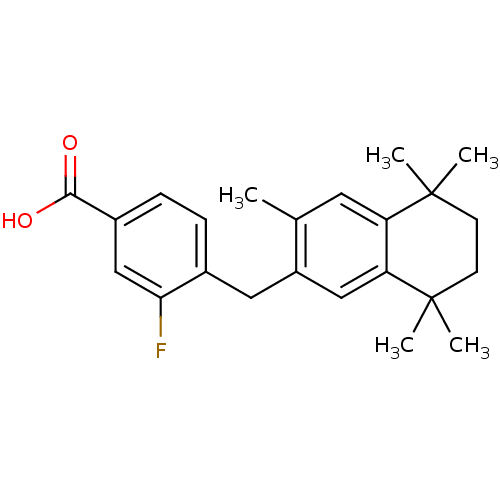 (3-Fluoro-4-(1-(1,2,3,4-tetrahydro-1,1,4,4,6-pentam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid form human RXRalpha expressed in human Caco2 cells after 16 hrs | J Med Chem 52: 5950-66 (2009) Article DOI: 10.1021/jm900496b BindingDB Entry DOI: 10.7270/Q2Q81D41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452511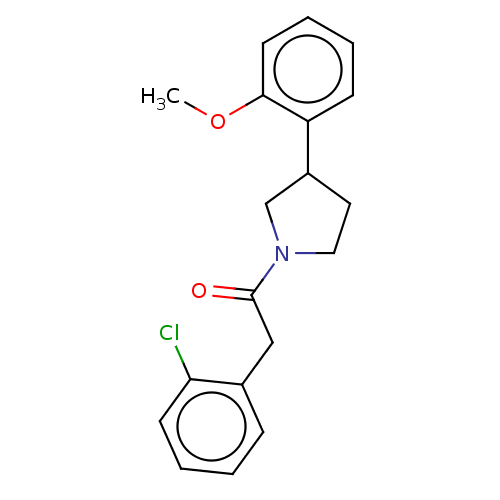 (CHEMBL4204517) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325659 (8-fluoro-N-phenyl-3,3a,4,9b-tetrahydro-1H-thiochro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325661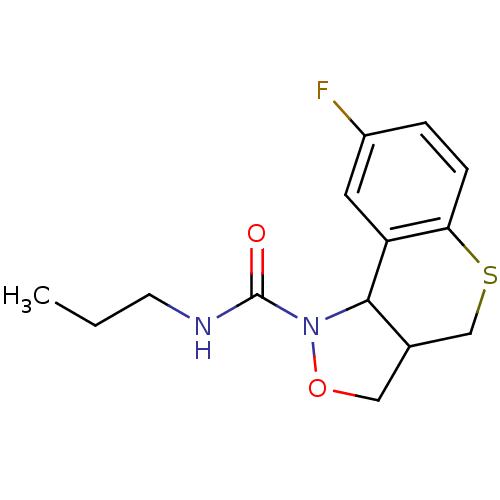 (8-fluoro-N-propyl-3,3a,4,9b-tetrahydro-1H-thiochro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452502 (CHEMBL4209594) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452501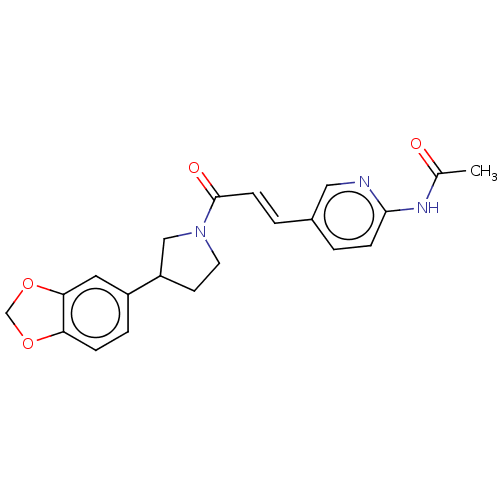 (CHEMBL4204415) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325663 (CHEMBL1224035 | N-butyl-8-fluoro-3,3a,4,9b-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 489 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50182486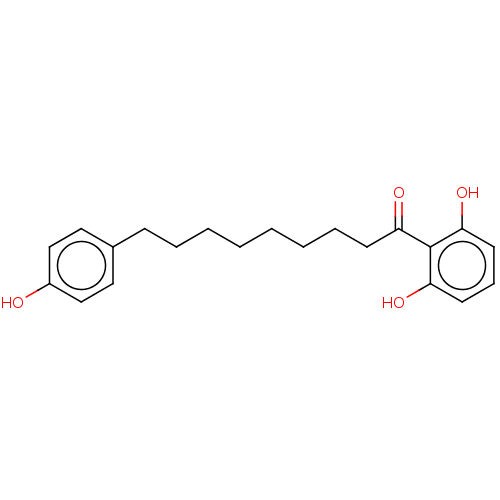 (MALABARICONE B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60 mi... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325660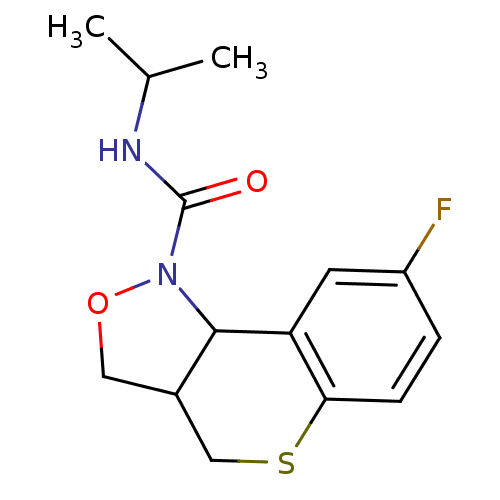 (8-fluoro-N-isopropyl-3,3a,4,9b-tetrahydro-1H-thioc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452494 (CHEMBL4204858) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452479 (CHEMBL4210008) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452512 (CHEMBL4205638) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452516 (CHEMBL4211939) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452506 (CHEMBL4216771) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452510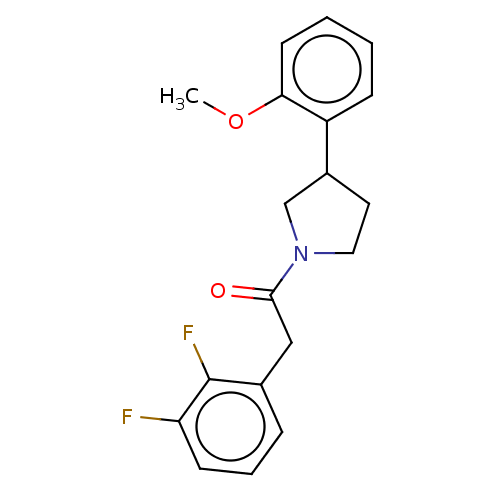 (CHEMBL4210599) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50303147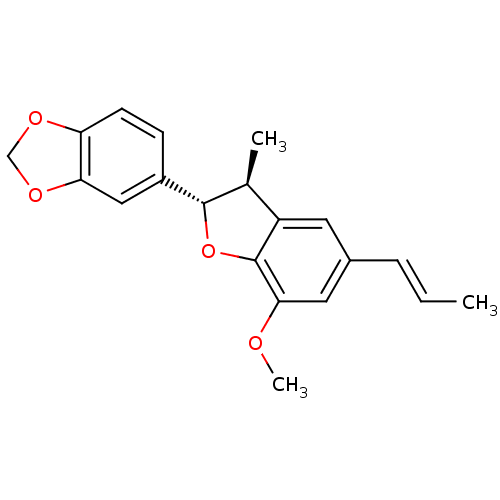 ((-)-licarin B | CHEMBL578403) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452496 (CHEMBL4217852) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452515 (CHEMBL4214096) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325664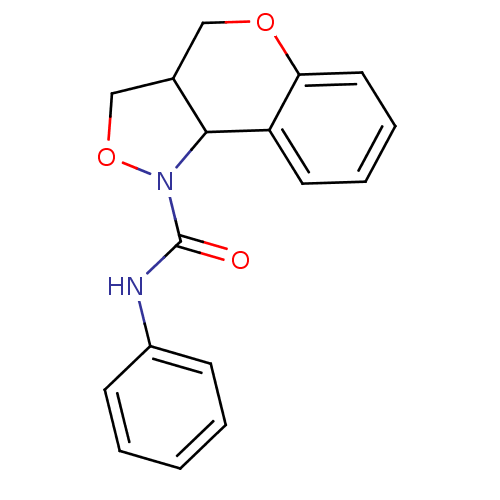 (CHEMBL1224036 | N-phenyl-3,3a,4,9b-tetrahydro-1H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452477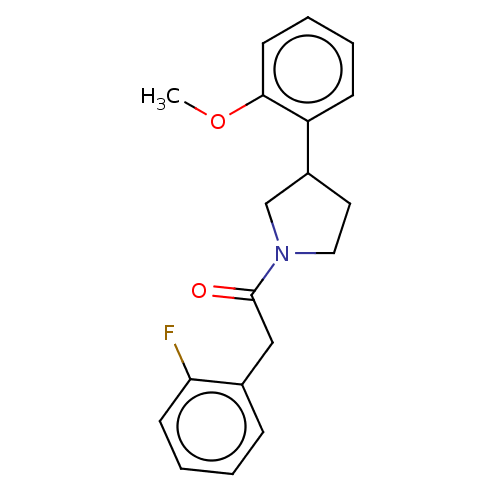 (CHEMBL4209685) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452482 (CHEMBL4208104) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325667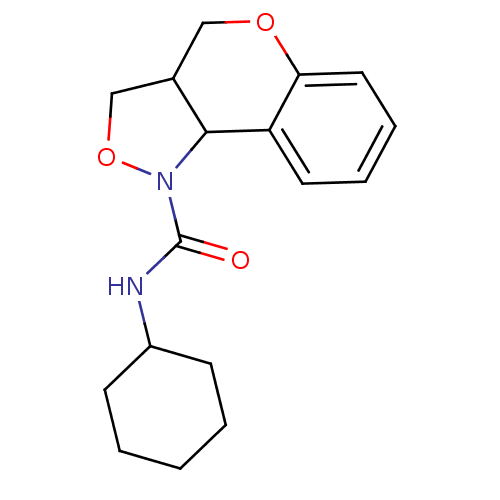 (CHEMBL1224103 | N-cyclohexyl-3,3a,4,9b-tetrahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452483 (CHEMBL4210639) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452495 (CHEMBL4218049) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452499 (CHEMBL4213777) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452507 (CHEMBL4205014) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50259983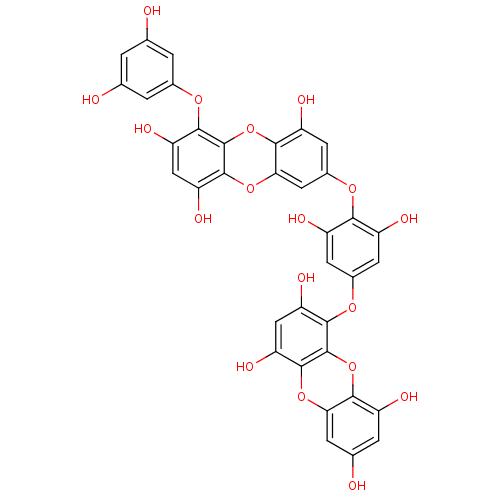 (CHEMBL508791 | US10106521, Compound Dieckol | diec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50259983 (CHEMBL508791 | US10106521, Compound Dieckol | diec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452509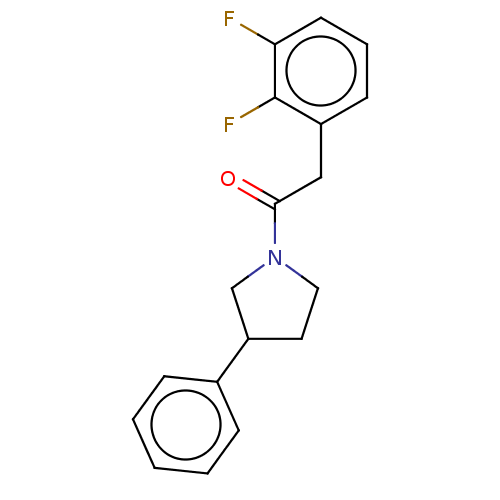 (CHEMBL4217672) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242962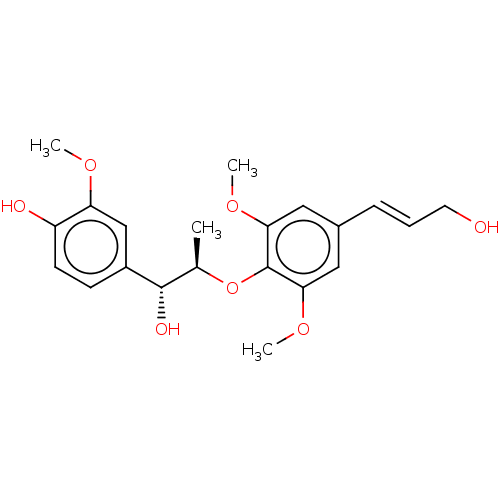 (CHEMBL4079519) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50157325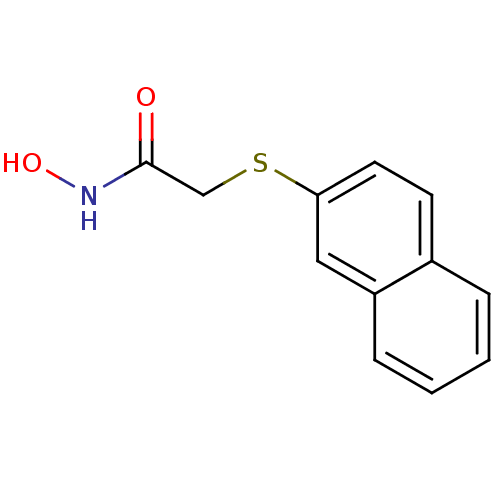 (CHEMBL182879 | N-Hydroxy-2-(naphthalen-2-ylsulfany...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity towards aminopeptidase N | Bioorg Med Chem Lett 15: 181-3 (2004) Article DOI: 10.1016/j.bmcl.2004.10.010 BindingDB Entry DOI: 10.7270/Q2BG2PRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325670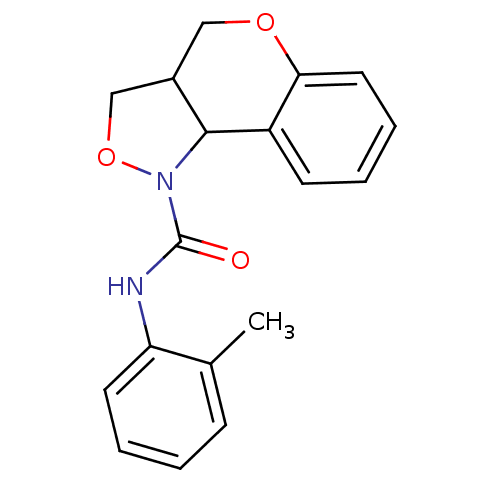 (CHEMBL1224106 | N-o-tolyl-3,3a,4,9b-tetrahydro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452497 (CHEMBL4205565) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242963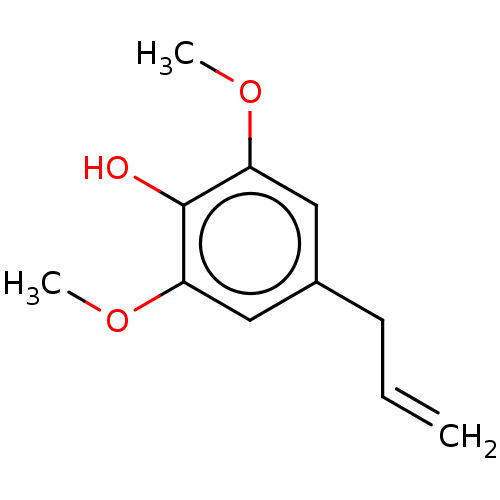 (CHEBI:86562 | Methoxyeugenol) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452508 (CHEMBL4209393) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242964 (CHEMBL4072450) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50325659 (8-fluoro-N-phenyl-3,3a,4,9b-tetrahydro-1H-thiochro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5HT2C receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50325666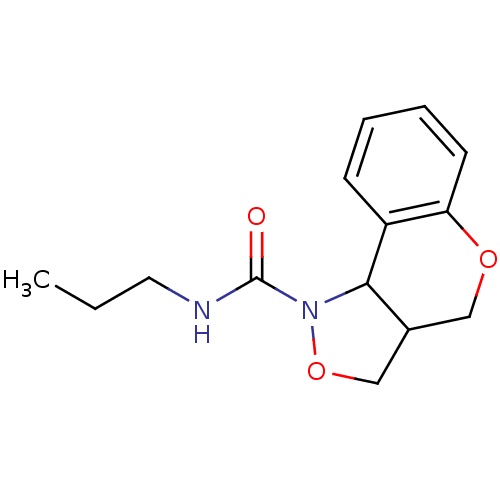 (CHEMBL1224102 | N-propyl-3,3a,4,9b-tetrahydro-1H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 5488-90 (2010) Article DOI: 10.1016/j.bmcl.2010.07.074 BindingDB Entry DOI: 10.7270/Q2M32VZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452481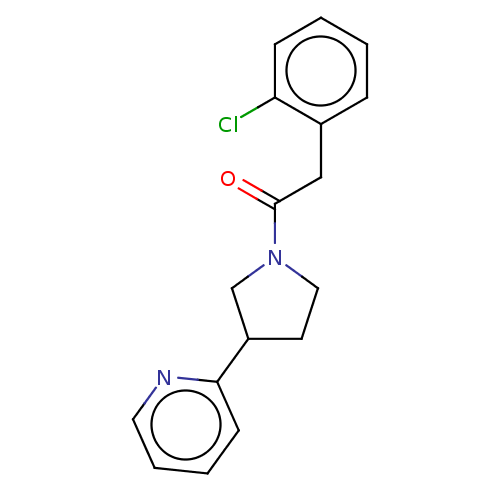 (CHEMBL4216697) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50259982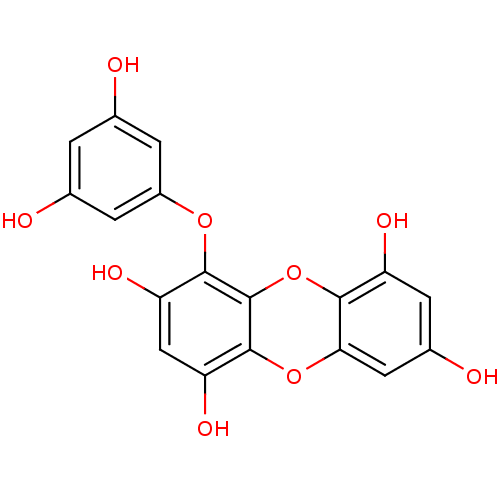 (CHEMBL471187 | US10106521, Compound Eckol | eckol) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1258 total ) | Next | Last >> |