

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040317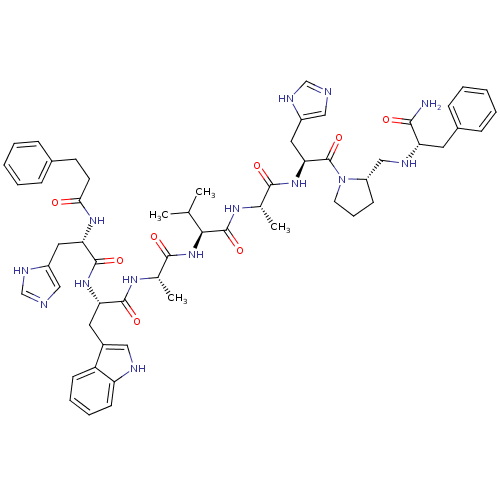 (CHEMBL262756 | PhCH2CH2(CO)His-Trp-Ala-Val-DAla-Hi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040307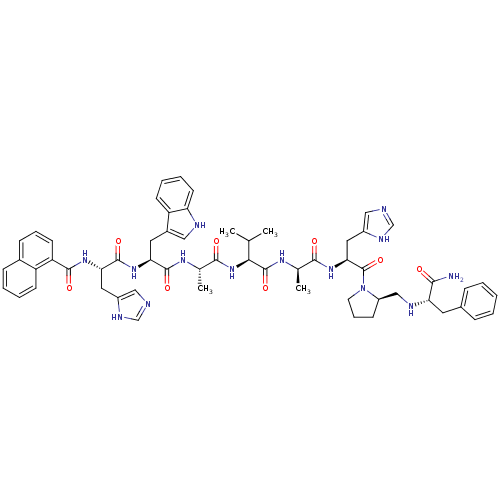 (1-naphthyl(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)Ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040299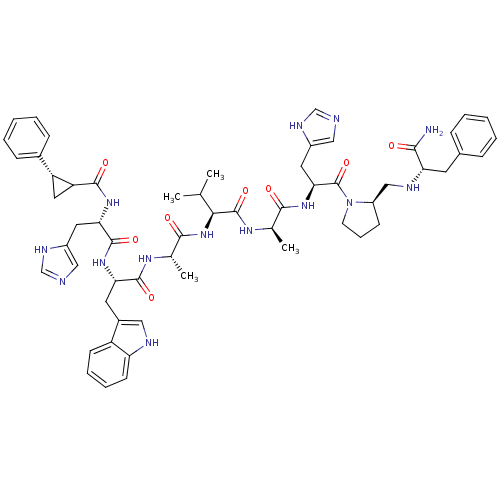 (CHEMBL410783 | cyclopropylbenzene(CO)His-Trp-Ala-V...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040325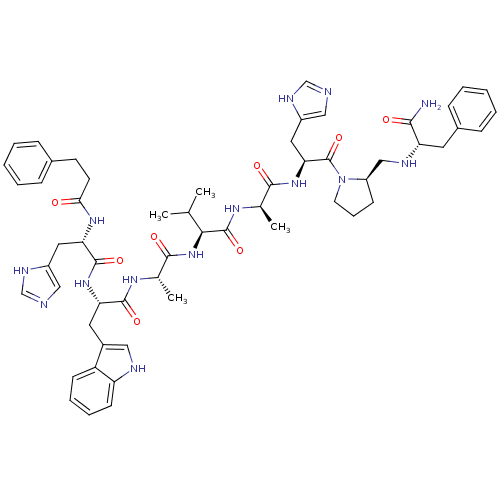 (CHEMBL268386 | PhCH2CH2(CO)His-Trp-Ala-Val-DAla-Hi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040299 (CHEMBL410783 | cyclopropylbenzene(CO)His-Trp-Ala-V...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040307 (1-naphthyl(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)Ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040301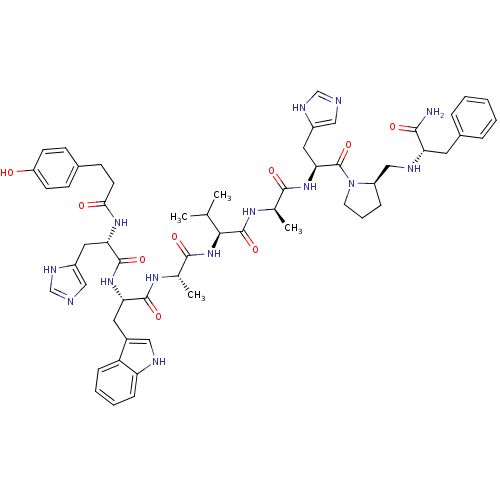 (4-OHPhCH2CH2(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040312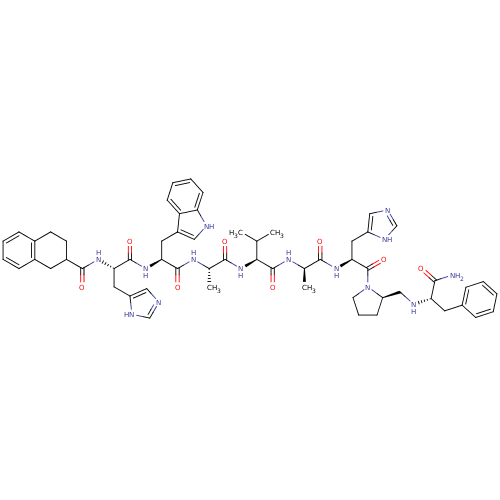 (2-tetrahydronaphthyl(CO)His-Trp-Ala-Val-DAla-His-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040308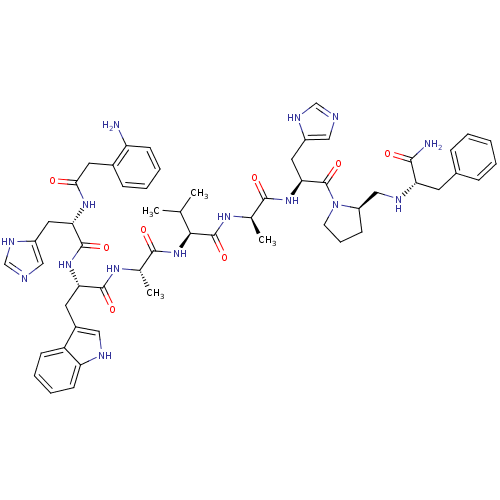 (2-aminoPhCH2(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040310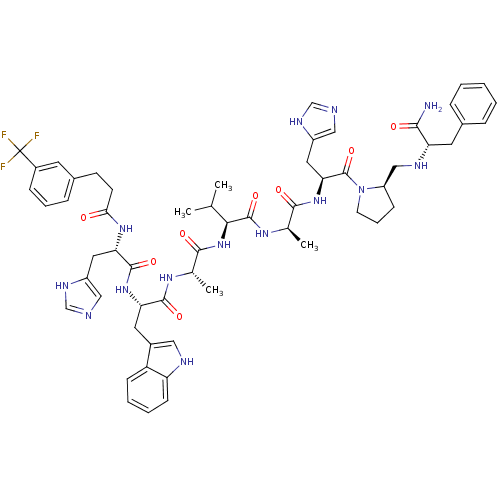 (3-CF3PhCH2CH2(CO)His-Trp-Ala-Val-DAla-His-DPro(psi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040315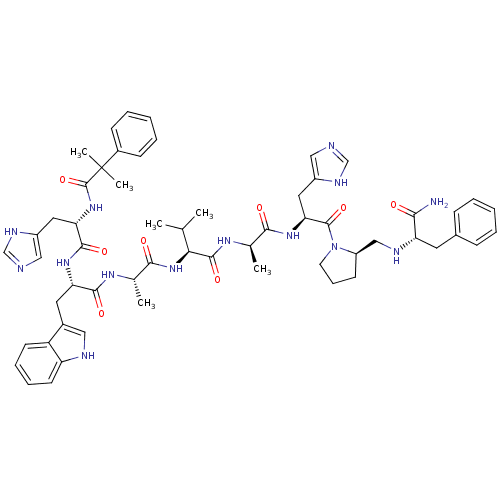 ((CH3)2PhC(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)Phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040315 ((CH3)2PhC(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)Phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040317 (CHEMBL262756 | PhCH2CH2(CO)His-Trp-Ala-Val-DAla-Hi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040301 (4-OHPhCH2CH2(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040312 (2-tetrahydronaphthyl(CO)His-Trp-Ala-Val-DAla-His-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040320 (CHEMBL264122 | PhCH2CH2(CO)His-Trp-Ala-Val-DAla-Hi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (RAT) | BDBM50034128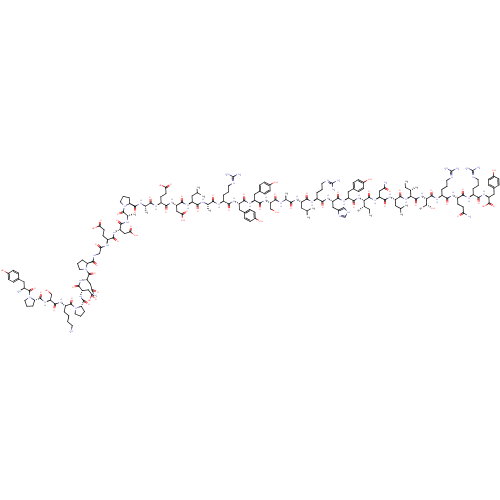 (CHEMBL441396 | Neuropeptide Y(NPY) | Tyr-Pro-Ser-L...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description 50% displacement of specifically bound [3H]-NPY2 from rat brain membranes. | J Med Chem 38: 1150-7 (1995) BindingDB Entry DOI: 10.7270/Q2513X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50407275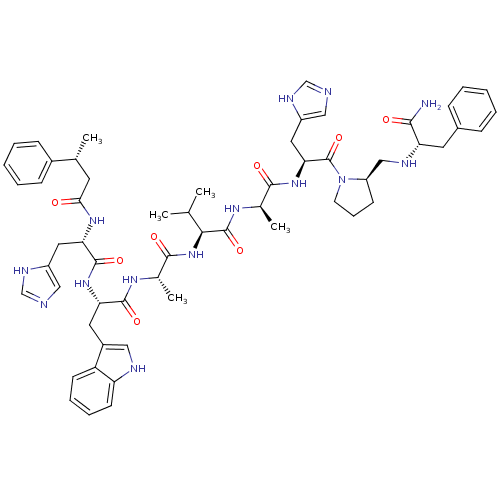 (CHEMBL2112786) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401571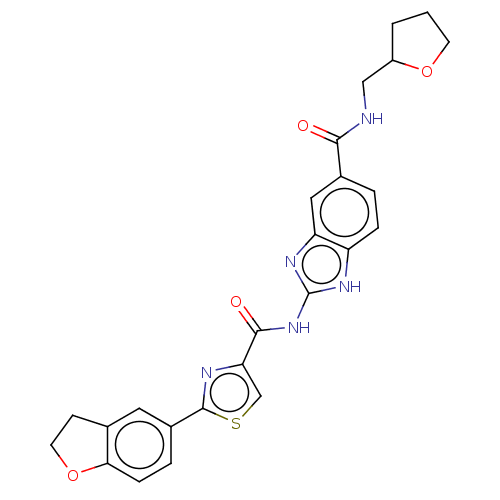 (US10005769, No. 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040316 (3-isoquinolyl(CO)His-Trp-Ala-Val-DAla-His-DPro(psi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401559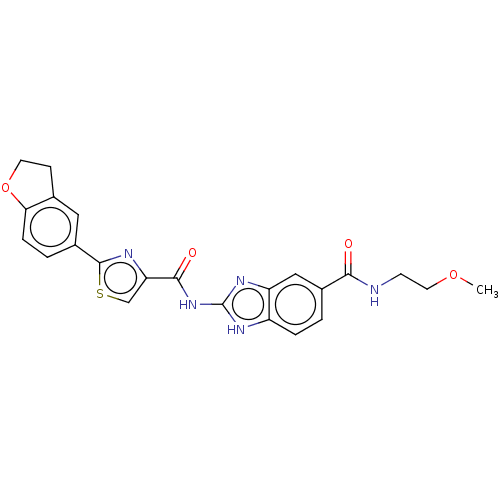 (US10005769, No. 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.699 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040324 (CHEMBL266516 | PhCH2CH2(CO)His-(BzthAla)-Ala-Val-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401558 (US10005769, No. 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.852 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50133864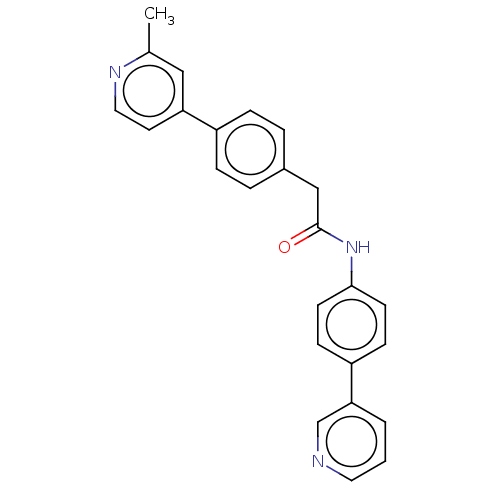 (CHEMBL2139947 | US10251893, Compound 59) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ulm University Hospital Curated by ChEMBL | Assay Description Inhibition of porcupine in HEK293T cells transfected with Wnt3A-expressing vector assessed as suppression of Wnt/beta-catenin signaling after 22 hrs ... | J Med Chem 61: 4087-4102 (2018) Article DOI: 10.1021/acs.jmedchem.8b00095 BindingDB Entry DOI: 10.7270/Q2GX4F5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040310 (3-CF3PhCH2CH2(CO)His-Trp-Ala-Val-DAla-His-DPro(psi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50407276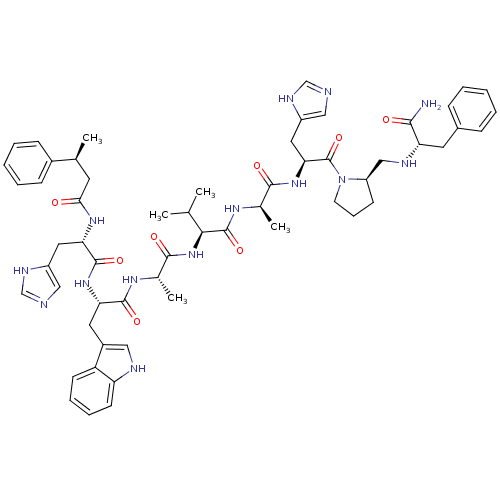 (CHEMBL2111952) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial [30-395] (Homo sapiens (Human)) | BDBM16117 (3-{[2,3,5,6-tetrafluoro-4-(2-methoxyphenyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 25 |
4SC AG | Assay Description The assays were carried out by using a colorimetric DCIP method, which uses the colorimetric reagent 2, 6-dichlorophenolindophenol as the final elect... | Bioorg Med Chem Lett 16: 267-70 (2006) Article DOI: 10.1016/j.bmcl.2005.10.011 BindingDB Entry DOI: 10.7270/Q2CJ8BQ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50407273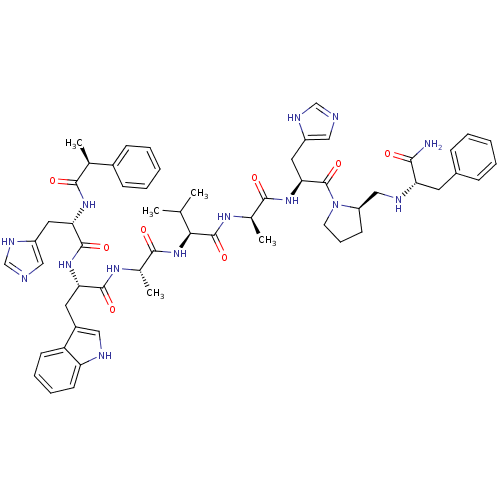 (CHEMBL2111953) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50407276 (CHEMBL2111952) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040314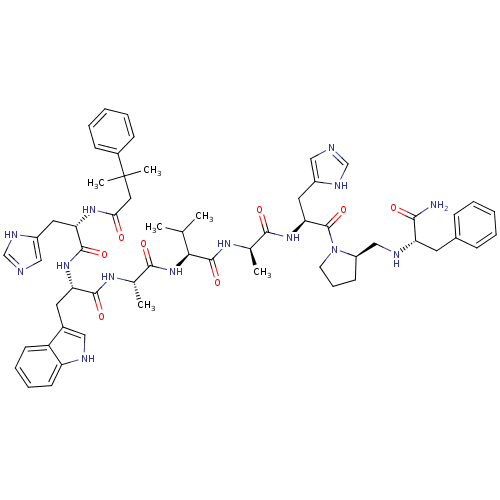 ((CH3)2PhCCH2(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50407273 (CHEMBL2111953) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401537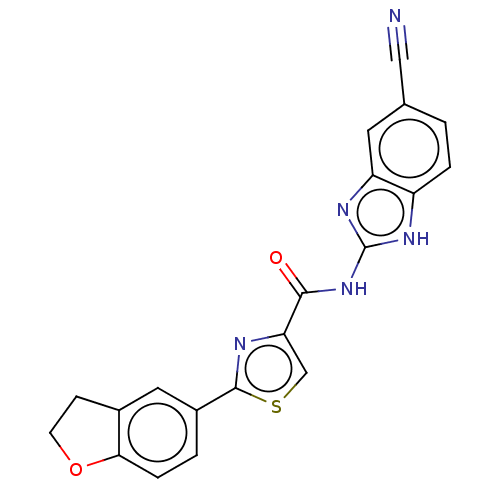 (US10005769, No. 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401539 (US10005769, No. 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401543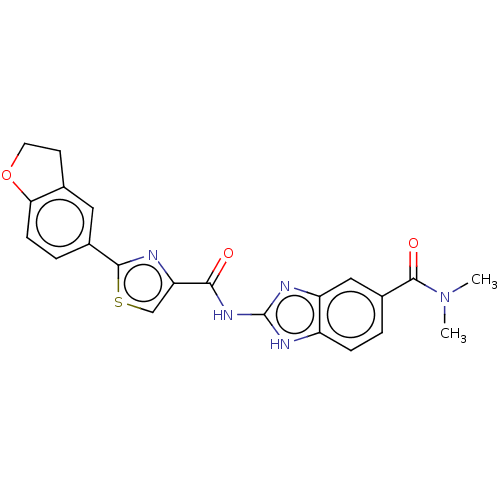 (US10005769, No. 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401542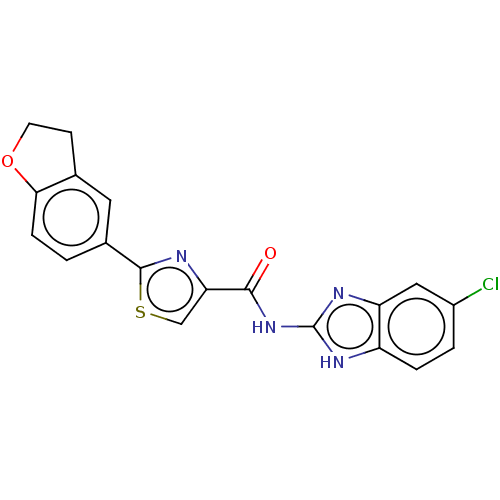 (US10005769, No. 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040323 (CHEMBL265736 | phenothiazoneCH2CH2(CO)His-Trp-Ala-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM15341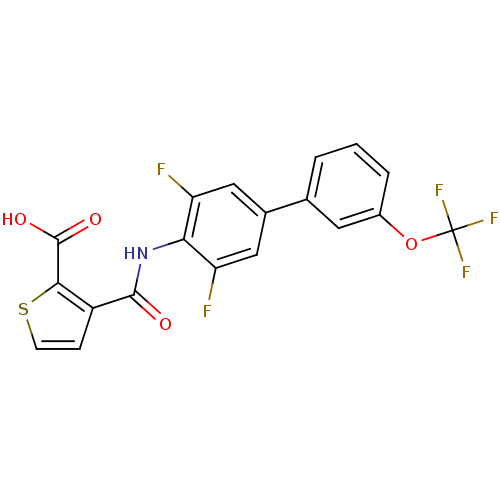 (3-({2,6-difluoro-4-[3-(trifluoromethoxy)phenyl]phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 30 |
4SC AG | Assay Description The assays were carried out by using a colorimetric DCIP method, which uses the colorimetric reagent 2, 6-dichlorophenolindophenol as the final elect... | J Med Chem 49: 1239-47 (2006) Article DOI: 10.1021/jm0506975 BindingDB Entry DOI: 10.7270/Q2571988 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040308 (2-aminoPhCH2(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040298 (CHEMBL386478 | PhCH2CH2(CO)His-Trp-Ala-Val-DAla-Hi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401578 (US10005769, No. 42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401583 (US10005769, No. 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401581 (US10005769, No. 45) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50407277 (CHEMBL2112785) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50407277 (CHEMBL2112785) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description The ability of the peptide to inhibit the binding of 50 pM [125I]-Gastrin releasing peptide to intact S-3T3 cells | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial [30-395] (Homo sapiens (Human)) | BDBM16115 (3-{[4-(3-ethoxyphenyl)-2,6-difluorophenyl]carbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
4SC AG | Assay Description The assays were carried out by using a colorimetric DCIP method, which uses the colorimetric reagent 2, 6-dichlorophenolindophenol as the final elect... | Bioorg Med Chem Lett 16: 267-70 (2006) Article DOI: 10.1016/j.bmcl.2005.10.011 BindingDB Entry DOI: 10.7270/Q2CJ8BQ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401544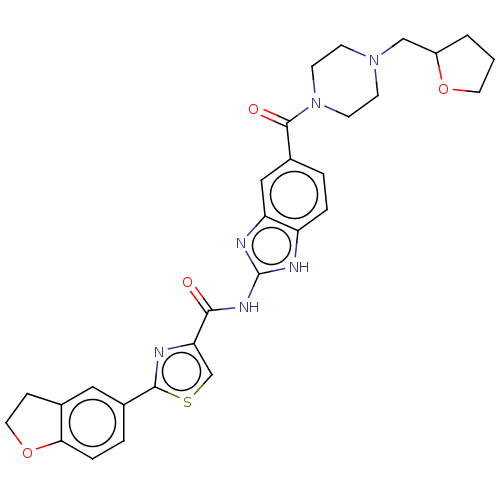 (US10005769, No. 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401561 (US10005769, No. 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401545 (US10005769, No. 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50040319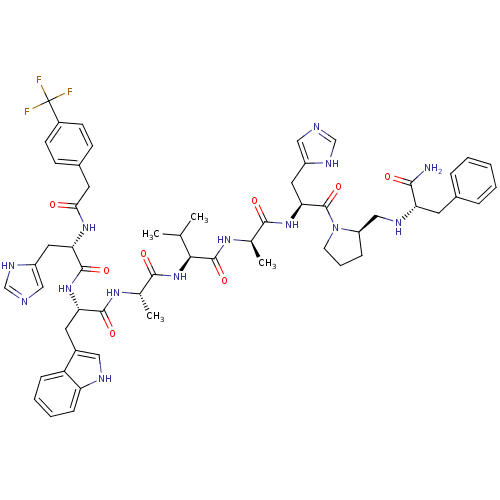 (4-CF3PhCH2(CO)His-Trp-Ala-Val-DAla-His-DPro(psi)Ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Ability of peptide to inhibit binding of 10 pM [125I]-gastrin releasing peptide to S-3T3 cell membrane. | J Med Chem 37: 439-45 (1994) BindingDB Entry DOI: 10.7270/Q2ST7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM401546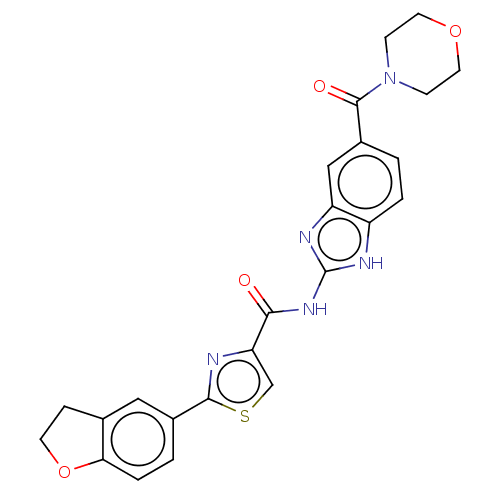 (US10005769, No. 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The substrate DYRKtide (synthetic peptide RRRFRPASPLRGPPK) was dissolved in freshly prepared Base Reaction Buffer (20 mM Hepes (pH 7.5), 10 mM MgCl2,... | Bioorg Med Chem Lett 19: 908-11 (2009) BindingDB Entry DOI: 10.7270/Q2F19224 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 487 total ) | Next | Last >> |