 Found 85 hits with Last Name = 'latham' and Initial = 'j'
Found 85 hits with Last Name = 'latham' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102222

(1-(2-Bromo-phenyl)-3-(7-cyano-3H-benzotriazol-4-yl...)Show InChI InChI=1S/C14H9BrN6O/c15-9-3-1-2-4-10(9)17-14(22)18-11-6-5-8(7-16)12-13(11)20-21-19-12/h1-6H,(H2,17,18,22)(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IL-8 from CHO cell membranes expressing human CX3C chemokine receptor 2 |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360871
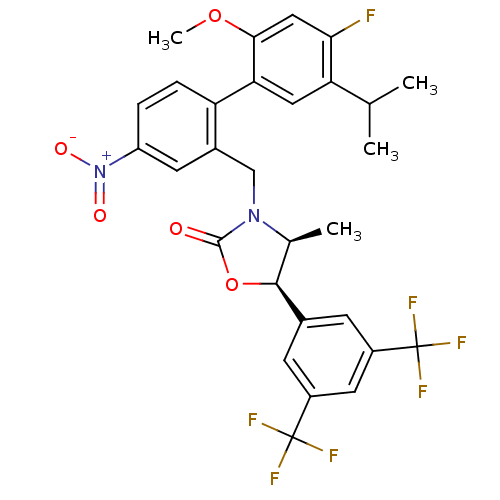
(CHEMBL1935001)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)[N+]([O-])=O)C(C)C |r| Show InChI InChI=1S/C29H25F7N2O5/c1-14(2)22-11-23(25(42-4)12-24(22)30)21-6-5-20(38(40)41)9-17(21)13-37-15(3)26(43-27(37)39)16-7-18(28(31,32)33)10-19(8-16)29(34,35)36/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360876

(CHEMBL1935006)Show SMILES COc1cc(F)c(cc1-c1ccc(SC)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H28F7NO3S/c1-15(2)23-12-24(26(40-4)13-25(23)31)22-7-6-21(42-5)10-18(22)14-38-16(3)27(41-28(38)39)17-8-19(29(32,33)34)11-20(9-17)30(35,36)37/h6-13,15-16,27H,14H2,1-5H3/t16-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360867

(CHEMBL1934997)Show SMILES COc1cc(F)c(cc1-c1ccc(Cl)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C29H25ClF7NO3/c1-14(2)22-11-23(25(40-4)12-24(22)31)21-6-5-20(30)9-17(21)13-38-15(3)26(41-27(38)39)16-7-18(28(32,33)34)10-19(8-16)29(35,36)37/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360873
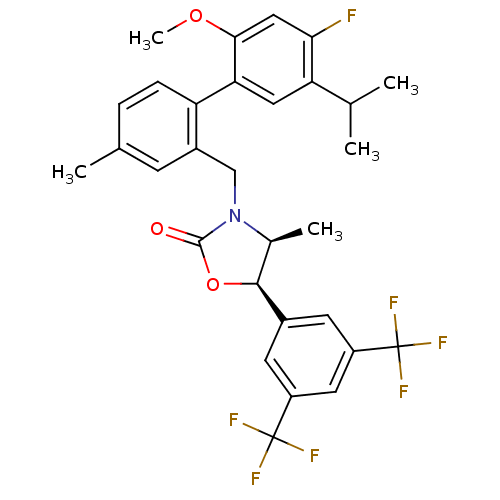
(CHEMBL1935003)Show SMILES COc1cc(F)c(cc1-c1ccc(C)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H28F7NO3/c1-15(2)23-12-24(26(40-5)13-25(23)31)22-7-6-16(3)8-19(22)14-38-17(4)27(41-28(38)39)18-9-20(29(32,33)34)11-21(10-18)30(35,36)37/h6-13,15,17,27H,14H2,1-5H3/t17-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50348228
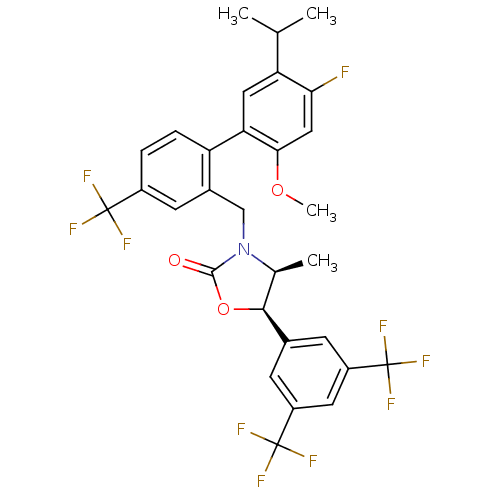
(CHEMBL1800807)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO3/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-18(28(32,33)34)9-17(21)13-41-15(3)26(44-27(41)42)16-7-19(29(35,36)37)10-20(8-16)30(38,39)40/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360881
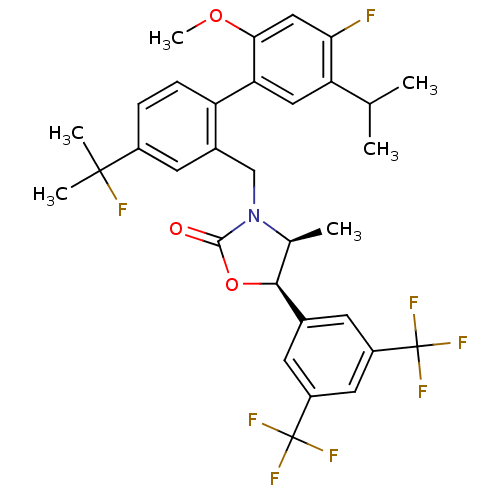
(CHEMBL1935011)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)(C)F)C(C)C |r| Show InChI InChI=1S/C32H31F8NO3/c1-16(2)24-13-25(27(43-6)14-26(24)33)23-8-7-20(30(4,5)34)11-19(23)15-41-17(3)28(44-29(41)42)18-9-21(31(35,36)37)12-22(10-18)32(38,39)40/h7-14,16-17,28H,15H2,1-6H3/t17-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360879
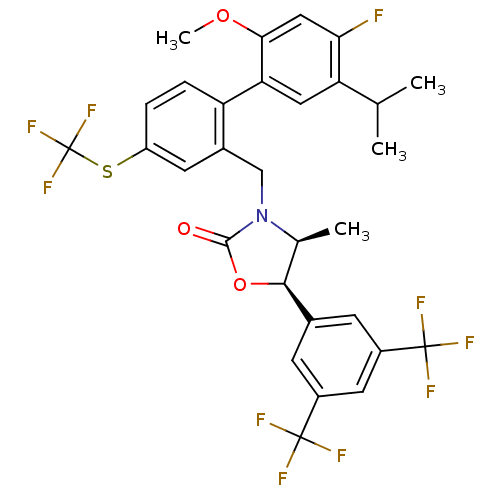
(CHEMBL1935009)Show SMILES COc1cc(F)c(cc1-c1ccc(SC(F)(F)F)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO3S/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-20(45-30(38,39)40)9-17(21)13-41-15(3)26(44-27(41)42)16-7-18(28(32,33)34)10-19(8-16)29(35,36)37/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360868
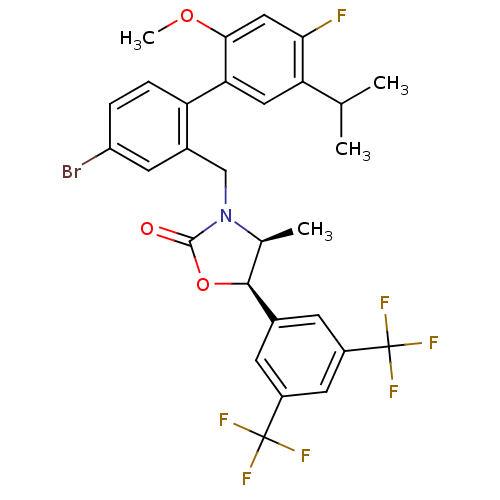
(CHEMBL1934998)Show SMILES COc1cc(F)c(cc1-c1ccc(Br)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C29H25BrF7NO3/c1-14(2)22-11-23(25(40-4)12-24(22)31)21-6-5-20(30)9-17(21)13-38-15(3)26(41-27(38)39)16-7-18(28(32,33)34)10-19(8-16)29(35,36)37/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360870
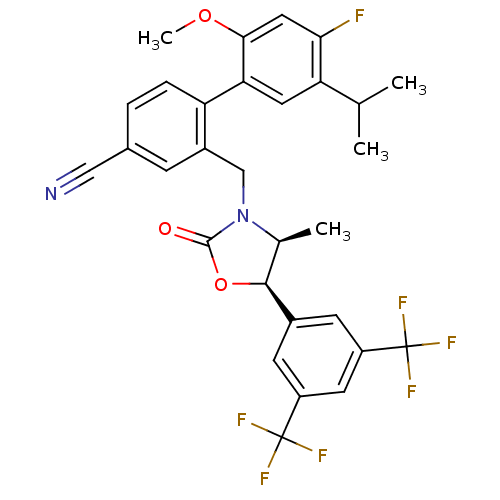
(CHEMBL1935000)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C#N)C(C)C |r| Show InChI InChI=1S/C30H25F7N2O3/c1-15(2)23-11-24(26(41-4)12-25(23)31)22-6-5-17(13-38)7-19(22)14-39-16(3)27(42-28(39)40)18-8-20(29(32,33)34)10-21(9-18)30(35,36)37/h5-12,15-16,27H,14H2,1-4H3/t16-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360869
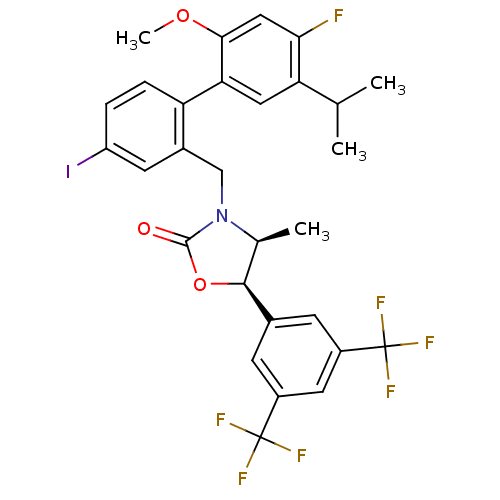
(CHEMBL1934999)Show SMILES COc1cc(F)c(cc1-c1ccc(I)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C29H25F7INO3/c1-14(2)22-11-23(25(40-4)12-24(22)30)21-6-5-20(37)9-17(21)13-38-15(3)26(41-27(38)39)16-7-18(28(31,32)33)10-19(8-16)29(34,35)36/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360883
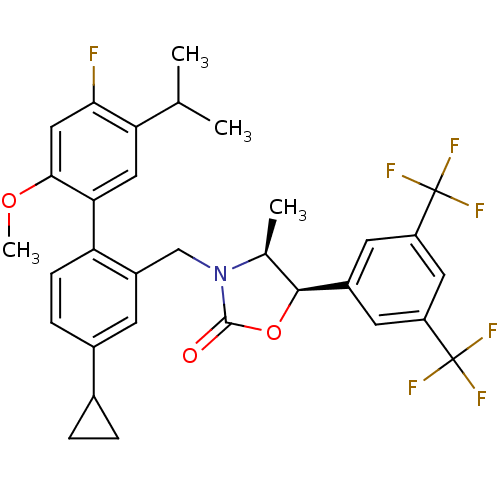
(CHEMBL1935013)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C1CC1)C(C)C |r| Show InChI InChI=1S/C32H30F7NO3/c1-16(2)25-13-26(28(42-4)14-27(25)33)24-8-7-19(18-5-6-18)9-21(24)15-40-17(3)29(43-30(40)41)20-10-22(31(34,35)36)12-23(11-20)32(37,38)39/h7-14,16-18,29H,5-6,15H2,1-4H3/t17-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360875
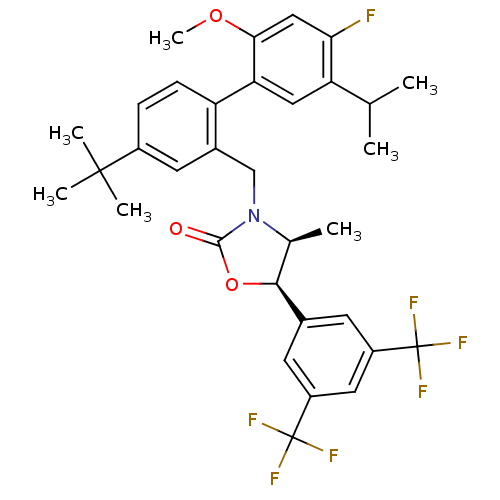
(CHEMBL1935005)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C33H34F7NO3/c1-17(2)25-14-26(28(43-7)15-27(25)34)24-9-8-21(31(4,5)6)12-20(24)16-41-18(3)29(44-30(41)42)19-10-22(32(35,36)37)13-23(11-19)33(38,39)40/h8-15,17-18,29H,16H2,1-7H3/t18-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360866
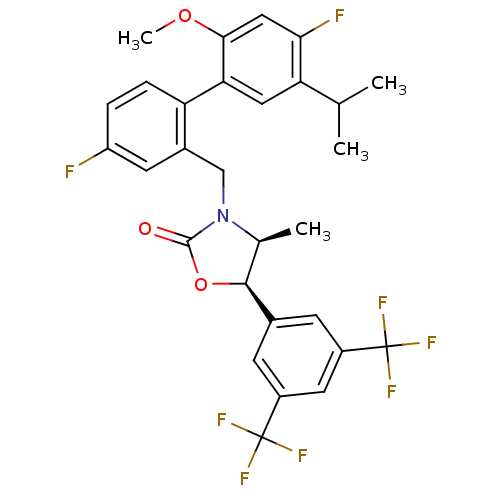
(CHEMBL1934996)Show SMILES COc1cc(F)c(cc1-c1ccc(F)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C29H25F8NO3/c1-14(2)22-11-23(25(40-4)12-24(22)31)21-6-5-20(30)9-17(21)13-38-15(3)26(41-27(38)39)16-7-18(28(32,33)34)10-19(8-16)29(35,36)37/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360887
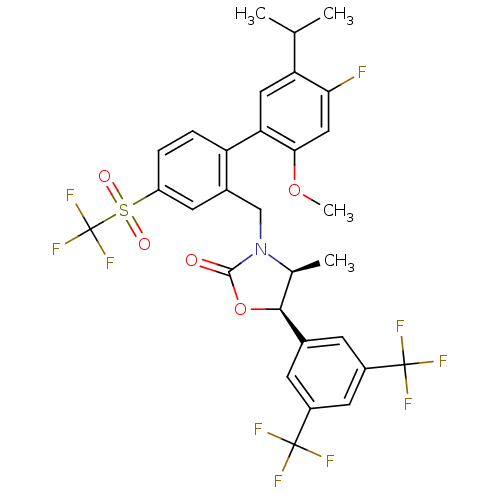
(CHEMBL1935099)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)S(=O)(=O)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO5S/c1-14(2)22-11-23(25(45-4)12-24(22)31)21-6-5-20(47(43,44)30(38,39)40)9-17(21)13-41-15(3)26(46-27(41)42)16-7-18(28(32,33)34)10-19(8-16)29(35,36)37/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360865
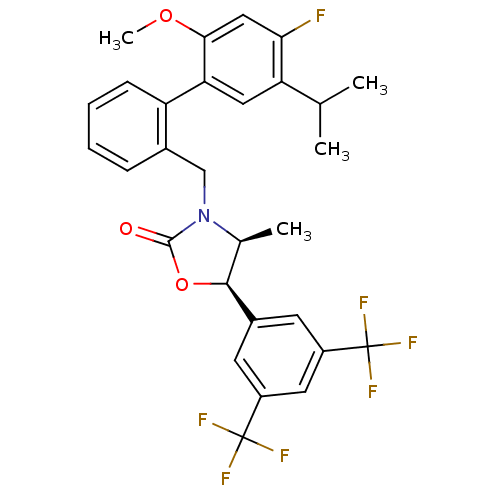
(CHEMBL1934995)Show SMILES COc1cc(F)c(cc1-c1ccccc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C29H26F7NO3/c1-15(2)22-12-23(25(39-4)13-24(22)30)21-8-6-5-7-17(21)14-37-16(3)26(40-27(37)38)18-9-19(28(31,32)33)11-20(10-18)29(34,35)36/h5-13,15-16,26H,14H2,1-4H3/t16-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50113628

(CHEMBL431511 | [5-(4-Fluoro-phenylcarbamoyl)-pyrid...)Show InChI InChI=1S/C15H13FN2O3S/c1-21-14(19)9-22-13-7-2-10(8-17-13)15(20)18-12-5-3-11(16)4-6-12/h2-8H,9H2,1H3,(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IL-8 from CHO cell membranes expressing human CX3C chemokine receptor 2 |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102217
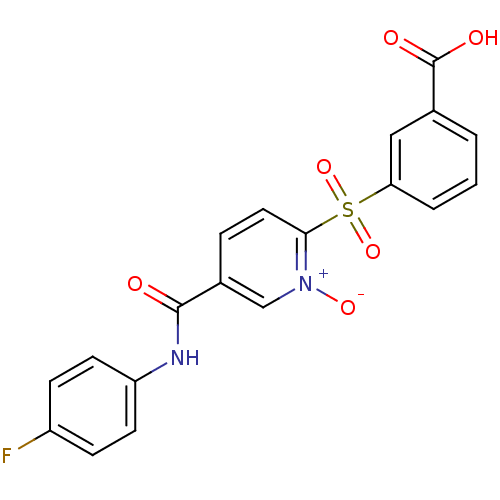
(3-[5-(4-Fluoro-phenylcarbamoyl)-1-oxy-pyridine-2-s...)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)c1ccc(c[n+]1[O-])C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C19H13FN2O6S/c20-14-5-7-15(8-6-14)21-18(23)13-4-9-17(22(26)11-13)29(27,28)16-3-1-2-12(10-16)19(24)25/h1-11H,(H,21,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Evaluated using an SPA assay with recombinant human [125I]-IL-8 and membranes preparedfrom Sf9 cells expressing human CXCR2 |
Bioorg Med Chem Lett 11: 1951-4 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DN0 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360884
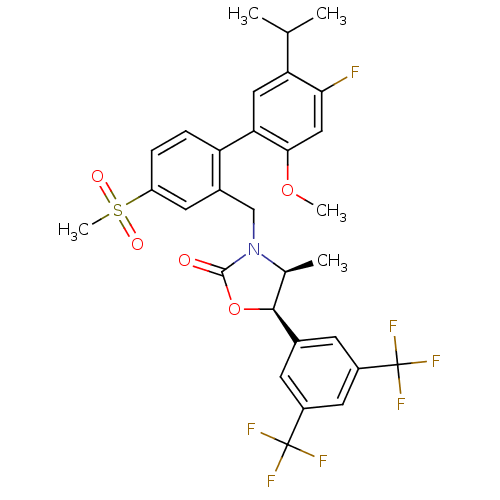
(CHEMBL1935014)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)S(C)(=O)=O)C(C)C |r| Show InChI InChI=1S/C30H28F7NO5S/c1-15(2)23-12-24(26(42-4)13-25(23)31)22-7-6-21(44(5,40)41)10-18(22)14-38-16(3)27(43-28(38)39)17-8-19(29(32,33)34)11-20(9-17)30(35,36)37/h6-13,15-16,27H,14H2,1-5H3/t16-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50113628

(CHEMBL431511 | [5-(4-Fluoro-phenylcarbamoyl)-pyrid...)Show InChI InChI=1S/C15H13FN2O3S/c1-21-14(19)9-22-13-7-2-10(8-17-13)15(20)18-12-5-3-11(16)4-6-12/h2-8H,9H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219799

(CHEMBL36535)Show SMILES Fc1ccc(NC(=O)c2ccc(SCC(=O)OCc3cccc(F)c3)nc2)cc1 Show InChI InChI=1S/C21H16F2N2O3S/c22-16-5-7-18(8-6-16)25-21(27)15-4-9-19(24-11-15)29-13-20(26)28-12-14-2-1-3-17(23)10-14/h1-11H,12-13H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219797

(CHEMBL36934)Show InChI InChI=1S/C17H17FN2O3S/c1-2-9-23-16(21)11-24-15-8-3-12(10-19-15)17(22)20-14-6-4-13(18)5-7-14/h3-8,10H,2,9,11H2,1H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219796
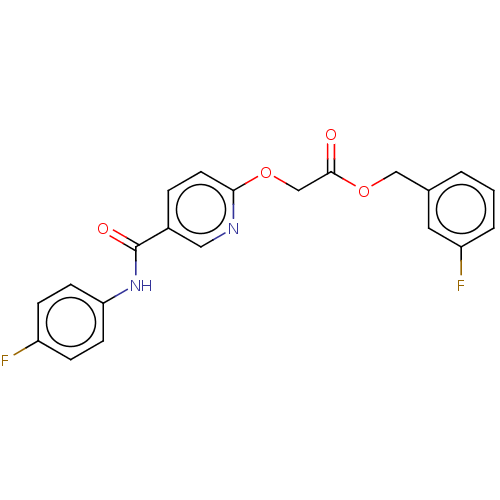
(CHEMBL39464)Show SMILES Fc1ccc(NC(=O)c2ccc(OCC(=O)OCc3cccc(F)c3)nc2)cc1 Show InChI InChI=1S/C21H16F2N2O4/c22-16-5-7-18(8-6-16)25-21(27)15-4-9-19(24-11-15)28-13-20(26)29-12-14-2-1-3-17(23)10-14/h1-11H,12-13H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360874
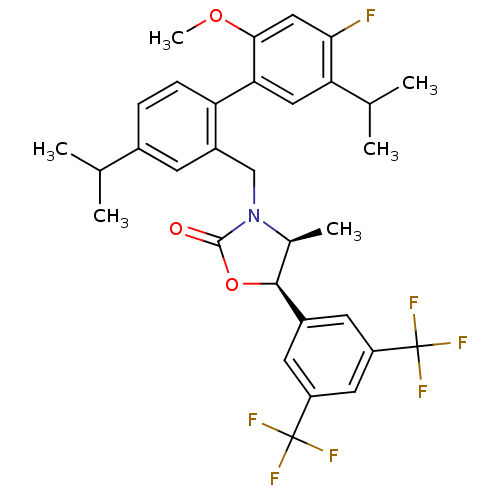
(CHEMBL1935004)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H32F7NO3/c1-16(2)19-7-8-24(26-13-25(17(3)4)27(33)14-28(26)42-6)21(9-19)15-40-18(5)29(43-30(40)41)20-10-22(31(34,35)36)12-23(11-20)32(37,38)39/h7-14,16-18,29H,15H2,1-6H3/t18-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360882

(CHEMBL1935012)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)(C)O)C(C)C |r| Show InChI InChI=1S/C32H32F7NO4/c1-16(2)24-13-25(27(43-6)14-26(24)33)23-8-7-20(30(4,5)42)11-19(23)15-40-17(3)28(44-29(40)41)18-9-21(31(34,35)36)12-22(10-18)32(37,38)39/h7-14,16-17,28,42H,15H2,1-6H3/t17-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219800
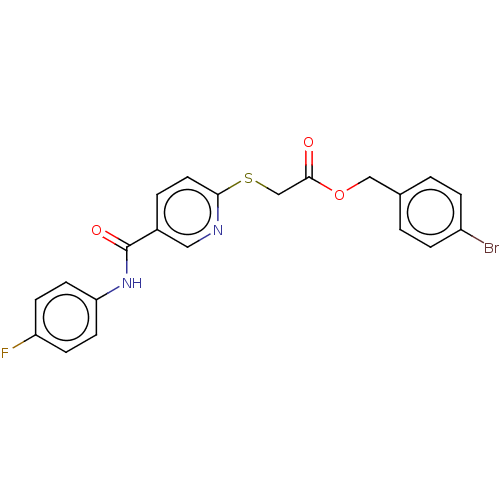
(CHEMBL432475)Show SMILES Fc1ccc(NC(=O)c2ccc(SCC(=O)OCc3ccc(Br)cc3)nc2)cc1 Show InChI InChI=1S/C21H16BrFN2O3S/c22-16-4-1-14(2-5-16)12-28-20(26)13-29-19-10-3-15(11-24-19)21(27)25-18-8-6-17(23)7-9-18/h1-11H,12-13H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102229
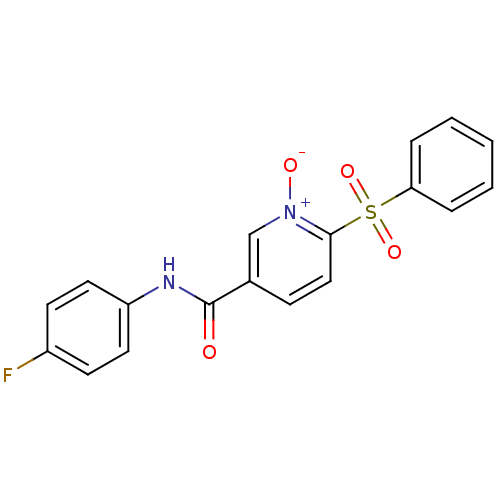
(6-Benzenesulfonyl-N-(4-fluoro-phenyl)-1-oxy-nicoti...)Show SMILES [O-][n+]1cc(ccc1S(=O)(=O)c1ccccc1)C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C18H13FN2O4S/c19-14-7-9-15(10-8-14)20-18(22)13-6-11-17(21(23)12-13)26(24,25)16-4-2-1-3-5-16/h1-12H,(H,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Evaluated using an SPA assay with recombinant human [125I]-IL-8 and membranes preparedfrom Sf9 cells expressing human CXCR2 |
Bioorg Med Chem Lett 11: 1951-4 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DN0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219806

(CHEMBL291140)Show SMILES Fc1ccc(NC(=O)c2ccc(OCC(=O)OCc3ccccc3)nc2)cc1 Show InChI InChI=1S/C21H17FN2O4/c22-17-7-9-18(10-8-17)24-21(26)16-6-11-19(23-12-16)27-14-20(25)28-13-15-4-2-1-3-5-15/h1-12H,13-14H2,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50331754
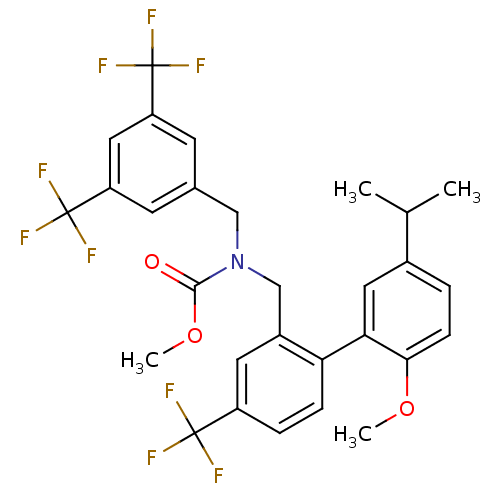
(CHEMBL1288516 | methyl 3,5-bis(trifluoromethyl)ben...)Show SMILES COC(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)Cc1cc(ccc1-c1cc(ccc1OC)C(C)C)C(F)(F)F Show InChI InChI=1S/C29H26F9NO3/c1-16(2)18-5-8-25(41-3)24(12-18)23-7-6-20(27(30,31)32)11-19(23)15-39(26(40)42-4)14-17-9-21(28(33,34)35)13-22(10-17)29(36,37)38/h5-13,16H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 20: 7469-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.019
BindingDB Entry DOI: 10.7270/Q2FF3SMV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102225

(CHEMBL39740 | N'-(3-[2,2']Bithiophenyl-5-yl-6,7-di...)Show SMILES CCN(CC)CCCCNc1nc2cc(Cl)c(Cl)cc2nc1-c1ccc(s1)-c1cccs1 Show InChI InChI=1S/C24H26Cl2N4S2/c1-3-30(4-2)12-6-5-11-27-24-23(22-10-9-21(32-22)20-8-7-13-31-20)28-18-14-16(25)17(26)15-19(18)29-24/h7-10,13-15H,3-6,11-12H2,1-2H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IL-8 from CHO cell membranes expressing human CX3C chemokine receptor 2 |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102225

(CHEMBL39740 | N'-(3-[2,2']Bithiophenyl-5-yl-6,7-di...)Show SMILES CCN(CC)CCCCNc1nc2cc(Cl)c(Cl)cc2nc1-c1ccc(s1)-c1cccs1 Show InChI InChI=1S/C24H26Cl2N4S2/c1-3-30(4-2)12-6-5-11-27-24-23(22-10-9-21(32-22)20-8-7-13-31-20)28-18-14-16(25)17(26)15-19(18)29-24/h7-10,13-15H,3-6,11-12H2,1-2H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Compound binding was evaluated using an SPA assay with recombinant human [125I]-IL-8 and membranes preparedfrom Sf9 cells expressing human C-X-C chem... |
Bioorg Med Chem Lett 11: 1951-4 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DN0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219809

(CHEMBL36643)Show InChI InChI=1S/C15H13FN2O4/c1-21-14(19)9-22-13-7-2-10(8-17-13)15(20)18-12-5-3-11(16)4-6-12/h2-8H,9H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50331755
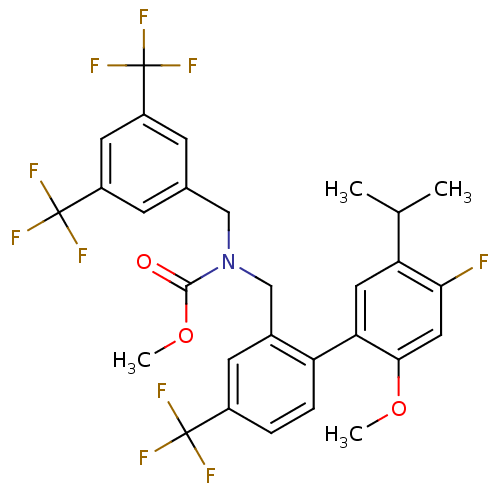
(CHEMBL1288517 | methyl 3,5-bis(trifluoromethyl)ben...)Show SMILES COC(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)Cc1cc(ccc1-c1cc(C(C)C)c(F)cc1OC)C(F)(F)F Show InChI InChI=1S/C29H25F10NO3/c1-15(2)22-11-23(25(42-3)12-24(22)30)21-6-5-18(27(31,32)33)9-17(21)14-40(26(41)43-4)13-16-7-19(28(34,35)36)10-20(8-16)29(37,38)39/h5-12,15H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 20: 7469-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.019
BindingDB Entry DOI: 10.7270/Q2FF3SMV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102219

(CHEMBL61835 | N-(4-Fluoro-phenyl)-6-methanesulfony...)Show SMILES CS(=O)(=O)c1ccc(c[n+]1[O-])C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C13H11FN2O4S/c1-21(19,20)12-7-2-9(8-16(12)18)13(17)15-11-5-3-10(14)4-6-11/h2-8H,1H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Evaluated using an SPA assay with recombinant human [125I]-IL-8 and membranes preparedfrom Sf9 cells expressing human CXCR2 |
Bioorg Med Chem Lett 11: 1951-4 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DN0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102216

(6-Ethanesulfonyl-N-(4-fluoro-phenyl)-1-oxy-nicotin...)Show SMILES CCS(=O)(=O)c1ccc(c[n+]1[O-])C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C14H13FN2O4S/c1-2-22(20,21)13-8-3-10(9-17(13)19)14(18)16-12-6-4-11(15)5-7-12/h3-9H,2H2,1H3,(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Evaluated using an SPA assay with recombinant human [125I]-IL-8 and membranes preparedfrom Sf9 cells expressing human CXCR2 |
Bioorg Med Chem Lett 11: 1951-4 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DN0 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50331759
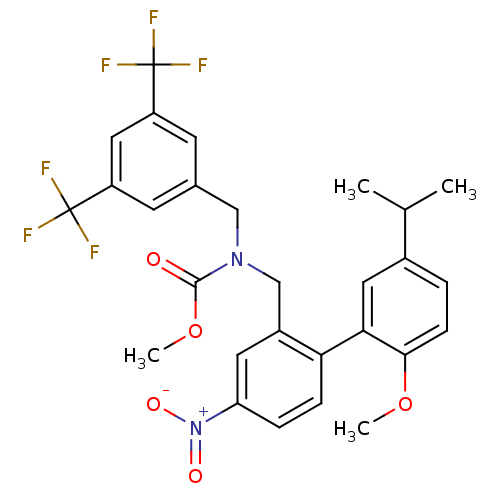
(CHEMBL1290417 | methyl 3,5-bis(trifluoromethyl)ben...)Show SMILES COC(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)Cc1cc(ccc1-c1cc(ccc1OC)C(C)C)[N+]([O-])=O Show InChI InChI=1S/C28H26F6N2O5/c1-16(2)18-5-8-25(40-3)24(12-18)23-7-6-22(36(38)39)11-19(23)15-35(26(37)41-4)14-17-9-20(27(29,30)31)13-21(10-17)28(32,33)34/h5-13,16H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 20: 7469-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.019
BindingDB Entry DOI: 10.7270/Q2FF3SMV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219811
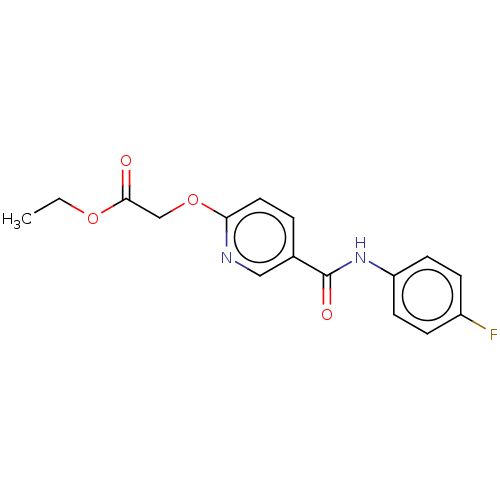
(CHEMBL39710)Show InChI InChI=1S/C16H15FN2O4/c1-2-22-15(20)10-23-14-8-3-11(9-18-14)16(21)19-13-6-4-12(17)5-7-13/h3-9H,2,10H2,1H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219812

(CHEMBL36842)Show InChI InChI=1S/C19H21FN2O4/c1-13(2)9-10-25-18(23)12-26-17-8-3-14(11-21-17)19(24)22-16-6-4-15(20)5-7-16/h3-8,11,13H,9-10,12H2,1-2H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50331753
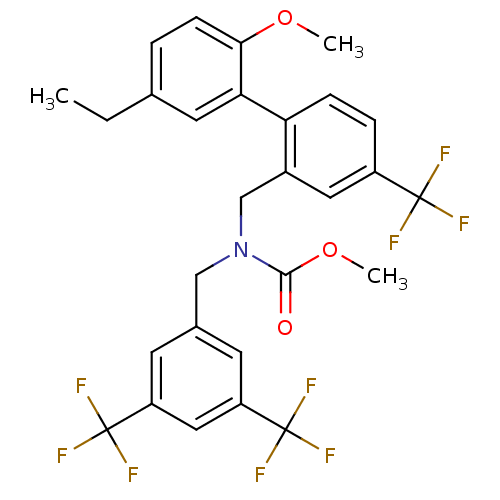
(CHEMBL1290190 | methyl 3,5-bis(trifluoromethyl)ben...)Show SMILES CCc1ccc(OC)c(c1)-c1ccc(cc1CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)OC)C(F)(F)F Show InChI InChI=1S/C28H24F9NO3/c1-4-16-5-8-24(40-2)23(11-16)22-7-6-19(26(29,30)31)12-18(22)15-38(25(39)41-3)14-17-9-20(27(32,33)34)13-21(10-17)28(35,36)37/h5-13H,4,14-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 20: 7469-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.019
BindingDB Entry DOI: 10.7270/Q2FF3SMV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219808
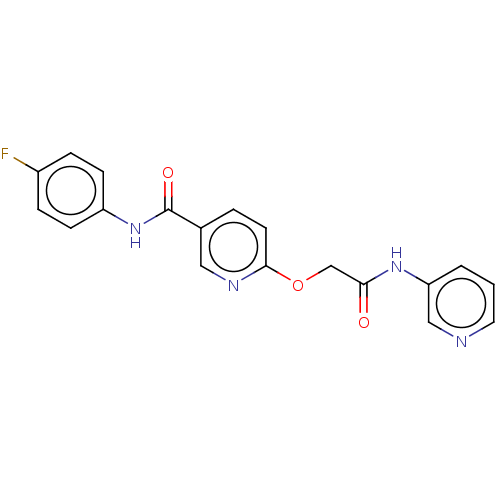
(CHEMBL288527)Show SMILES Fc1ccc(NC(=O)c2ccc(OCC(=O)Nc3cccnc3)nc2)cc1 Show InChI InChI=1S/C19H15FN4O3/c20-14-4-6-15(7-5-14)24-19(26)13-3-8-18(22-10-13)27-12-17(25)23-16-2-1-9-21-11-16/h1-11H,12H2,(H,23,25)(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219794
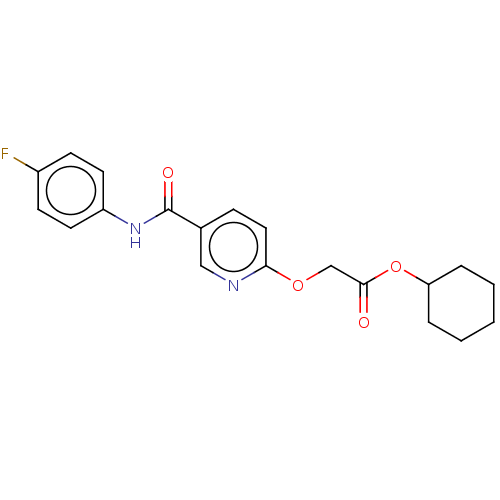
(CHEMBL289656)Show SMILES Fc1ccc(NC(=O)c2ccc(OCC(=O)OC3CCCCC3)nc2)cc1 Show InChI InChI=1S/C20H21FN2O4/c21-15-7-9-16(10-8-15)23-20(25)14-6-11-18(22-12-14)26-13-19(24)27-17-4-2-1-3-5-17/h6-12,17H,1-5,13H2,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50331762
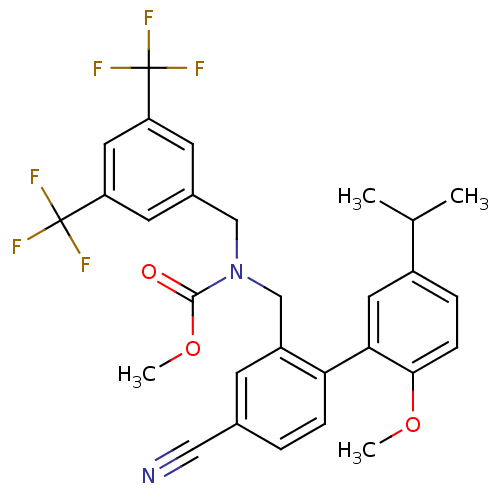
(CHEMBL1290759 | methyl 3,5-bis(trifluoromethyl)ben...)Show SMILES COC(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)Cc1cc(ccc1-c1cc(ccc1OC)C(C)C)C#N Show InChI InChI=1S/C29H26F6N2O3/c1-17(2)20-6-8-26(39-3)25(12-20)24-7-5-18(14-36)9-21(24)16-37(27(38)40-4)15-19-10-22(28(30,31)32)13-23(11-19)29(33,34)35/h5-13,17H,15-16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 20: 7469-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.019
BindingDB Entry DOI: 10.7270/Q2FF3SMV |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360880
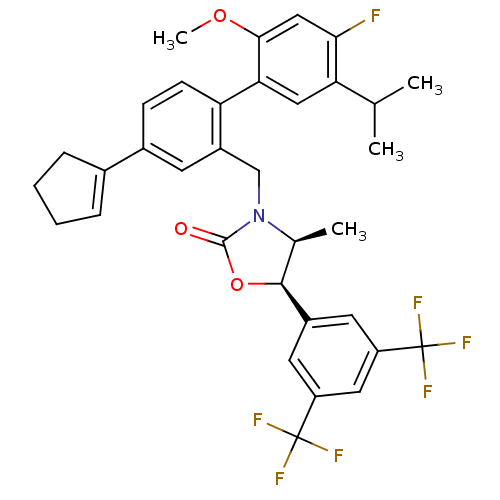
(CHEMBL1935010)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C1=CCCC1)C(C)C |r,t:41| Show InChI InChI=1S/C34H32F7NO3/c1-18(2)27-15-28(30(44-4)16-29(27)35)26-10-9-21(20-7-5-6-8-20)11-23(26)17-42-19(3)31(45-32(42)43)22-12-24(33(36,37)38)14-25(13-22)34(39,40)41/h7,9-16,18-19,31H,5-6,8,17H2,1-4H3/t19-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102226
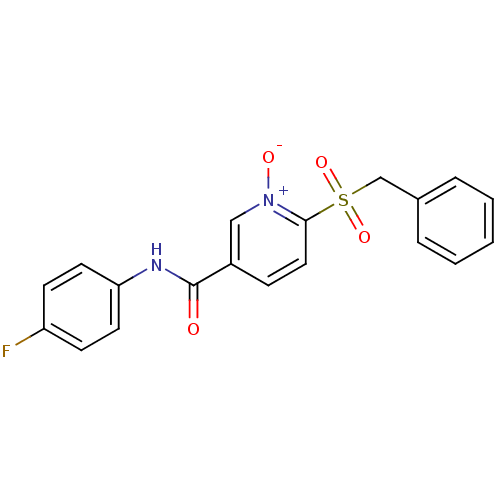
(CHEMBL58619 | N-(4-Fluoro-phenyl)-1-oxy-6-phenylme...)Show SMILES [O-][n+]1cc(ccc1S(=O)(=O)Cc1ccccc1)C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C19H15FN2O4S/c20-16-7-9-17(10-8-16)21-19(23)15-6-11-18(22(24)12-15)27(25,26)13-14-4-2-1-3-5-14/h1-12H,13H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Evaluated using an SPA assay with recombinant human [125I]-IL-8 and membranes preparedfrom Sf9 cells expressing human CXCR2 |
Bioorg Med Chem Lett 11: 1951-4 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DN0 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360872

(CHEMBL1935002)Show SMILES COc1cc(F)c(cc1-c1ccc(N)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C29H27F7N2O3/c1-14(2)22-11-23(25(40-4)12-24(22)30)21-6-5-20(37)9-17(21)13-38-15(3)26(41-27(38)39)16-7-18(28(31,32)33)10-19(8-16)29(34,35)36/h5-12,14-15,26H,13,37H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219810
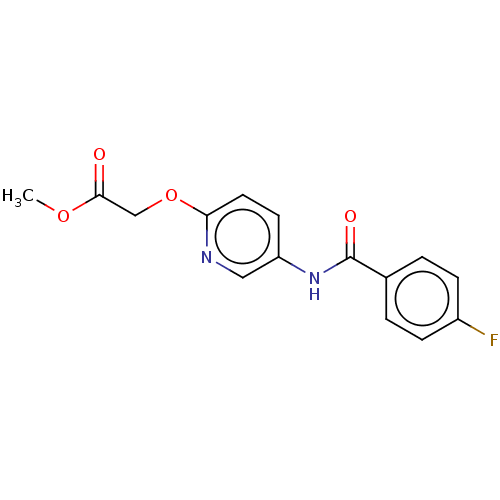
(CHEMBL288876)Show InChI InChI=1S/C15H13FN2O4/c1-21-14(19)9-22-13-7-6-12(8-17-13)18-15(20)10-2-4-11(16)5-3-10/h2-8H,9H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50331761
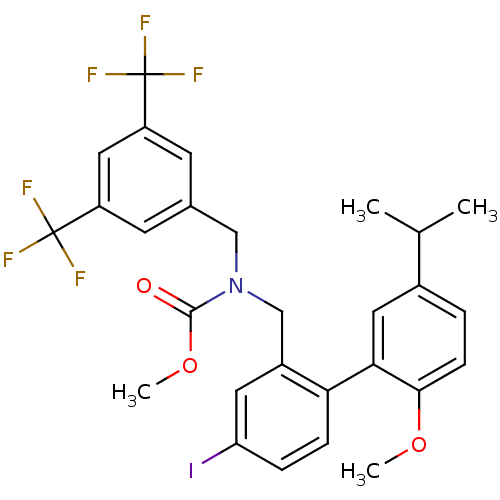
(CHEMBL1290643 | methyl 3,5-bis(trifluoromethyl)ben...)Show SMILES COC(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)Cc1cc(I)ccc1-c1cc(ccc1OC)C(C)C Show InChI InChI=1S/C28H26F6INO3/c1-16(2)18-5-8-25(38-3)24(12-18)23-7-6-22(35)11-19(23)15-36(26(37)39-4)14-17-9-20(27(29,30)31)13-21(10-17)28(32,33)34/h5-13,16H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 20: 7469-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.019
BindingDB Entry DOI: 10.7270/Q2FF3SMV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50102218
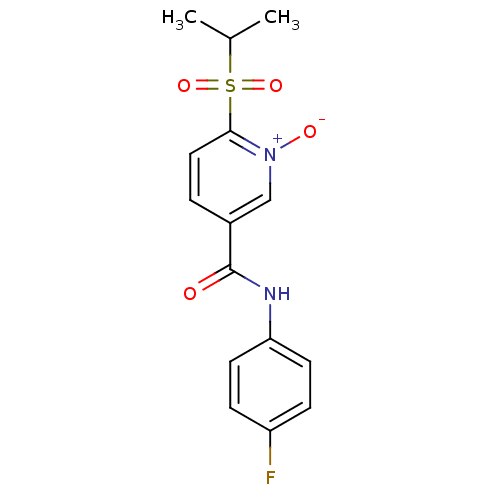
(CHEMBL61655 | N-(4-Fluoro-phenyl)-1-oxy-6-(propane...)Show SMILES CC(C)S(=O)(=O)c1ccc(c[n+]1[O-])C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C15H15FN2O4S/c1-10(2)23(21,22)14-8-3-11(9-18(14)20)15(19)17-13-6-4-12(16)5-7-13/h3-10H,1-2H3,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Evaluated using an SPA assay with recombinant human [125I]-IL-8 and membranes preparedfrom Sf9 cells expressing human CXCR2 |
Bioorg Med Chem Lett 11: 1951-4 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DN0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1/2
(Homo sapiens (Human)) | BDBM50219795
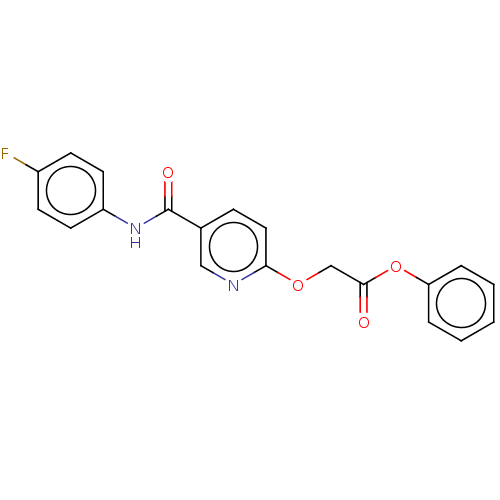
(CHEMBL40080)Show SMILES Fc1ccc(NC(=O)c2ccc(OCC(=O)Oc3ccccc3)nc2)cc1 Show InChI InChI=1S/C20H15FN2O4/c21-15-7-9-16(10-8-15)23-20(25)14-6-11-18(22-12-14)26-13-19(24)27-17-4-2-1-3-5-17/h1-12H,13H2,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Inc.
Curated by ChEMBL
| Assay Description
Inhibition against GRO-alpha driven human neutrophil chemotaxis |
Bioorg Med Chem Lett 12: 1517-20 (2002)
BindingDB Entry DOI: 10.7270/Q2JM28ZD |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50331751
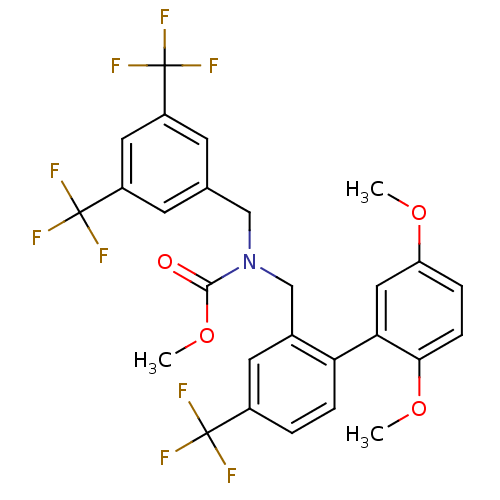
(CHEMBL1289987 | methyl 3,5-bis(trifluoromethyl)ben...)Show SMILES COC(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)Cc1cc(ccc1-c1cc(OC)ccc1OC)C(F)(F)F Show InChI InChI=1S/C27H22F9NO4/c1-39-20-5-7-23(40-2)22(12-20)21-6-4-17(25(28,29)30)10-16(21)14-37(24(38)41-3)13-15-8-18(26(31,32)33)11-19(9-15)27(34,35)36/h4-12H,13-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 444 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 20: 7469-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.019
BindingDB Entry DOI: 10.7270/Q2FF3SMV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data