 Found 296 hits with Last Name = 'leon' and Initial = 'l'
Found 296 hits with Last Name = 'leon' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50500145

(CHEMBL1235987)Show SMILES NC(=O)[C@@H](CS)NC(=O)CCCCCNC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C32H41F4N5O13P2S/c33-31(34,55(49,50)51)20-9-5-18(6-10-20)14-22(29(47)38-13-3-1-2-4-25(42)40-24(17-57)28(37)46)41-30(48)23(16-27(44)45)39-26(43)15-19-7-11-21(12-8-19)32(35,36)56(52,53)54/h5-12,22-24,57H,1-4,13-17H2,(H2,37,46)(H,38,47)(H,39,43)(H,40,42)(H,41,48)(H,44,45)(H2,49,50,51)(H2,52,53,54)/t22-,23+,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219566
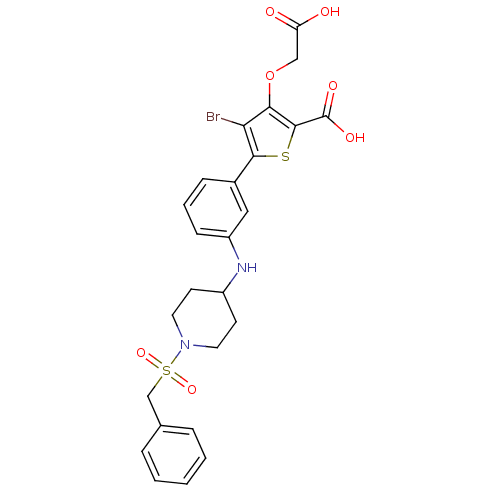
(4-bromo-3-carboxymethoxy-5-[3-(1-phenylmethanesulf...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384445
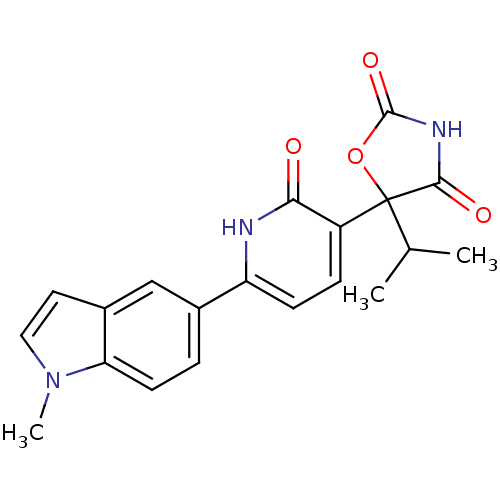
(CHEMBL2035508)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C20H19N3O4/c1-11(2)20(18(25)22-19(26)27-20)14-5-6-15(21-17(14)24)12-4-7-16-13(10-12)8-9-23(16)3/h4-11H,1-3H3,(H,21,24)(H,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384445

(CHEMBL2035508)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C20H19N3O4/c1-11(2)20(18(25)22-19(26)27-20)14-5-6-15(21-17(14)24)12-4-7-16-13(10-12)8-9-23(16)3/h4-11H,1-3H3,(H,21,24)(H,22,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384446

(CHEMBL2035509)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc(C)c(C)c1 Show InChI InChI=1S/C19H20N2O4/c1-10(2)19(17(23)21-18(24)25-19)14-7-8-15(20-16(14)22)13-6-5-11(3)12(4)9-13/h5-10H,1-4H3,(H,20,22)(H,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384446

(CHEMBL2035509)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc(C)c(C)c1 Show InChI InChI=1S/C19H20N2O4/c1-10(2)19(17(23)21-18(24)25-19)14-7-8-15(20-16(14)22)13-6-5-11(3)12(4)9-13/h5-10H,1-4H3,(H,20,22)(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384442
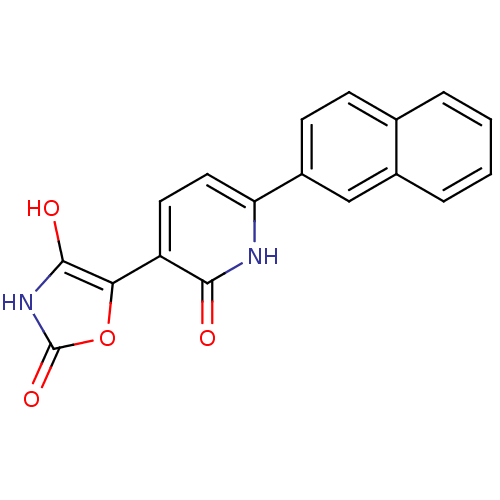
(CHEMBL2035507)Show SMILES Oc1[nH]c(=O)oc1-c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C18H12N2O4/c21-16-13(15-17(22)20-18(23)24-15)7-8-14(19-16)12-6-5-10-3-1-2-4-11(10)9-12/h1-9,22H,(H,19,21)(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23232
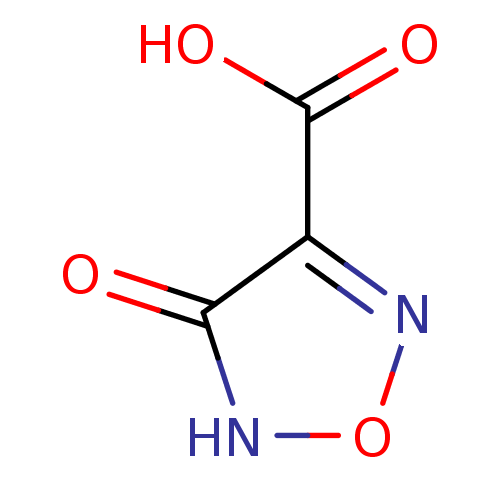
(1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...)Show InChI InChI=1S/C3H2N2O4/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 210 | -38.1 | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23251
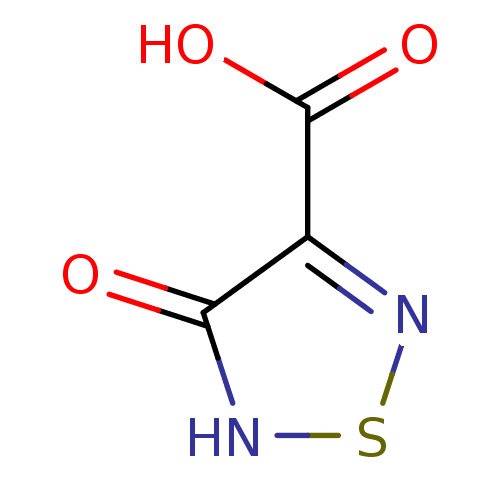
(1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...)Show InChI InChI=1S/C3H2N2O3S/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 290 | -37.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384442

(CHEMBL2035507)Show SMILES Oc1[nH]c(=O)oc1-c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C18H12N2O4/c21-16-13(15-17(22)20-18(23)24-15)7-8-14(19-16)12-6-5-10-3-1-2-4-11(10)9-12/h1-9,22H,(H,19,21)(H,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23242
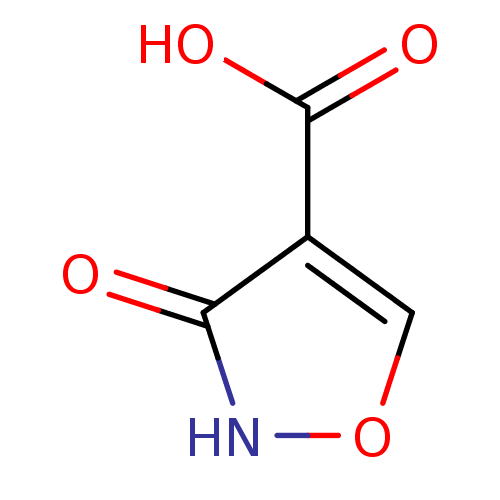
(1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...)Show InChI InChI=1S/C4H3NO4/c6-3-2(4(7)8)1-9-5-3/h1H,(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 470 | -36.1 | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50384442

(CHEMBL2035507)Show SMILES Oc1[nH]c(=O)oc1-c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C18H12N2O4/c21-16-13(15-17(22)20-18(23)24-15)7-8-14(19-16)12-6-5-10-3-1-2-4-11(10)9-12/h1-9,22H,(H,19,21)(H,20,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs by FLIPR assay |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Glucose-1-phosphate cytidylyltransferase
(Salmonella typhi) | BDBM50454133

(CHEBI:17677 | Cystetine Triphosphate)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H16N3O14P3/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(24-8)3-23-28(19,20)26-29(21,22)25-27(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,21,22)(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Binding affinity to Salmonella typhi glucose-1-phosphate cytidylyl-transferase assessed as dissociation constant by spectrophotometry |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs by FLIPR assay |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070228

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2,2-dimet...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C18H34N2O3S/c1-6-7-8-9-10-11-13(14(21)12-24)16(22)20-15(17(23)19-5)18(2,3)4/h13,15,24H,6-12H2,1-5H3,(H,19,23)(H,20,22)/t13?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070247

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2-cyclohe...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C28H44N2O3S/c1-2-3-4-5-12-17-24(26(31)21-34)27(32)30-25(20-23-15-10-7-11-16-23)28(33)29-19-18-22-13-8-6-9-14-22/h6,8-9,13-14,23-25,34H,2-5,7,10-12,15-21H2,1H3,(H,29,33)(H,30,32)/t24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070227
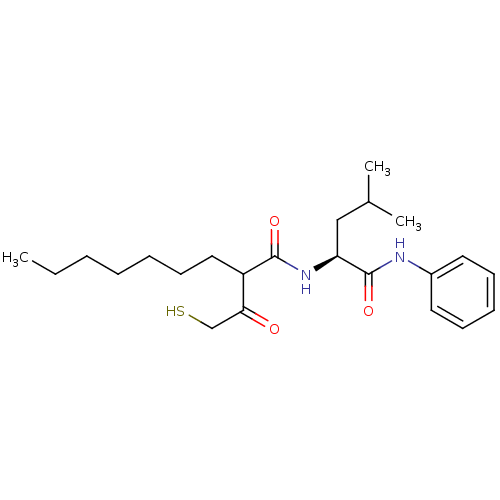
(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C23H36N2O3S/c1-4-5-6-7-11-14-19(21(26)16-29)22(27)25-20(15-17(2)3)23(28)24-18-12-9-8-10-13-18/h8-10,12-13,17,19-20,29H,4-7,11,14-16H2,1-3H3,(H,24,28)(H,25,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070250

(2-(1-Hydroxy-2-mercapto-ethyl)-5-phenyl-pentanoic ...)Show SMILES OC(CS)C(CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H42N2O3S/c33-28(22-36)26(18-10-17-23-11-4-1-5-12-23)29(34)32-27(21-25-15-8-3-9-16-25)30(35)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-28,33,36H,3,8-10,15-22H2,(H,31,35)(H,32,34)/t26?,27-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50236304

(1-(2-(5-bromo-2-(4-fluorophenyl)-1H-indol-3-yl)eth...)Show SMILES Fc1ccc(cc1)-c1[nH]c2ccc(Br)cc2c1CCNC(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C23H19BrFN3O3S/c24-16-8-11-21-20(14-16)19(22(27-21)15-6-9-17(25)10-7-15)12-13-26-23(29)28-32(30,31)18-4-2-1-3-5-18/h1-11,14,27H,12-13H2,(H2,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned CXCR2 by calcium flux FLIPR assay |
Bioorg Med Chem Lett 18: 1926-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.127
BindingDB Entry DOI: 10.7270/Q20P10V3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070226
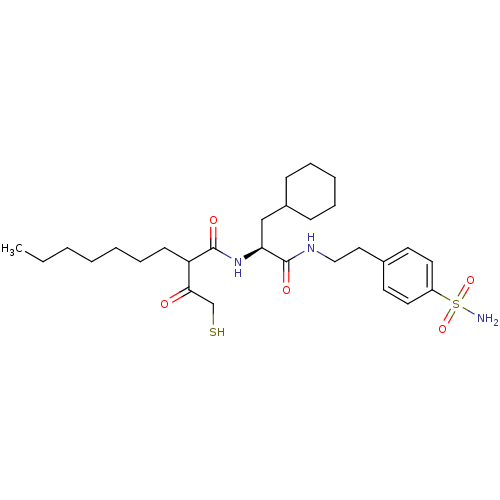
(2-(2-Mercapto-acetyl)-nonanoic acid {(S)-2-cyclohe...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C28H45N3O5S2/c1-2-3-4-5-9-12-24(26(32)20-37)27(33)31-25(19-22-10-7-6-8-11-22)28(34)30-18-17-21-13-15-23(16-14-21)38(29,35)36/h13-16,22,24-25,37H,2-12,17-20H2,1H3,(H,30,34)(H,31,33)(H2,29,35,36)/t24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50236292
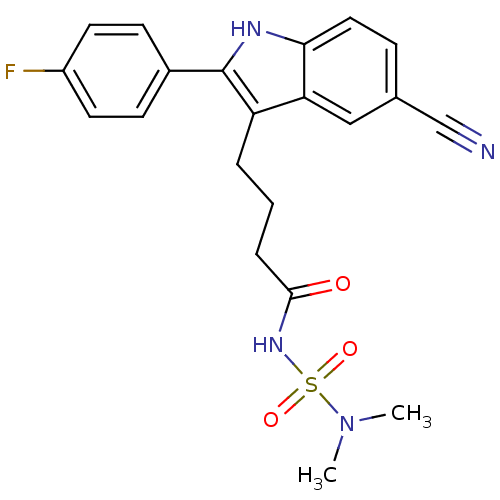
(4-[5-cyano-2-(4-fluorophenyl)-1H-indol-3-yl]-N-(di...)Show SMILES CN(C)S(=O)(=O)NC(=O)CCCc1c([nH]c2ccc(cc12)C#N)-c1ccc(F)cc1 Show InChI InChI=1S/C21H21FN4O3S/c1-26(2)30(28,29)25-20(27)5-3-4-17-18-12-14(13-23)6-11-19(18)24-21(17)15-7-9-16(22)10-8-15/h6-12,24H,3-5H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned CXCR2 by calcium flux FLIPR assay |
Bioorg Med Chem Lett 18: 1926-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.127
BindingDB Entry DOI: 10.7270/Q20P10V3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070223
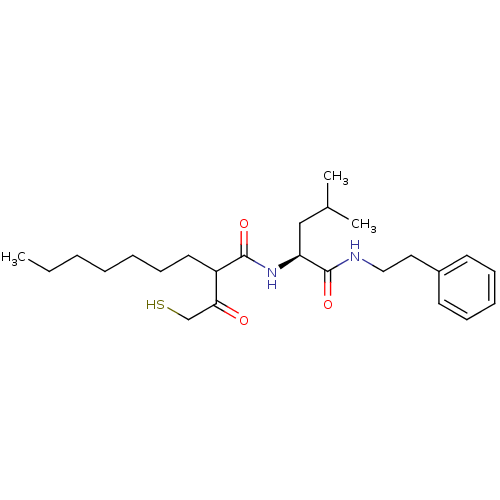
(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C25H40N2O3S/c1-4-5-6-7-11-14-21(23(28)18-31)24(29)27-22(17-19(2)3)25(30)26-16-15-20-12-9-8-10-13-20/h8-10,12-13,19,21-22,31H,4-7,11,14-18H2,1-3H3,(H,26,30)(H,27,29)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070229

(2-(2-Mercapto-acetyl)-4-methyl-pentanoic acid ((S)...)Show SMILES CNC(=O)[C@@H](NC(=O)C(CC(C)C)C(=O)CS)C(C)(C)C Show InChI InChI=1S/C15H28N2O3S/c1-9(2)7-10(11(18)8-21)13(19)17-12(14(20)16-6)15(3,4)5/h9-10,12,21H,7-8H2,1-6H3,(H,16,20)(H,17,19)/t10?,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070239
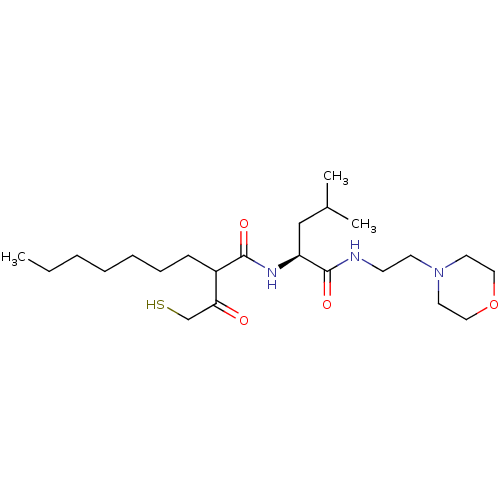
(2-(2-Mercapto-acetyl)-nonanoic acid [(S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C23H43N3O4S/c1-4-5-6-7-8-9-19(21(27)17-31)22(28)25-20(16-18(2)3)23(29)24-10-11-26-12-14-30-15-13-26/h18-20,31H,4-17H2,1-3H3,(H,24,29)(H,25,28)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50500147
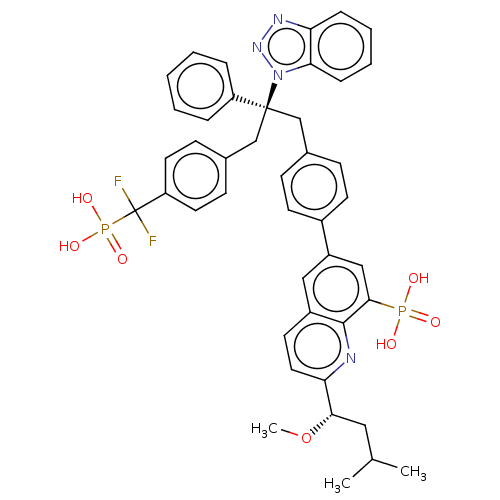
(CHEMBL3746639)Show SMILES CO[C@@H](CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(C[C@](Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 |r| Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55)/t39-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070241

(2-(2-Mercapto-acetyl)-nonanoic acid [(S)-1-(2-ethy...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NC(=O)C(CC(C)C)NCC Show InChI InChI=1S/C25H47N3O4S/c1-7-9-10-11-12-13-19(22(29)16-33)23(30)27-21(15-18(5)6)25(32)28-24(31)20(26-8-2)14-17(3)4/h17-21,26,33H,7-16H2,1-6H3,(H,27,30)(H,28,31,32)/t19?,20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against collagenase-1(MMP-1). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070235

(2-(1-Hydroxy-2-mercapto-ethyl)-nonanoic acid [(S)-...)Show SMILES CCCCCCCC(C(O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C23H45N3O4S/c1-4-5-6-7-8-9-19(21(27)17-31)22(28)25-20(16-18(2)3)23(29)24-10-11-26-12-14-30-15-13-26/h18-21,27,31H,4-17H2,1-3H3,(H,24,29)(H,25,28)/t19?,20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against collagenase-1(MMP-1). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070233

(2-(1-Hydroxy-2-mercapto-ethyl)-nonanoic acid [(S)-...)Show SMILES CCCCCCCC(C(O)CS)C(=O)N[C@@H](CCCCNS(=O)(=O)c1ccc(C)cc1)C(=O)NC(=O)C(CC(C)C)NCC Show InChI InChI=1S/C32H56N4O6S2/c1-6-8-9-10-11-14-26(29(37)22-43)30(38)35-27(31(39)36-32(40)28(33-7-2)21-23(3)4)15-12-13-20-34-44(41,42)25-18-16-24(5)17-19-25/h16-19,23,26-29,33-34,37,43H,6-15,20-22H2,1-5H3,(H,35,38)(H,36,39,40)/t26?,27-,28?,29?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against collagenase-1(MMP-1). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070240
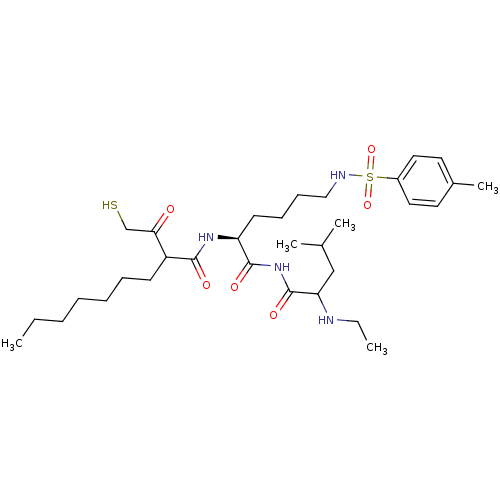
(2-(2-Mercapto-acetyl)-nonanoic acid [(S)-1-(2-ethy...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CCCCNS(=O)(=O)c1ccc(C)cc1)C(=O)NC(=O)C(CC(C)C)NCC Show InChI InChI=1S/C32H54N4O6S2/c1-6-8-9-10-11-14-26(29(37)22-43)30(38)35-27(31(39)36-32(40)28(33-7-2)21-23(3)4)15-12-13-20-34-44(41,42)25-18-16-24(5)17-19-25/h16-19,23,26-28,33-34,43H,6-15,20-22H2,1-5H3,(H,35,38)(H,36,39,40)/t26?,27-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50236295
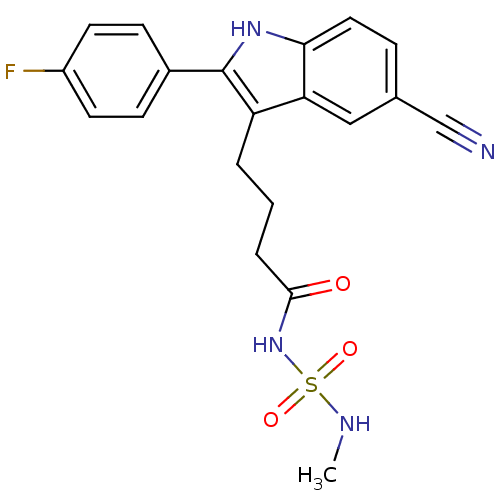
(4-[5-cyano-2-(4-fluorophenyl)-1H-indol-3-yl]-N-[(m...)Show SMILES CNS(=O)(=O)NC(=O)CCCc1c([nH]c2ccc(cc12)C#N)-c1ccc(F)cc1 Show InChI InChI=1S/C20H19FN4O3S/c1-23-29(27,28)25-19(26)4-2-3-16-17-11-13(12-22)5-10-18(17)24-20(16)14-6-8-15(21)9-7-14/h5-11,23-24H,2-4H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned CXCR2 by calcium flux FLIPR assay |
Bioorg Med Chem Lett 18: 1926-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.127
BindingDB Entry DOI: 10.7270/Q20P10V3 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070229

(2-(2-Mercapto-acetyl)-4-methyl-pentanoic acid ((S)...)Show SMILES CNC(=O)[C@@H](NC(=O)C(CC(C)C)C(=O)CS)C(C)(C)C Show InChI InChI=1S/C15H28N2O3S/c1-9(2)7-10(11(18)8-21)13(19)17-12(14(20)16-6)15(3,4)5/h9-10,12,21H,7-8H2,1-6H3,(H,16,20)(H,17,19)/t10?,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against collagenase-1(MMP-1). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070228

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2,2-dimet...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C18H34N2O3S/c1-6-7-8-9-10-11-13(14(21)12-24)16(22)20-15(17(23)19-5)18(2,3)4/h13,15,24H,6-12H2,1-5H3,(H,19,23)(H,20,22)/t13?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against collagenase-1(MMP-1). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070228

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2,2-dimet...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C18H34N2O3S/c1-6-7-8-9-10-11-13(14(21)12-24)16(22)20-15(17(23)19-5)18(2,3)4/h13,15,24H,6-12H2,1-5H3,(H,19,23)(H,20,22)/t13?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50236312
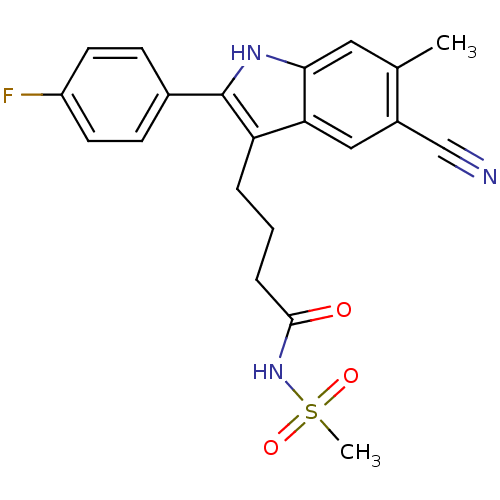
(4-(5-cyano-2-(4-fluorophenyl)-6-methyl-1H-indol-3-...)Show SMILES Cc1cc2[nH]c(c(CCCC(=O)NS(C)(=O)=O)c2cc1C#N)-c1ccc(F)cc1 Show InChI InChI=1S/C21H20FN3O3S/c1-13-10-19-18(11-15(13)12-23)17(4-3-5-20(26)25-29(2,27)28)21(24-19)14-6-8-16(22)9-7-14/h6-11,24H,3-5H2,1-2H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of europium labeled-human IL8 from human cloned CXCR2 expressed in Sf9 membrane with Galphai3-beta-1-gamma-2 by DELFIA binding assay |
Bioorg Med Chem Lett 18: 1926-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.127
BindingDB Entry DOI: 10.7270/Q20P10V3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070234

(2-(2-Mercapto-acetyl)-4-methyl-pentanoic acid [(S)...)Show SMILES CC(C)CC(C(=O)CS)C(=O)N[C@H](C(=O)Nc1ccccn1)C(C)(C)C Show InChI InChI=1S/C19H29N3O3S/c1-12(2)10-13(14(23)11-26)17(24)22-16(19(3,4)5)18(25)21-15-8-6-7-9-20-15/h6-9,12-13,16,26H,10-11H2,1-5H3,(H,22,24)(H,20,21,25)/t13?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070223

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C25H40N2O3S/c1-4-5-6-7-11-14-21(23(28)18-31)24(29)27-22(17-19(2)3)25(30)26-16-15-20-12-9-8-10-13-20/h8-10,12-13,19,21-22,31H,4-7,11,14-18H2,1-3H3,(H,26,30)(H,27,29)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against collagenase-1(MMP-1). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070244

(2-(1-Hydroxy-2-mercapto-ethyl)-nonanoic acid [(S)-...)Show SMILES CCCCCCCC(C(O)CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C26H49N3O4S/c1-2-3-4-5-9-12-22(24(30)20-34)25(31)28-23(19-21-10-7-6-8-11-21)26(32)27-13-14-29-15-17-33-18-16-29/h21-24,30,34H,2-20H2,1H3,(H,27,32)(H,28,31)/t22?,23-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against collagenase-1(MMP-1). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070225
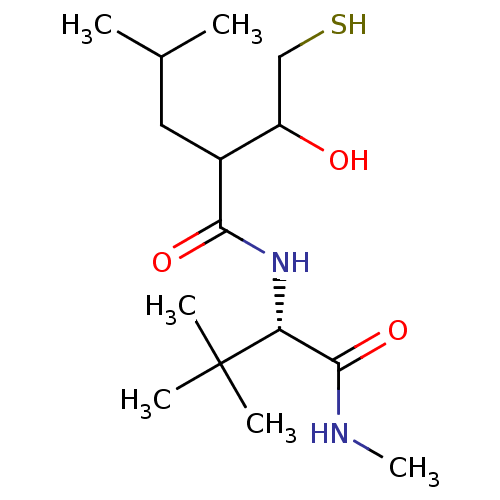
(2-(1-Hydroxy-2-mercapto-ethyl)-4-methyl-pentanoic ...)Show SMILES CNC(=O)[C@@H](NC(=O)C(CC(C)C)C(O)CS)C(C)(C)C Show InChI InChI=1S/C15H30N2O3S/c1-9(2)7-10(11(18)8-21)13(19)17-12(14(20)16-6)15(3,4)5/h9-12,18,21H,7-8H2,1-6H3,(H,16,20)(H,17,19)/t10?,11?,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070230
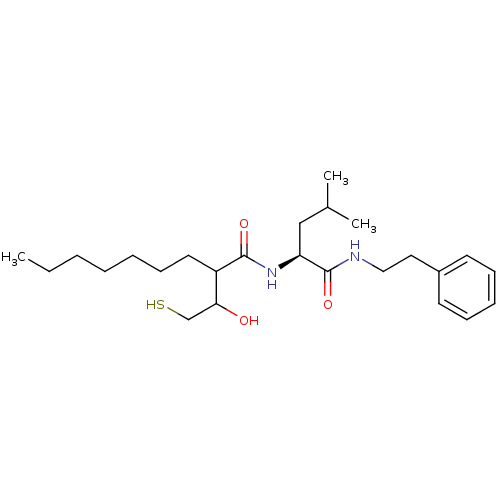
(2-(1-Hydroxy-2-mercapto-ethyl)-nonanoic acid ((S)-...)Show SMILES CCCCCCCC(C(O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C25H42N2O3S/c1-4-5-6-7-11-14-21(23(28)18-31)24(29)27-22(17-19(2)3)25(30)26-16-15-20-12-9-8-10-13-20/h8-10,12-13,19,21-23,28,31H,4-7,11,14-18H2,1-3H3,(H,26,30)(H,27,29)/t21?,22-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against collagenase-1(MMP-1). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070236
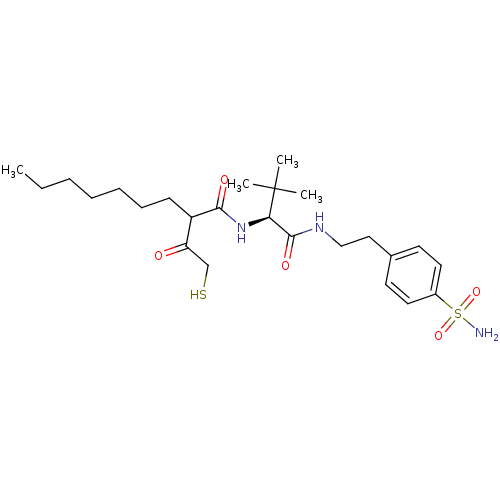
(2-(2-Mercapto-acetyl)-nonanoic acid {(S)-2,2-dimet...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@H](C(=O)NCCc1ccc(cc1)S(N)(=O)=O)C(C)(C)C Show InChI InChI=1S/C25H41N3O5S2/c1-5-6-7-8-9-10-20(21(29)17-34)23(30)28-22(25(2,3)4)24(31)27-16-15-18-11-13-19(14-12-18)35(26,32)33/h11-14,20,22,34H,5-10,15-17H2,1-4H3,(H,27,31)(H,28,30)(H2,26,32,33)/t20?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50236318
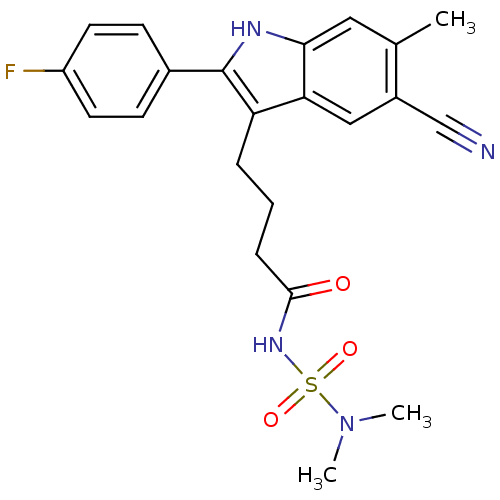
(4-[5-cyano-2-(4-fluorophenyl)-6-methyl-1H-indol-3-...)Show SMILES CN(C)S(=O)(=O)NC(=O)CCCc1c([nH]c2cc(C)c(cc12)C#N)-c1ccc(F)cc1 Show InChI InChI=1S/C22H23FN4O3S/c1-14-11-20-19(12-16(14)13-24)18(22(25-20)15-7-9-17(23)10-8-15)5-4-6-21(28)26-31(29,30)27(2)3/h7-12,25H,4-6H2,1-3H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of europium labeled-human IL8 from human cloned CXCR2 expressed in Sf9 membrane with Galphai3-beta-1-gamma-2 by DELFIA binding assay |
Bioorg Med Chem Lett 18: 1926-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.127
BindingDB Entry DOI: 10.7270/Q20P10V3 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070241

(2-(2-Mercapto-acetyl)-nonanoic acid [(S)-1-(2-ethy...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NC(=O)C(CC(C)C)NCC Show InChI InChI=1S/C25H47N3O4S/c1-7-9-10-11-12-13-19(22(29)16-33)23(30)27-21(15-18(5)6)25(32)28-24(31)20(26-8-2)14-17(3)4/h17-21,26,33H,7-16H2,1-6H3,(H,27,30)(H,28,31,32)/t19?,20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070242
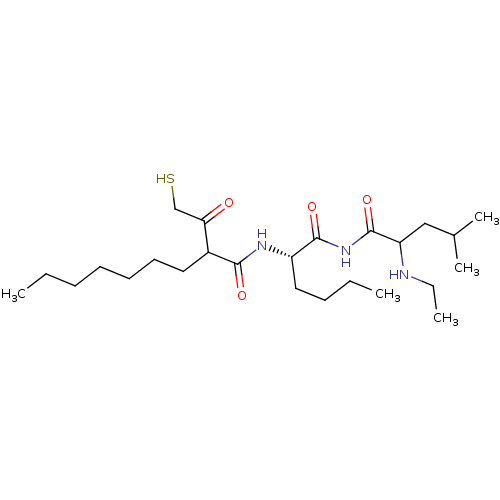
(2-(2-Mercapto-acetyl)-nonanoic acid [(S)-1-(2-ethy...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CCCC)C(=O)NC(=O)C(CC(C)C)NCC Show InChI InChI=1S/C25H47N3O4S/c1-6-9-11-12-13-14-19(22(29)17-33)23(30)27-20(15-10-7-2)24(31)28-25(32)21(26-8-3)16-18(4)5/h18-21,26,33H,6-17H2,1-5H3,(H,27,30)(H,28,31,32)/t19?,20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070241

(2-(2-Mercapto-acetyl)-nonanoic acid [(S)-1-(2-ethy...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NC(=O)C(CC(C)C)NCC Show InChI InChI=1S/C25H47N3O4S/c1-7-9-10-11-12-13-19(22(29)16-33)23(30)27-21(15-18(5)6)25(32)28-24(31)20(26-8-2)14-17(3)4/h17-21,26,33H,7-16H2,1-6H3,(H,27,30)(H,28,31,32)/t19?,20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50236305
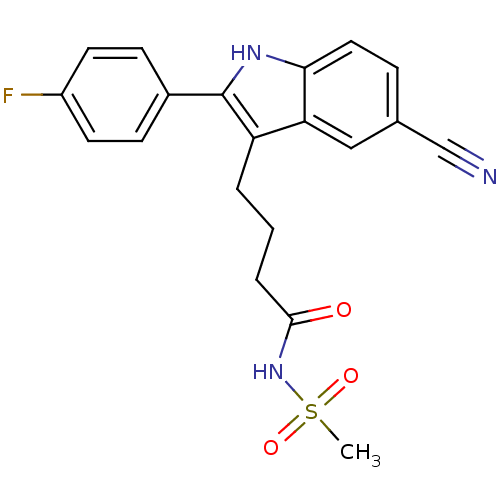
(4-(5-cyano-2-(4-fluorophenyl)-1H-indol-3-yl)-N-(me...)Show SMILES CS(=O)(=O)NC(=O)CCCc1c([nH]c2ccc(cc12)C#N)-c1ccc(F)cc1 Show InChI InChI=1S/C20H18FN3O3S/c1-28(26,27)24-19(25)4-2-3-16-17-11-13(12-22)5-10-18(17)23-20(16)14-6-8-15(21)9-7-14/h5-11,23H,2-4H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned CXCR2 by calcium flux FLIPR assay |
Bioorg Med Chem Lett 18: 1926-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.127
BindingDB Entry DOI: 10.7270/Q20P10V3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data