 Found 391 hits with Last Name = 'léger' and Initial = 's'
Found 391 hits with Last Name = 'léger' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50306307

((S)-2-((S)-1-(4'-((S)-1-amino-1-oxopropan-2-yl)bip...)Show SMILES C[C@H](C(N)=O)c1ccc(cc1)-c1ccc(cc1)[C@H](N[C@@H](CC(C)(C)F)C(=O)NC1(CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C27H30F4N4O2/c1-16(23(33)36)17-4-6-18(7-5-17)19-8-10-20(11-9-19)22(27(29,30)31)34-21(14-25(2,3)28)24(37)35-26(15-32)12-13-26/h4-11,16,21-22,34H,12-14H2,1-3H3,(H2,33,36)(H,35,37)/t16-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50233032
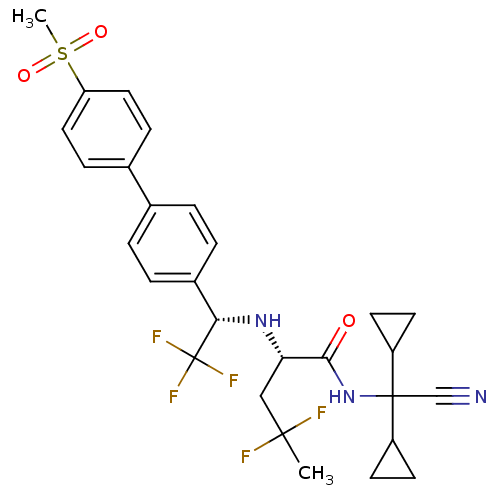
((S)-4,4-difluoro-2-[(S)-2,2,2-trifluoro-1-(4'-meth...)Show SMILES CC(F)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC(C#N)(C1CC1)C1CC1 Show InChI InChI=1S/C28H30F5N3O3S/c1-26(29,30)15-23(25(37)36-27(16-34,20-9-10-20)21-11-12-21)35-24(28(31,32)33)19-5-3-17(4-6-19)18-7-13-22(14-8-18)40(2,38)39/h3-8,13-14,20-21,23-24,35H,9-12,15H2,1-2H3,(H,36,37)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753
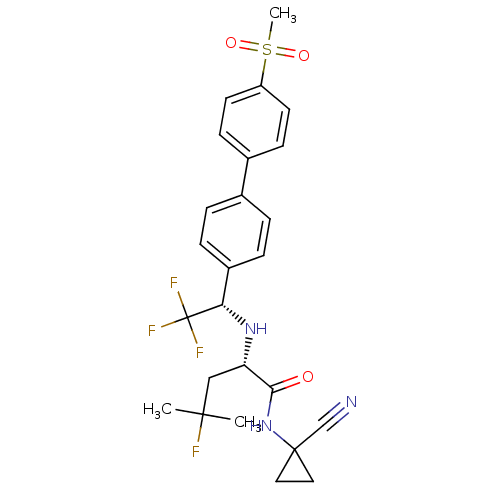
(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50214543

((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C#N Show InChI InChI=1S/C26H32F3N3O3S2/c1-17(2)15-23(25(33)31-21(16-30)13-14-36-3)32-24(26(27,28)29)20-7-5-18(6-8-20)19-9-11-22(12-10-19)37(4,34)35/h5-12,17,21,23-24,32H,13-15H2,1-4H3,(H,31,33)/t21-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 17: 4328-32 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.024
BindingDB Entry DOI: 10.7270/Q2TQ618K |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489
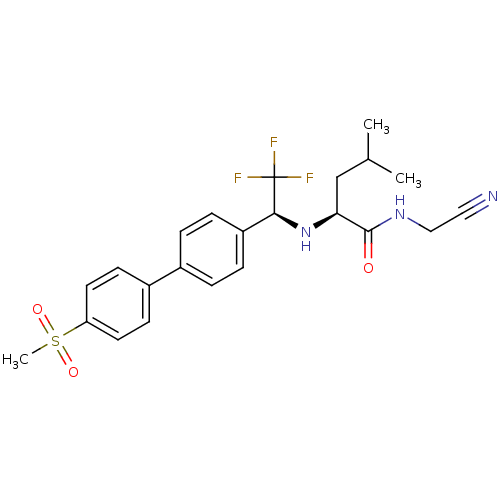
((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306306
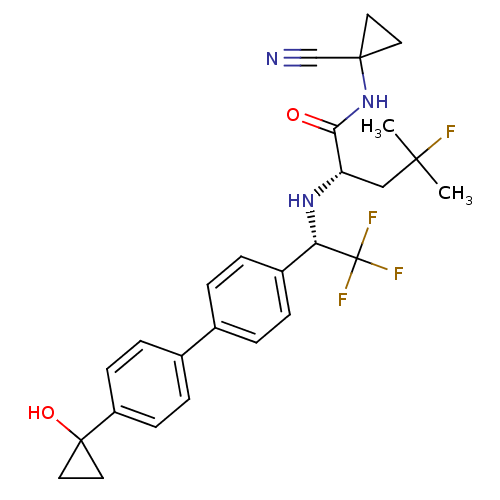
((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(O)CC1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H29F4N3O2/c1-24(2,28)15-21(23(35)34-25(16-32)11-12-25)33-22(27(29,30)31)19-5-3-17(4-6-19)18-7-9-20(10-8-18)26(36)13-14-26/h3-10,21-22,33,36H,11-15H2,1-2H3,(H,34,35)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489

((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306309

(1-(4'-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-fl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C28H30F4N4O2/c1-25(2,29)15-21(23(37)36-26(16-33)11-12-26)35-22(28(30,31)32)19-5-3-17(4-6-19)18-7-9-20(10-8-18)27(13-14-27)24(34)38/h3-10,21-22,35H,11-15H2,1-2H3,(H2,34,38)(H,36,37)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306304

((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C(C)(C)O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H31F4N3O2/c1-24(2,28)15-21(23(35)34-26(16-32)13-14-26)33-22(27(29,30)31)19-7-5-17(6-8-19)18-9-11-20(12-10-18)25(3,4)36/h5-12,21-22,33,36H,13-15H2,1-4H3,(H,34,35)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489

((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489

((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 17: 4328-32 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.024
BindingDB Entry DOI: 10.7270/Q2TQ618K |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306305
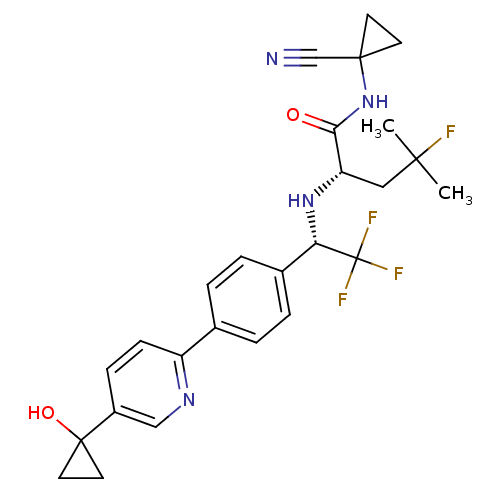
((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cn1)C1(O)CC1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H28F4N4O2/c1-23(2,27)13-20(22(35)34-24(15-31)9-10-24)33-21(26(28,29)30)17-5-3-16(4-6-17)19-8-7-18(14-32-19)25(36)11-12-25/h3-8,14,20-21,33,36H,9-13H2,1-2H3,(H,34,35)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50233036
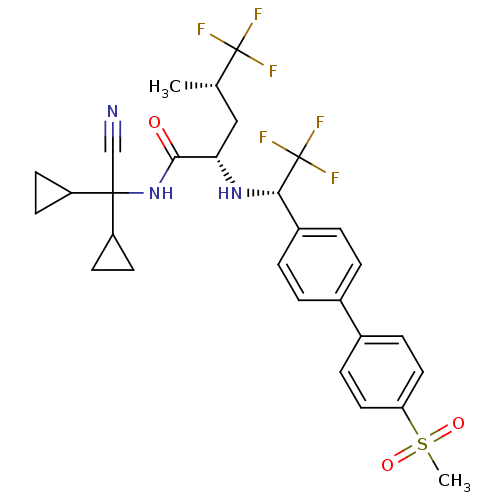
((2S,4S)-5,5,5-trifluoro-4-methyl-2-[(S)-2,2,2-trif...)Show SMILES C[C@@H](C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC(C#N)(C1CC1)C1CC1)C(F)(F)F Show InChI InChI=1S/C29H31F6N3O3S/c1-17(28(30,31)32)15-24(26(39)38-27(16-36,21-9-10-21)22-11-12-22)37-25(29(33,34)35)20-5-3-18(4-6-20)19-7-13-23(14-8-19)42(2,40)41/h3-8,13-14,17,21-22,24-25,37H,9-12,15H2,1-2H3,(H,38,39)/t17-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50233039
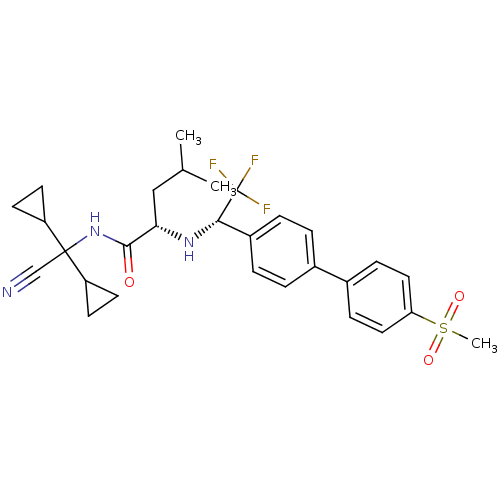
((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC(C#N)(C1CC1)C1CC1 Show InChI InChI=1S/C29H34F3N3O3S/c1-18(2)16-25(27(36)35-28(17-33,22-10-11-22)23-12-13-23)34-26(29(30,31)32)21-6-4-19(5-7-21)20-8-14-24(15-9-20)39(3,37)38/h4-9,14-15,18,22-23,25-26,34H,10-13,16H2,1-3H3,(H,35,36)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336095
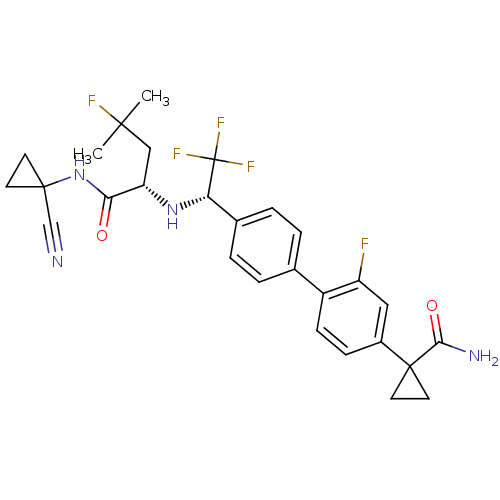
(1-(4'-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-fl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1F)C1(CC1)C(N)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C28H29F5N4O2/c1-25(2,30)14-21(23(38)37-26(15-34)9-10-26)36-22(28(31,32)33)17-5-3-16(4-6-17)19-8-7-18(13-20(19)29)27(11-12-27)24(35)39/h3-8,13,21-22,36H,9-12,14H2,1-2H3,(H2,35,39)(H,37,38)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336094

(1-(4'-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-fl...)Show SMILES CC(C)(F)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1F)C1(CC1)C(N)=O)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C28H30F4N4O2/c1-26(2,32)14-21(24(37)36-27(15-33)9-10-27)35-22(23(30)31)17-5-3-16(4-6-17)19-8-7-18(13-20(19)29)28(11-12-28)25(34)38/h3-8,13,21-23,35H,9-12,14H2,1-2H3,(H2,34,38)(H,36,37)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336091
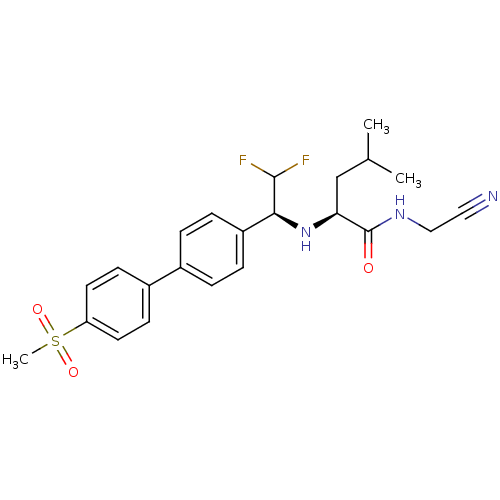
((S)-N-(cyanomethyl)-2-((S)-2,2-difluoro-1-(4'-(met...)Show SMILES CC(C)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)NCC#N |r| Show InChI InChI=1S/C23H27F2N3O3S/c1-15(2)14-20(23(29)27-13-12-26)28-21(22(24)25)18-6-4-16(5-7-18)17-8-10-19(11-9-17)32(3,30)31/h4-11,15,20-22,28H,13-14H2,1-3H3,(H,27,29)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19491
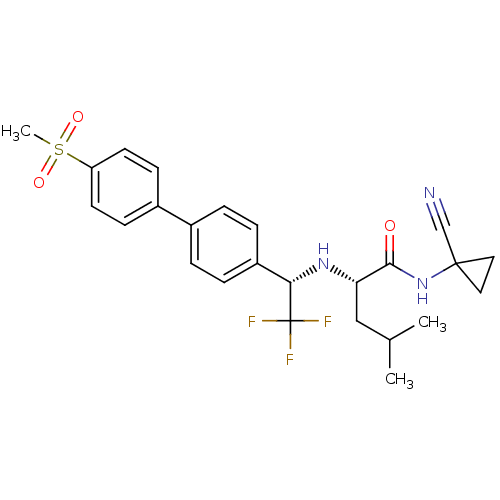
((2S)-N-(1-cyanocyclopropyl)-4-methyl-2-{[(1S)-2,2,...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H28F3N3O3S/c1-16(2)14-21(23(32)31-24(15-29)12-13-24)30-22(25(26,27)28)19-6-4-17(5-7-19)18-8-10-20(11-9-18)35(3,33)34/h4-11,16,21-22,30H,12-14H2,1-3H3,(H,31,32)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306310

((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES Cc1csc(n1)-c1ccc(cc1)[C@H](N[C@@H](CC(C)(C)F)C(=O)NC1(CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C22H24F4N4OS/c1-13-11-32-19(28-13)15-6-4-14(5-7-15)17(22(24,25)26)29-16(10-20(2,3)23)18(31)30-21(12-27)8-9-21/h4-7,11,16-17,29H,8-10H2,1-3H3,(H,30,31)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19847

((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-[4-(meth...)Show SMILES CSc1ccc(cc1)-c1ccccc1[C@@H]1CCC(F)(F)C[C@H]1C(=O)NCC#N |r| Show InChI InChI=1S/C22H22F2N2OS/c1-28-16-8-6-15(7-9-16)17-4-2-3-5-18(17)19-10-11-22(23,24)14-20(19)21(27)26-13-12-25/h2-9,19-20H,10-11,13-14H2,1H3,(H,26,27)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rabbit cathepsin K |
J Med Chem 51: 6410-20 (2008)
Article DOI: 10.1021/jm800610j
BindingDB Entry DOI: 10.7270/Q261105F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50240981
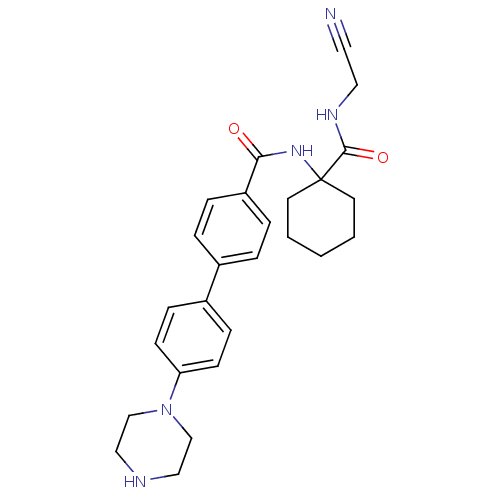
(4'-piperazin-1-yl-biphenyl-4-carboxylic acid [1-(c...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C26H31N5O2/c27-14-15-29-25(33)26(12-2-1-3-13-26)30-24(32)22-6-4-20(5-7-22)21-8-10-23(11-9-21)31-18-16-28-17-19-31/h4-11,28H,1-3,12-13,15-19H2,(H,29,33)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306311
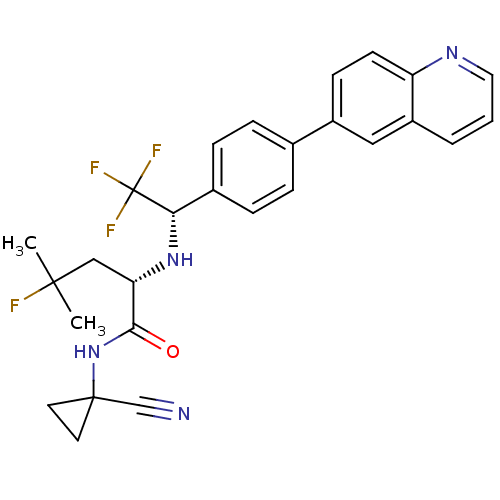
((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc2ncccc2c1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H26F4N4O/c1-25(2,28)15-22(24(36)35-26(16-32)11-12-26)34-23(27(29,30)31)18-7-5-17(6-8-18)19-9-10-21-20(14-19)4-3-13-33-21/h3-10,13-14,22-23,34H,11-12,15H2,1-2H3,(H,35,36)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306302

((S)-N-(1-cyanocyclopropyl)-2-((S)-1-(4'-((R)-2,2-d...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)[C@@H](O)C(F)F)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H27F6N3O2/c1-24(2,29)13-19(23(37)35-25(14-33)11-12-25)34-21(26(30,31)32)18-9-5-16(6-10-18)15-3-7-17(8-4-15)20(36)22(27)28/h3-10,19-22,34,36H,11-13H2,1-2H3,(H,35,37)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306303

((S)-N-(1-cyanocyclopropyl)-2-((S)-1-(4'-((S)-2,2-d...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)[C@H](O)C(F)F)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H27F6N3O2/c1-24(2,29)13-19(23(37)35-25(14-33)11-12-25)34-21(26(30,31)32)18-9-5-16(6-10-18)15-3-7-17(8-4-15)20(36)22(27)28/h3-10,19-22,34,36H,11-13H2,1-2H3,(H,35,37)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50233034

((2S,4R)-5,5,5-trifluoro-4-methyl-2-[(S)-2,2,2-trif...)Show SMILES C[C@H](C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC(C#N)(C1CC1)C1CC1)C(F)(F)F Show InChI InChI=1S/C29H31F6N3O3S/c1-17(28(30,31)32)15-24(26(39)38-27(16-36,21-9-10-21)22-11-12-22)37-25(29(33,34)35)20-5-3-18(4-6-20)19-7-13-23(14-8-19)42(2,40)41/h3-8,13-14,17,21-22,24-25,37H,9-12,15H2,1-2H3,(H,38,39)/t17-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19909

((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{1-methyl-4...)Show SMILES CSc1ccc(cc1)-c1cn(C)nc1[C@@H]1CCC(F)(F)C[C@H]1C(=O)NCC#N |r| Show InChI InChI=1S/C20H22F2N4OS/c1-26-12-17(13-3-5-14(28-2)6-4-13)18(25-26)15-7-8-20(21,22)11-16(15)19(27)24-10-9-23/h3-6,12,15-16H,7-8,10-11H2,1-2H3,(H,24,27)/t15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rabbit cathepsin K |
J Med Chem 51: 6410-20 (2008)
Article DOI: 10.1021/jm800610j
BindingDB Entry DOI: 10.7270/Q261105F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306308
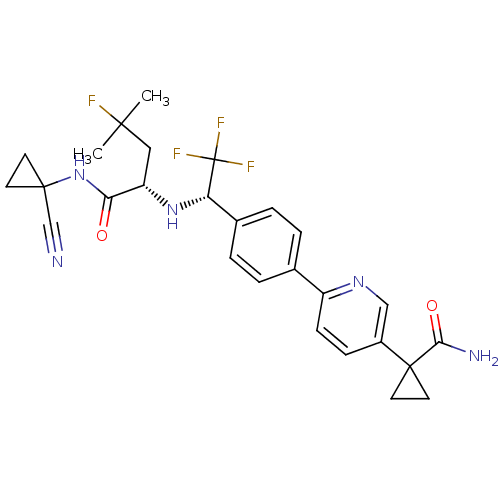
(1-(6-(4-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cn1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H29F4N5O2/c1-24(2,28)13-20(22(37)36-25(15-32)9-10-25)35-21(27(29,30)31)17-5-3-16(4-6-17)19-8-7-18(14-34-19)26(11-12-26)23(33)38/h3-8,14,20-21,35H,9-13H2,1-2H3,(H2,33,38)(H,36,37)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19855
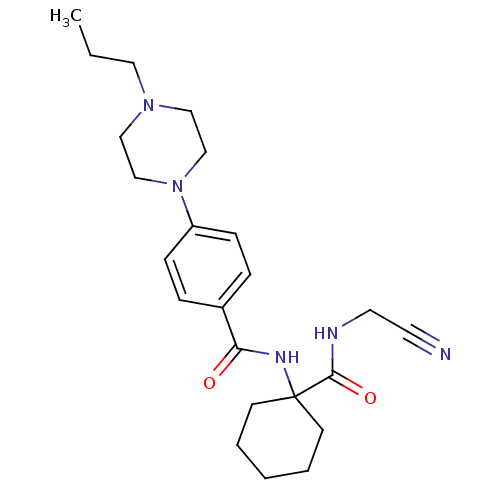
(Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...)Show SMILES CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H33N5O2/c1-2-14-27-15-17-28(18-16-27)20-8-6-19(7-9-20)21(29)26-23(10-4-3-5-11-23)22(30)25-13-12-24/h6-9H,2-5,10-11,13-18H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50214542
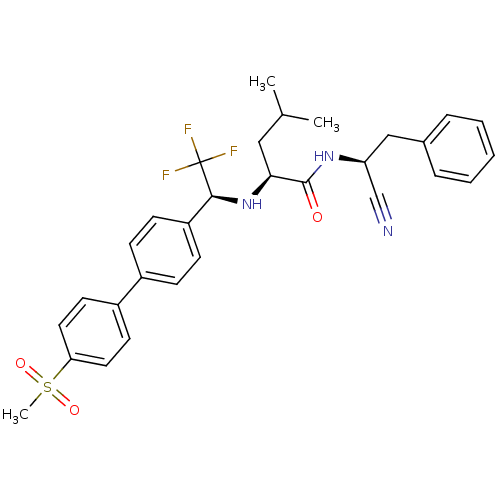
((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N Show InChI InChI=1S/C30H32F3N3O3S/c1-20(2)17-27(29(37)35-25(19-34)18-21-7-5-4-6-8-21)36-28(30(31,32)33)24-11-9-22(10-12-24)23-13-15-26(16-14-23)40(3,38)39/h4-16,20,25,27-28,36H,17-18H2,1-3H3,(H,35,37)/t25-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 17: 4328-32 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.024
BindingDB Entry DOI: 10.7270/Q2TQ618K |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50233030
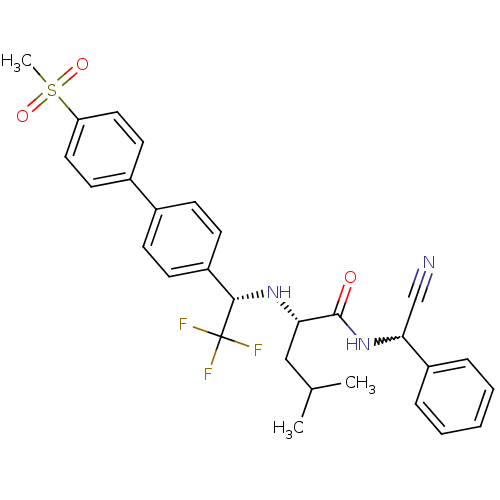
((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC(C#N)c1ccccc1 |w:30.31| Show InChI InChI=1S/C29H30F3N3O3S/c1-19(2)17-25(28(36)35-26(18-33)22-7-5-4-6-8-22)34-27(29(30,31)32)23-11-9-20(10-12-23)21-13-15-24(16-14-21)39(3,37)38/h4-16,19,25-27,34H,17H2,1-3H3,(H,35,36)/t25-,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50214542

((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N Show InChI InChI=1S/C30H32F3N3O3S/c1-20(2)17-27(29(37)35-25(19-34)18-21-7-5-4-6-8-21)36-28(30(31,32)33)24-11-9-22(10-12-24)23-13-15-26(16-14-23)40(3,38)39/h4-16,20,25,27-28,36H,17-18H2,1-3H3,(H,35,37)/t25-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19489

((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336089
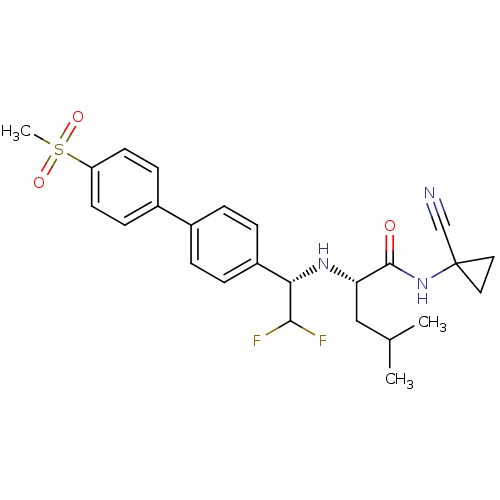
((S)-N-(1-cyanocyclopropyl)-2-((S)-2,2-difluoro-1-(...)Show SMILES CC(C)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H29F2N3O3S/c1-16(2)14-21(24(31)30-25(15-28)12-13-25)29-22(23(26)27)19-6-4-17(5-7-19)18-8-10-20(11-9-18)34(3,32)33/h4-11,16,21-23,29H,12-14H2,1-3H3,(H,30,31)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173405
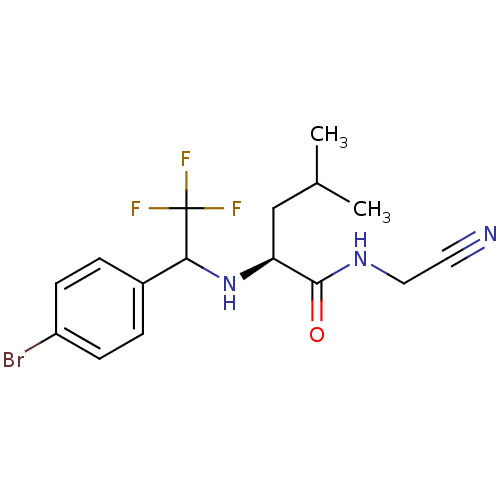
((S)-2-[1-(4-Bromo-phenyl)-2,2,2-trifluoro-ethylami...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C16H19BrF3N3O/c1-10(2)9-13(15(24)22-8-7-21)23-14(16(18,19)20)11-3-5-12(17)6-4-11/h3-6,10,13-14,23H,8-9H2,1-2H3,(H,22,24)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K from humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50214540
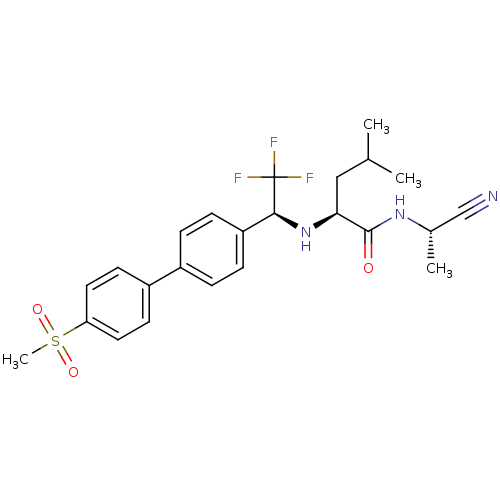
((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](C)C#N Show InChI InChI=1S/C24H28F3N3O3S/c1-15(2)13-21(23(31)29-16(3)14-28)30-22(24(25,26)27)19-7-5-17(6-8-19)18-9-11-20(12-10-18)34(4,32)33/h5-12,15-16,21-22,30H,13H2,1-4H3,(H,29,31)/t16-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 17: 4328-32 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.024
BindingDB Entry DOI: 10.7270/Q2TQ618K |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50150528

((S)-4-Methyl-2-[4-(4-piperazin-1-yl-phenyl)-thioph...)Show SMILES CC(C)C[C@H](Nc1cscc1-c1ccc(cc1)N1CCNCC1)C(=O)NCC#N Show InChI InChI=1S/C22H29N5OS/c1-16(2)13-20(22(28)25-8-7-23)26-21-15-29-14-19(21)17-3-5-18(6-4-17)27-11-9-24-10-12-27/h3-6,14-16,20,24,26H,8-13H2,1-2H3,(H,25,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336090
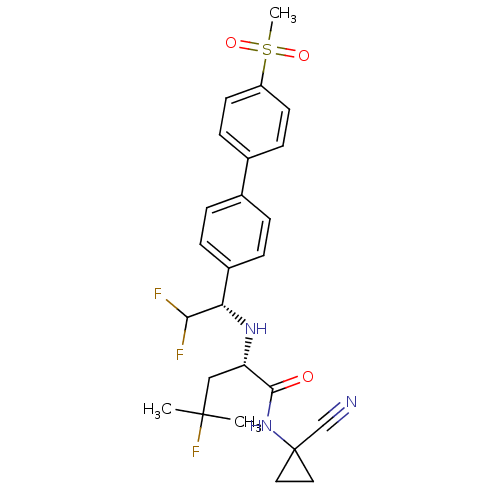
((S)-N-(1-cyanocyclopropyl)-2-((S)-2,2-difluoro-1-(...)Show SMILES CC(C)(F)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H28F3N3O3S/c1-24(2,28)14-20(23(32)31-25(15-29)12-13-25)30-21(22(26)27)18-6-4-16(5-7-18)17-8-10-19(11-9-17)35(3,33)34/h4-11,20-22,30H,12-14H2,1-3H3,(H,31,32)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50214540

((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](C)C#N Show InChI InChI=1S/C24H28F3N3O3S/c1-15(2)13-21(23(31)29-16(3)14-28)30-22(24(25,26)27)19-7-5-17(6-8-19)18-9-11-20(12-10-18)34(4,32)33/h5-12,15-16,21-22,30H,13H2,1-4H3,(H,29,31)/t16-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173405

((S)-2-[1-(4-Bromo-phenyl)-2,2,2-trifluoro-ethylami...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C16H19BrF3N3O/c1-10(2)9-13(15(24)22-8-7-21)23-14(16(18,19)20)11-3-5-12(17)6-4-11/h3-6,10,13-14,23H,8-9H2,1-2H3,(H,22,24)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
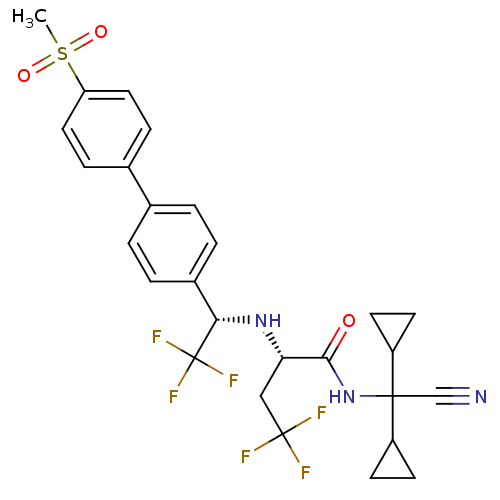
(Homo sapiens (Human)) | BDBM50233031
((S)-n-(cyano-dicyclopropyl-methyl)-4,4,4-trifluoro...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)[C@H](N[C@@H](CC(F)(F)F)C(=O)NC(C#N)(C1CC1)C1CC1)C(F)(F)F Show InChI InChI=1S/C27H27F6N3O3S/c1-40(38,39)21-12-6-17(7-13-21)16-2-4-18(5-3-16)23(27(31,32)33)35-22(14-26(28,29)30)24(37)36-25(15-34,19-8-9-19)20-10-11-20/h2-7,12-13,19-20,22-23,35H,8-11,14H2,1H3,(H,36,37)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336093

((2S)-N-(1-cyanocyclopropyl)-4-methyl-2-((1S)-2,2,2...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C(O)C(F)(F)F)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H27F6N3O2/c1-15(2)13-20(23(37)35-24(14-33)11-12-24)34-21(25(27,28)29)18-7-3-16(4-8-18)17-5-9-19(10-6-17)22(36)26(30,31)32/h3-10,15,20-22,34,36H,11-13H2,1-2H3,(H,35,37)/t20-,21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
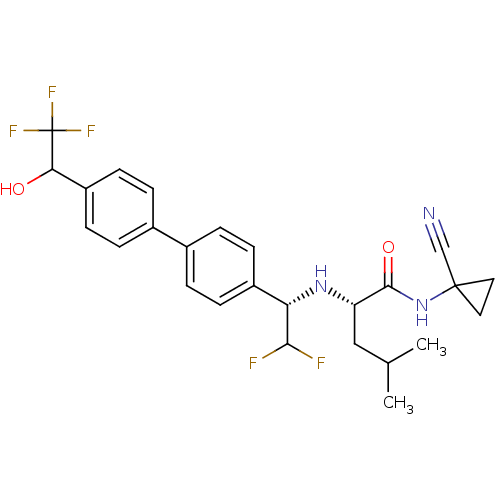
(Homo sapiens (Human)) | BDBM50336092
((2S)-N-(1-cyanocyclopropyl)-2-((1S)-2,2-difluoro-1...)Show SMILES CC(C)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1)C(O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H28F5N3O2/c1-15(2)13-20(24(36)34-25(14-32)11-12-25)33-21(23(27)28)18-7-3-16(4-8-18)17-5-9-19(10-6-17)22(35)26(29,30)31/h3-10,15,20-23,33,35H,11-13H2,1-2H3,(H,34,36)/t20-,21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50080261

(3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-([1,...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4cccnc4n3)cc12 Show InChI InChI=1S/C33H34ClN3O3S/c1-32(2,3)41-29-26-17-25(40-20-24-13-10-22-7-6-16-35-30(22)36-24)14-15-27(26)37(19-21-8-11-23(34)12-9-21)28(29)18-33(4,5)31(38)39/h6-17H,18-20H2,1-5H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. |
Bioorg Med Chem Lett 9: 2391-6 (1999)
BindingDB Entry DOI: 10.7270/Q26T0KTJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data