 Found 2718 hits with Last Name = 'levis' and Initial = 'm'
Found 2718 hits with Last Name = 'levis' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300970
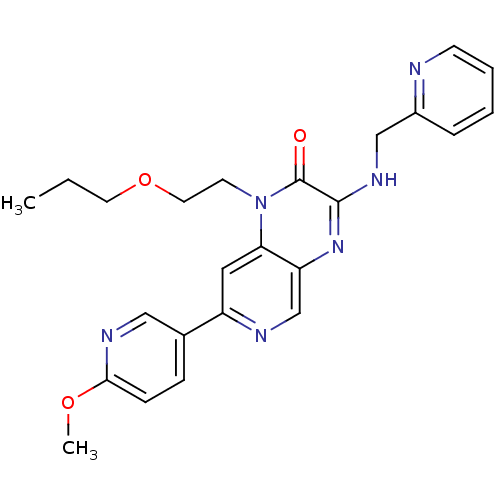
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCc2ccccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H26N6O3/c1-3-11-33-12-10-30-21-13-19(17-7-8-22(32-2)27-14-17)26-16-20(21)29-23(24(30)31)28-15-18-6-4-5-9-25-18/h4-9,13-14,16H,3,10-12,15H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300964
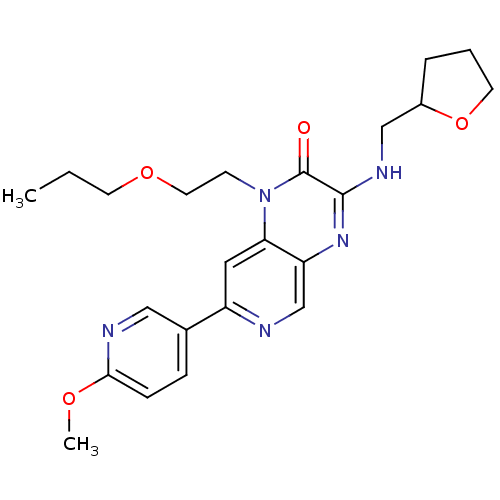
(CHEMBL584270 | rac-7-(6-methoxypyridin-3-yl)-1-(2-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC2CCCO2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H29N5O4/c1-3-9-31-11-8-28-20-12-18(16-6-7-21(30-2)25-13-16)24-15-19(20)27-22(23(28)29)26-14-17-5-4-10-32-17/h6-7,12-13,15,17H,3-5,8-11,14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300953
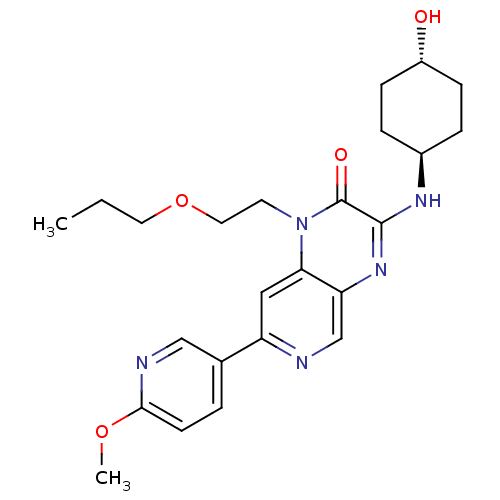
(3-[(trans-4-hydroxycyclohexyl)amino]-7-(6-methoxyp...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H]2CC[C@H](O)CC2)c1=O)-c1ccc(OC)nc1 |r,wU:16.16,wD:19.20,(24.97,-.51,;23.64,-1.28,;23.64,-2.82,;22.3,-3.59,;22.3,-5.13,;20.97,-5.9,;20.97,-7.44,;22.3,-8.21,;23.62,-7.45,;24.95,-8.21,;24.95,-9.75,;23.62,-10.52,;22.3,-9.75,;20.97,-10.52,;19.62,-9.75,;18.29,-10.51,;16.96,-9.74,;15.63,-10.51,;14.29,-9.73,;14.3,-8.19,;12.97,-7.41,;15.64,-7.43,;16.97,-8.2,;19.64,-8.21,;18.31,-7.44,;26.28,-7.43,;27.61,-8.2,;28.94,-7.43,;28.94,-5.89,;30.27,-5.11,;31.61,-5.87,;27.59,-5.12,;26.27,-5.9,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-21-13-19(16-4-9-22(32-2)26-14-16)25-15-20(21)28-23(24(29)31)27-17-5-7-18(30)8-6-17/h4,9,13-15,17-18,30H,3,5-8,10-12H2,1-2H3,(H,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300972
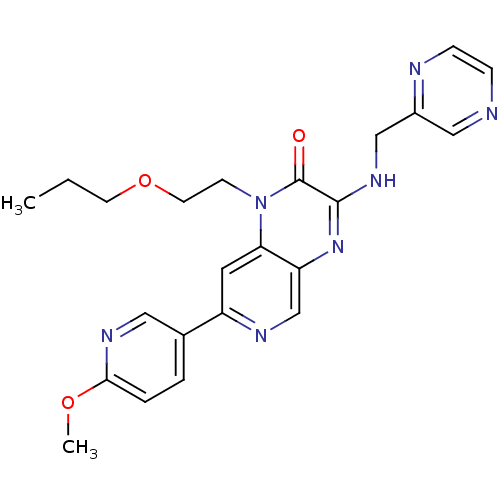
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCc2cnccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H25N7O3/c1-3-9-33-10-8-30-20-11-18(16-4-5-21(32-2)27-12-16)26-15-19(20)29-22(23(30)31)28-14-17-13-24-6-7-25-17/h4-7,11-13,15H,3,8-10,14H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300976
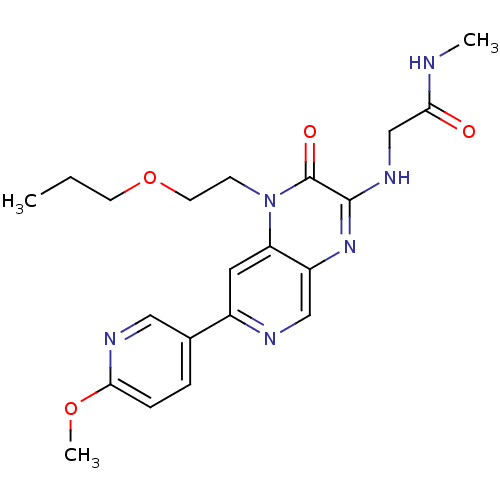
(2-(7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyeth...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)NC)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H26N6O4/c1-4-8-31-9-7-27-17-10-15(14-5-6-19(30-3)24-11-14)23-12-16(17)26-20(21(27)29)25-13-18(28)22-2/h5-6,10-12H,4,7-9,13H2,1-3H3,(H,22,28)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50296256
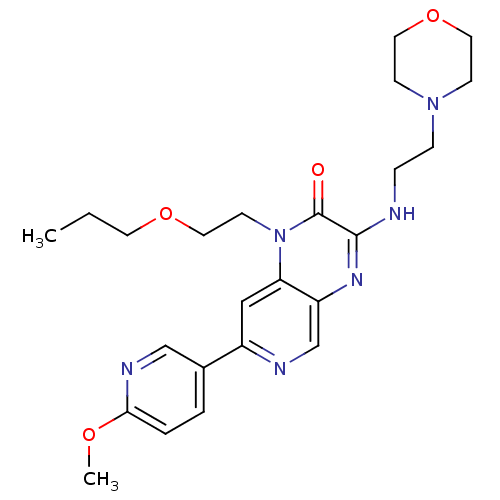
(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-19(18-4-5-22(32-2)27-16-18)26-17-20(21)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300983
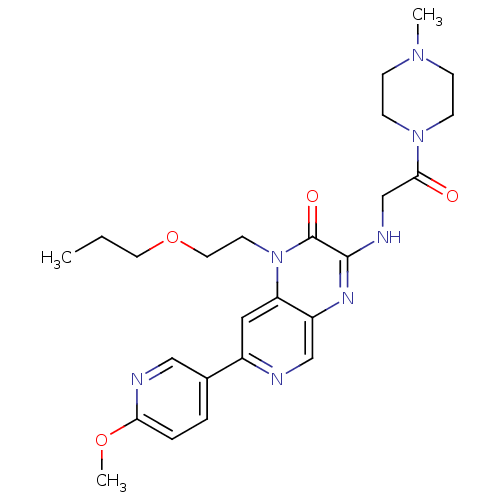
(7-(6-methoxypyridin-3-yl)-3-(2-(4-methylpiperazin-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCN(C)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H33N7O4/c1-4-12-36-13-11-32-21-14-19(18-5-6-22(35-3)27-15-18)26-16-20(21)29-24(25(32)34)28-17-23(33)31-9-7-30(2)8-10-31/h5-6,14-16H,4,7-13,17H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50296256

(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-19(18-4-5-22(32-2)27-16-18)26-17-20(21)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 4092-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.004
BindingDB Entry DOI: 10.7270/Q2ZG6S9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300977
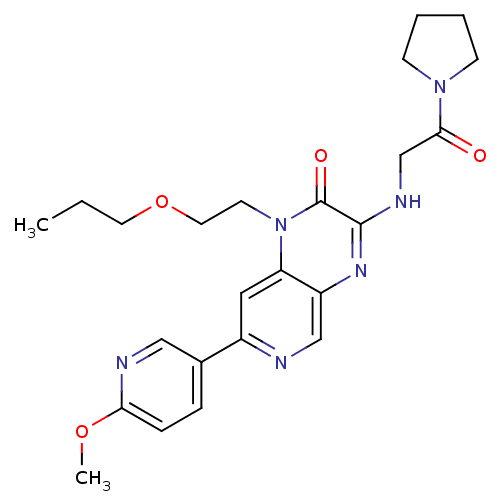
(7-(6-methoxypyridin-3-yl)-3-(2-oxo-2-(pyrrolidin-1...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O4/c1-3-11-34-12-10-30-20-13-18(17-6-7-21(33-2)26-14-17)25-15-19(20)28-23(24(30)32)27-16-22(31)29-8-4-5-9-29/h6-7,13-15H,3-5,8-12,16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300989
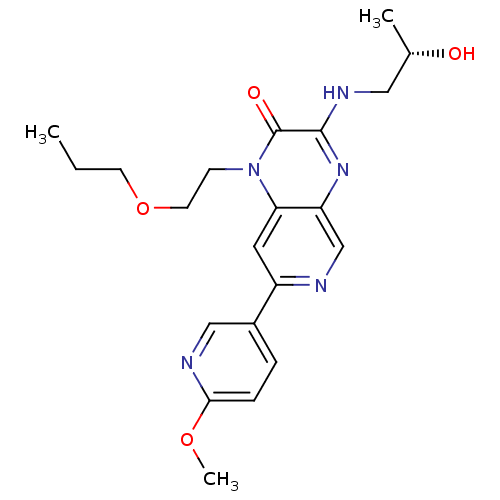
((S)-3-(2-hydroxypropylamino)-7-(6-methoxypyridin-3...)Show SMILES CCCOCCn1c2cc(ncc2nc(NC[C@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-12-15)22-13-17(18)25-20(21(26)28)24-11-14(2)27/h5-6,10,12-14,27H,4,7-9,11H2,1-3H3,(H,24,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300991
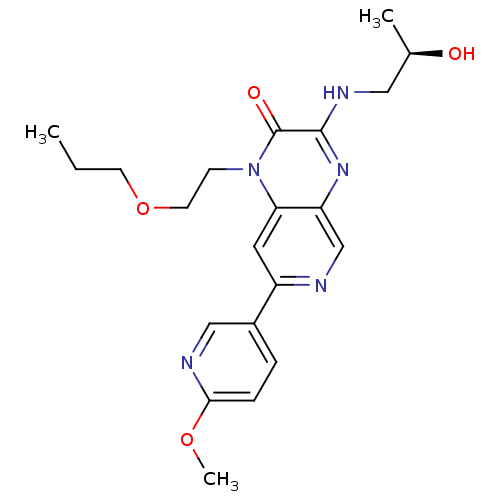
((R)-3-(2-hydroxypropylamino)-7-(6-methoxypyridin-3...)Show SMILES CCCOCCn1c2cc(ncc2nc(NC[C@@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-12-15)22-13-17(18)25-20(21(26)28)24-11-14(2)27/h5-6,10,12-14,27H,4,7-9,11H2,1-3H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300962
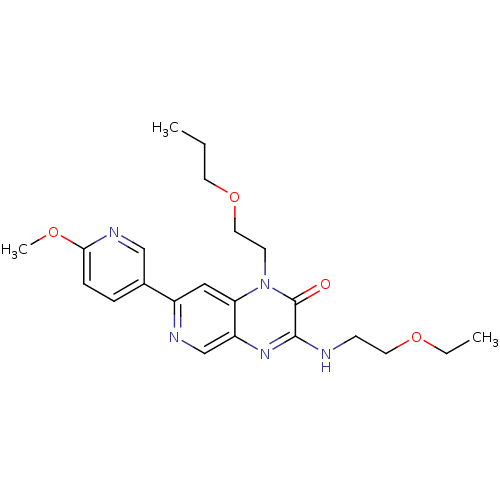
(3-(2-ethoxyethylamino)-7-(6-methoxypyridin-3-yl)-1...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCOCC)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C22H29N5O4/c1-4-10-31-12-9-27-19-13-17(16-6-7-20(29-3)25-14-16)24-15-18(19)26-21(22(27)28)23-8-11-30-5-2/h6-7,13-15H,4-5,8-12H2,1-3H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300978
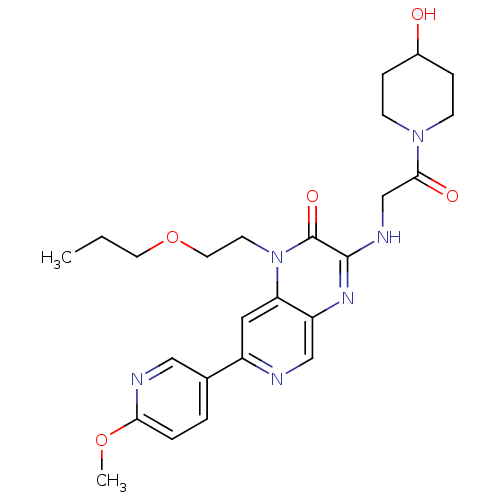
(3-(2-(4-hydroxypiperidin-1-yl)-2-oxoethylamino)-7-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCC(O)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H32N6O5/c1-3-11-36-12-10-31-21-13-19(17-4-5-22(35-2)27-14-17)26-15-20(21)29-24(25(31)34)28-16-23(33)30-8-6-18(32)7-9-30/h4-5,13-15,18,32H,3,6-12,16H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300985
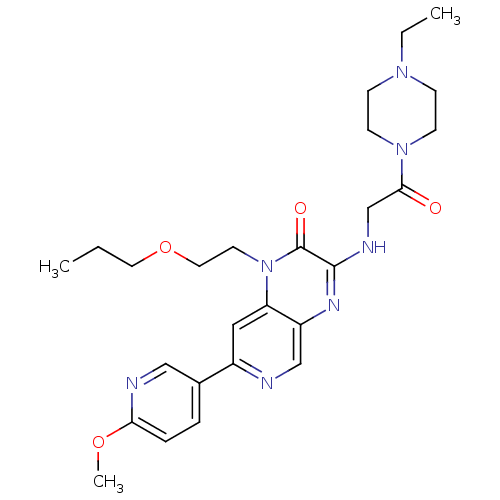
(3-(2-(4-ethylpiperazin-1-yl)-2-oxoethylamino)-7-(6...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCN(CC)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C26H35N7O4/c1-4-13-37-14-12-33-22-15-20(19-6-7-23(36-3)28-16-19)27-17-21(22)30-25(26(33)35)29-18-24(34)32-10-8-31(5-2)9-11-32/h6-7,15-17H,4-5,8-14,18H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300963
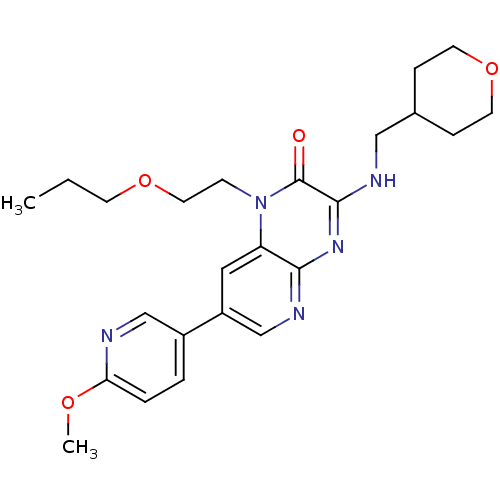
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-((t...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCC2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H31N5O4/c1-3-9-32-12-8-29-20-13-19(18-4-5-21(31-2)25-15-18)16-27-22(20)28-23(24(29)30)26-14-17-6-10-33-11-7-17/h4-5,13,15-17H,3,6-12,14H2,1-2H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300948
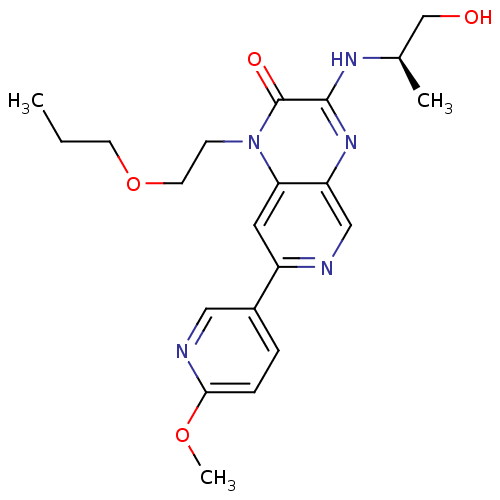
((R)-3-(1-hydroxypropan-2-ylamino)-7-(6-methoxypyri...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H](C)CO)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-11-15)22-12-17(18)25-20(21(26)28)24-14(2)13-27/h5-6,10-12,14,27H,4,7-9,13H2,1-3H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300980
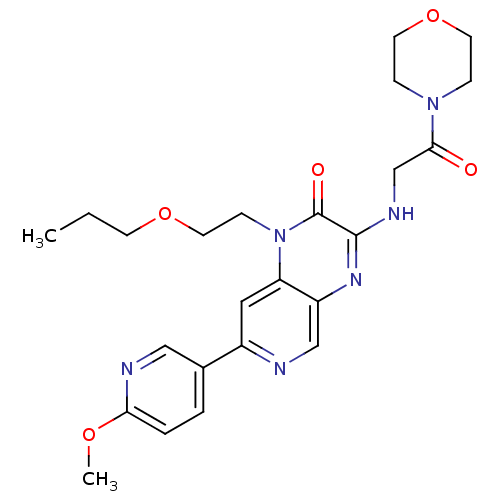
(7-(6-methoxypyridin-3-yl)-3-(2-morpholino-2-oxoeth...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O5/c1-3-9-34-12-8-30-20-13-18(17-4-5-21(33-2)26-14-17)25-15-19(20)28-23(24(30)32)27-16-22(31)29-6-10-35-11-7-29/h4-5,13-15H,3,6-12,16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300961
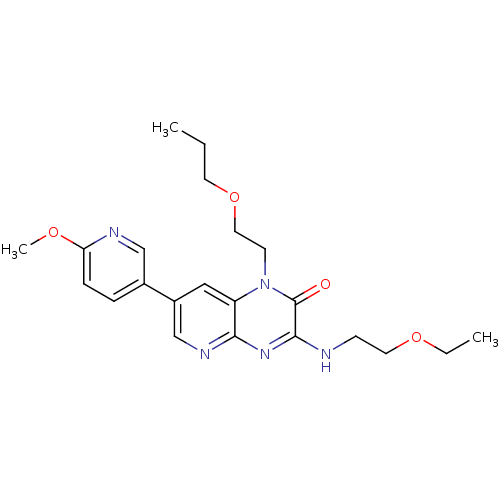
(3-(2-ethoxyethylamino)-7-(6-methoxypyridin-3-yl)-1...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCCOCC)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C22H29N5O4/c1-4-10-31-12-9-27-18-13-17(16-6-7-19(29-3)24-14-16)15-25-20(18)26-21(22(27)28)23-8-11-30-5-2/h6-7,13-15H,4-5,8-12H2,1-3H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308556
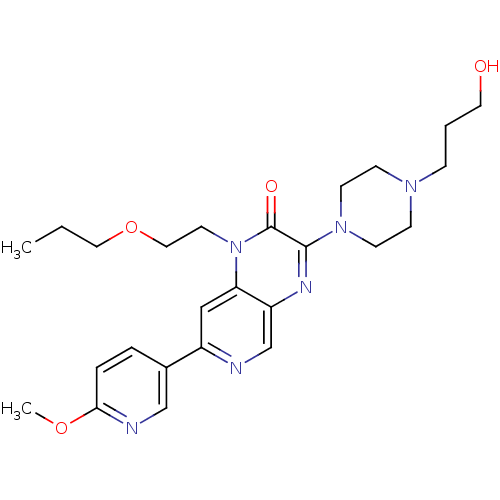
(3-(4-(3-hydroxypropyl)piperazin-1-yl)-1-(2-propoxy...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CCCO)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H34N6O4/c1-3-14-35-15-12-31-22-16-20(19-5-6-23(34-2)27-17-19)26-18-21(22)28-24(25(31)33)30-10-8-29(9-11-30)7-4-13-32/h5-6,16-18,32H,3-4,7-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308557
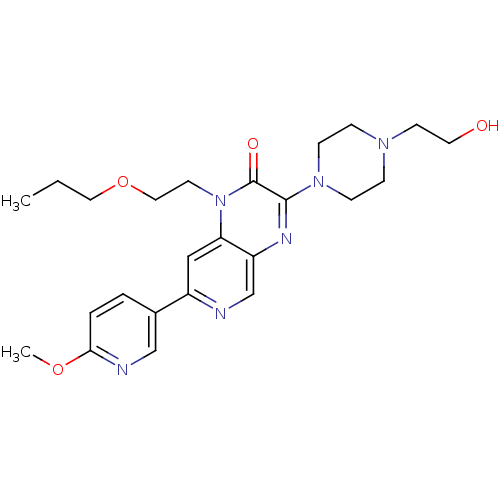
(3-(4-(2-hydroxyethyl)piperazin-1-yl)-1-(2-propoxye...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CCO)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-13-34-14-11-30-21-15-19(18-4-5-22(33-2)26-16-18)25-17-20(21)27-23(24(30)32)29-8-6-28(7-9-29)10-12-31/h4-5,15-17,31H,3,6-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308563
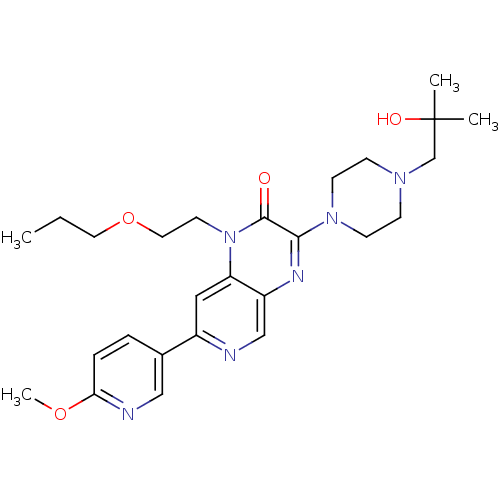
(3-(4-(2-hydroxy-2-methylpropyl)piperazin-1-yl)-1-(...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CC(C)(C)O)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C26H36N6O4/c1-5-13-36-14-12-32-22-15-20(19-6-7-23(35-4)28-16-19)27-17-21(22)29-24(25(32)33)31-10-8-30(9-11-31)18-26(2,3)34/h6-7,15-17,34H,5,8-14,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308558

(3-(4-ethylpiperazin-1-yl)-1-(2-propoxyethyl)-7-(6-...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CC)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O3/c1-4-13-33-14-12-30-21-15-19(18-6-7-22(32-3)26-16-18)25-17-20(21)27-23(24(30)31)29-10-8-28(5-2)9-11-29/h6-7,15-17H,4-5,8-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308564

((R)-3-(4-(2-hydroxypropyl)piperazin-1-yl)-1-(2-iso...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(C[C@@H](C)O)CC2)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C25H34N6O4/c1-4-12-35-13-11-31-22-14-20(19-5-6-23(34-3)27-15-19)26-16-21(22)28-24(25(31)33)30-9-7-29(8-10-30)17-18(2)32/h5-6,14-16,18,32H,4,7-13,17H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300950
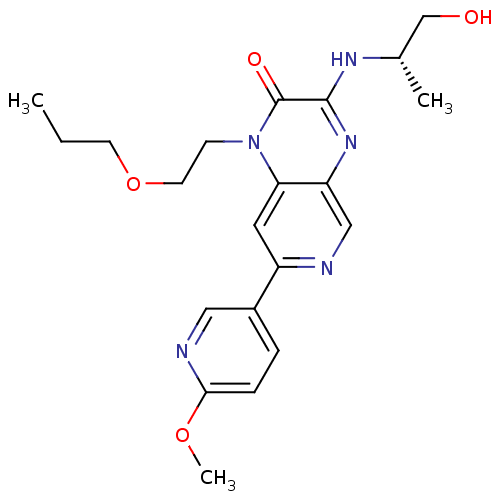
((S)-3-(1-hydroxypropan-2-ylamino)-7-(6-methoxypyri...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@@H](C)CO)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-11-15)22-12-17(18)25-20(21(26)28)24-14(2)13-27/h5-6,10-12,14,27H,4,7-9,13H2,1-3H3,(H,24,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300951
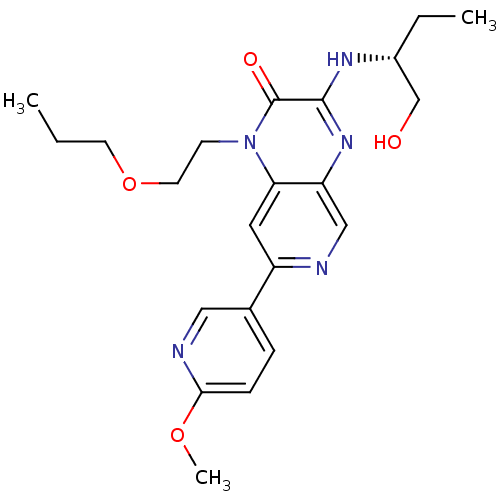
((R)-3-(1-hydroxybutan-2-ylamino)-7-(6-methoxypyrid...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H](CC)CO)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C22H29N5O4/c1-4-9-31-10-8-27-19-11-17(15-6-7-20(30-3)24-12-15)23-13-18(19)26-21(22(27)29)25-16(5-2)14-28/h6-7,11-13,16,28H,4-5,8-10,14H2,1-3H3,(H,25,26)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300957
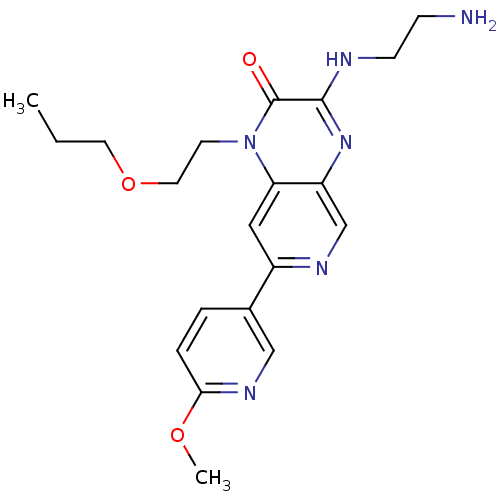
(3-(2-aminoethylamino)-7-(6-methoxypyridin-3-yl)-1-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C20H26N6O3/c1-3-9-29-10-8-26-17-11-15(14-4-5-18(28-2)24-12-14)23-13-16(17)25-19(20(26)27)22-7-6-21/h4-5,11-13H,3,6-10,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300957

(3-(2-aminoethylamino)-7-(6-methoxypyridin-3-yl)-1-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C20H26N6O3/c1-3-9-29-10-8-26-17-11-15(14-4-5-18(28-2)24-12-14)23-13-16(17)25-19(20(26)27)22-7-6-21/h4-5,11-13H,3,6-10,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300987
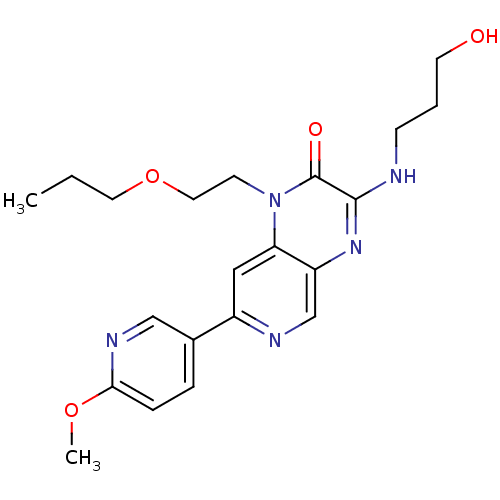
(3-(3-hydroxypropylamino)-7-(6-methoxypyridin-3-yl)...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCCO)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H27N5O4/c1-3-10-30-11-8-26-18-12-16(15-5-6-19(29-2)24-13-15)23-14-17(18)25-20(21(26)28)22-7-4-9-27/h5-6,12-14,27H,3-4,7-11H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300971
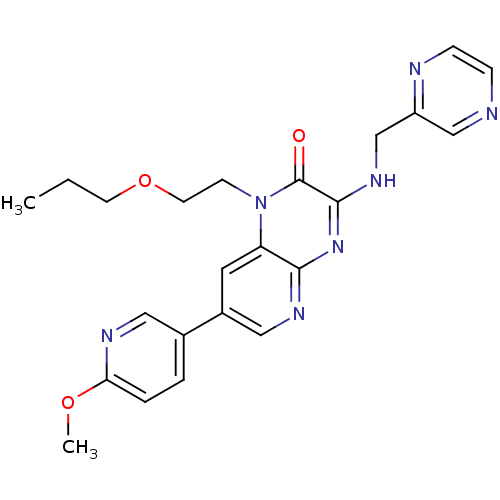
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCc2cnccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H25N7O3/c1-3-9-33-10-8-30-19-11-17(16-4-5-20(32-2)26-12-16)13-27-21(19)29-22(23(30)31)28-15-18-14-24-6-7-25-18/h4-7,11-14H,3,8-10,15H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308561
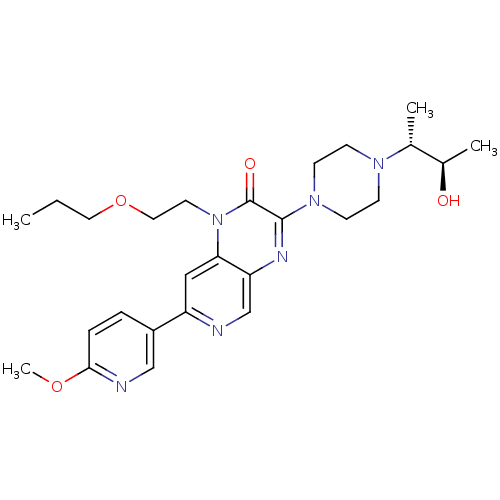
((+/-)-3-(4-((2R,3R)-3-hydroxybutan-2-yl)piperazin-...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CC2)[C@H](C)[C@@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C26H36N6O4/c1-5-13-36-14-12-32-23-15-21(20-6-7-24(35-4)28-16-20)27-17-22(23)29-25(26(32)34)31-10-8-30(9-11-31)18(2)19(3)33/h6-7,15-19,33H,5,8-14H2,1-4H3/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50297795
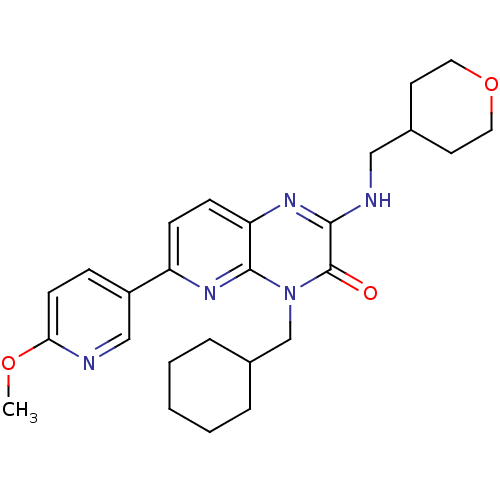
(4-(cyclohexylmethyl)-6-(6-methoxypyridin-3-yl)-2-(...)Show SMILES COc1ccc(cn1)-c1ccc2nc(NCC3CCOCC3)c(=O)n(CC3CCCCC3)c2n1 Show InChI InChI=1S/C26H33N5O3/c1-33-23-10-7-20(16-27-23)21-8-9-22-25(30-21)31(17-19-5-3-2-4-6-19)26(32)24(29-22)28-15-18-11-13-34-14-12-18/h7-10,16,18-19H,2-6,11-15,17H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 4088-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.012
BindingDB Entry DOI: 10.7270/Q23R0SXR |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50297794
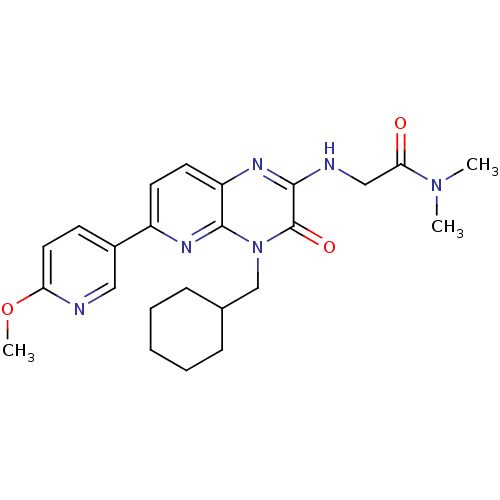
(2-(4-(cyclohexylmethyl)-6-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-c1ccc2nc(NCC(=O)N(C)C)c(=O)n(CC3CCCCC3)c2n1 Show InChI InChI=1S/C24H30N6O3/c1-29(2)21(31)14-26-22-24(32)30(15-16-7-5-4-6-8-16)23-19(27-22)11-10-18(28-23)17-9-12-20(33-3)25-13-17/h9-13,16H,4-8,14-15H2,1-3H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 4088-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.012
BindingDB Entry DOI: 10.7270/Q23R0SXR |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50297796

(4-(cyclohexylmethyl)-6-(2-methoxypyrimidin-5-yl)-2...)Show SMILES COc1ncc(cn1)-c1ccc2nc(NCC3CCOCC3)c(=O)n(CC3CCCCC3)c2n1 Show InChI InChI=1S/C25H32N6O3/c1-33-25-27-14-19(15-28-25)20-7-8-21-23(30-20)31(16-18-5-3-2-4-6-18)24(32)22(29-21)26-13-17-9-11-34-12-10-17/h7-8,14-15,17-18H,2-6,9-13,16H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 4088-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.012
BindingDB Entry DOI: 10.7270/Q23R0SXR |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308553

((S)-3-(4-(2-hydroxypropyl)piperazin-1-yl)-1-(2-iso...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(C[C@H](C)O)CC2)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C25H34N6O4/c1-4-12-35-13-11-31-22-14-20(19-5-6-23(34-3)27-15-19)26-16-21(22)28-24(25(31)33)30-9-7-29(8-10-30)17-18(2)32/h5-6,14-16,18,32H,4,7-13,17H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300982
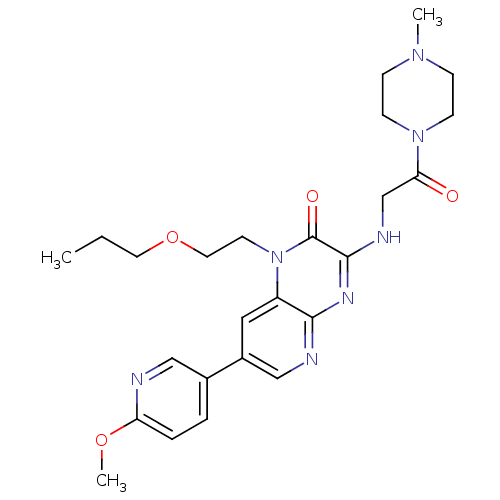
(7-(6-methoxypyridin-3-yl)-3-(2-(4-methylpiperazin-...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCC(=O)N2CCN(C)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H33N7O4/c1-4-12-36-13-11-32-20-14-19(18-5-6-21(35-3)26-15-18)16-27-23(20)29-24(25(32)34)28-17-22(33)31-9-7-30(2)8-10-31/h5-6,14-16H,4,7-13,17H2,1-3H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300952
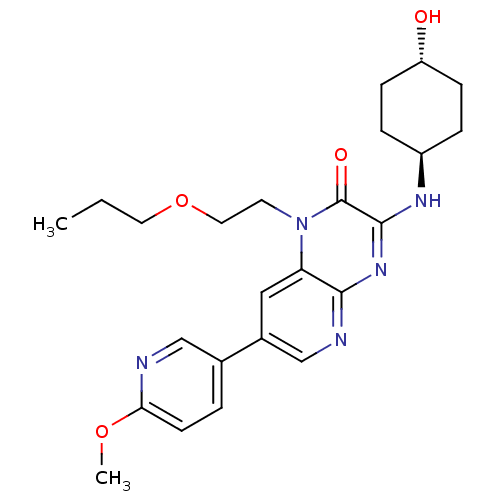
(3-(trans-4-hydroxycyclohexylamino)-7-(6-methoxypyr...)Show SMILES CCCOCCn1c2cc(cnc2nc(N[C@H]2CC[C@H](O)CC2)c1=O)-c1ccc(OC)nc1 |r,wU:16.16,wD:19.20,(3.98,.08,;2.65,-.69,;2.65,-2.23,;1.32,-3,;1.32,-4.54,;-.02,-5.31,;-.02,-6.85,;1.31,-7.63,;2.64,-6.86,;3.96,-7.62,;3.97,-9.17,;2.64,-9.93,;1.31,-9.17,;-.02,-9.93,;-1.36,-9.16,;-2.7,-9.93,;-4.03,-9.15,;-5.37,-9.93,;-6.7,-9.15,;-6.69,-7.6,;-8.02,-6.82,;-5.35,-6.84,;-4.03,-7.62,;-1.35,-7.63,;-2.68,-6.86,;5.29,-6.85,;6.63,-7.62,;7.96,-6.84,;7.95,-5.3,;9.28,-4.53,;10.62,-5.29,;6.61,-4.54,;5.28,-5.31,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-20-13-17(16-4-9-21(32-2)25-14-16)15-26-22(20)28-23(24(29)31)27-18-5-7-19(30)8-6-18/h4,9,13-15,18-19,30H,3,5-8,10-12H2,1-2H3,(H,26,27,28)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308562

((+/-)-3-(4-((2S,3R)-3-hydroxybutan-2-yl)piperazin-...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CC2)[C@@H](C)[C@@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C26H36N6O4/c1-5-13-36-14-12-32-23-15-21(20-6-7-24(35-4)28-16-20)27-17-22(23)29-25(26(32)34)31-10-8-30(9-11-31)18(2)19(3)33/h6-7,15-19,33H,5,8-14H2,1-4H3/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50296257
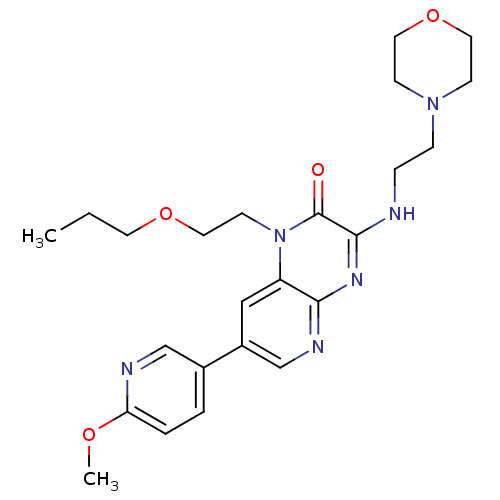
(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-20-15-19(18-4-5-21(32-2)26-16-18)17-27-22(20)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50296257

(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-20-15-19(18-4-5-21(32-2)26-16-18)17-27-22(20)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 4092-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.004
BindingDB Entry DOI: 10.7270/Q2ZG6S9Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300988

((S)-3-(2-hydroxypropylamino)-7-(6-methoxypyridin-3...)Show SMILES CCCOCCn1c2cc(cnc2nc(NC[C@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-17-10-16(15-5-6-18(29-3)22-12-15)13-24-19(17)25-20(21(26)28)23-11-14(2)27/h5-6,10,12-14,27H,4,7-9,11H2,1-3H3,(H,23,24,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300969
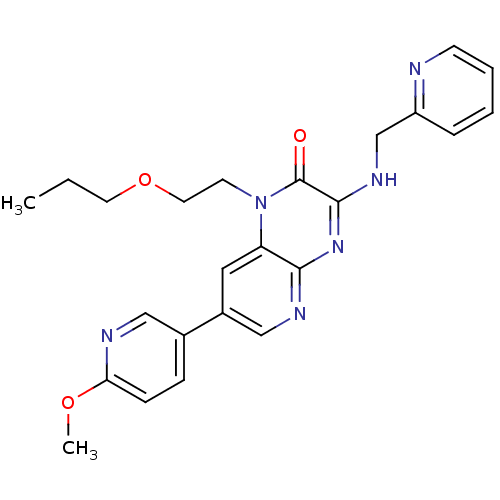
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCc2ccccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H26N6O3/c1-3-11-33-12-10-30-20-13-18(17-7-8-21(32-2)26-14-17)15-27-22(20)29-23(24(30)31)28-16-19-6-4-5-9-25-19/h4-9,13-15H,3,10-12,16H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300984

(3-(2-(4-ethylpiperazin-1-yl)-2-oxoethylamino)-7-(6...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCC(=O)N2CCN(CC)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C26H35N7O4/c1-4-13-37-14-12-33-21-15-20(19-6-7-22(36-3)27-16-19)17-28-24(21)30-25(26(33)35)29-18-23(34)32-10-8-31(5-2)9-11-32/h6-7,15-17H,4-5,8-14,18H2,1-3H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50297797
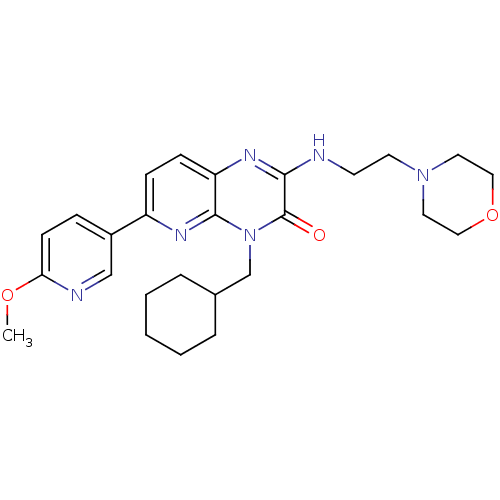
(4-(cyclohexylmethyl)-6-(6-methoxypyridin-3-yl)-2-(...)Show SMILES COc1ccc(cn1)-c1ccc2nc(NCCN3CCOCC3)c(=O)n(CC3CCCCC3)c2n1 Show InChI InChI=1S/C26H34N6O3/c1-34-23-10-7-20(17-28-23)21-8-9-22-25(30-21)32(18-19-5-3-2-4-6-19)26(33)24(29-22)27-11-12-31-13-15-35-16-14-31/h7-10,17,19H,2-6,11-16,18H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 4088-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.012
BindingDB Entry DOI: 10.7270/Q23R0SXR |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300975
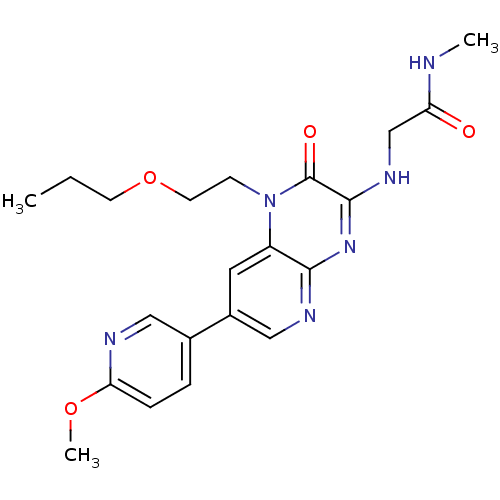
(2-(7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyeth...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCC(=O)NC)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H26N6O4/c1-4-8-31-9-7-27-16-10-15(14-5-6-18(30-3)23-11-14)12-24-19(16)26-20(21(27)29)25-13-17(28)22-2/h5-6,10-12H,4,7-9,13H2,1-3H3,(H,22,28)(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300990
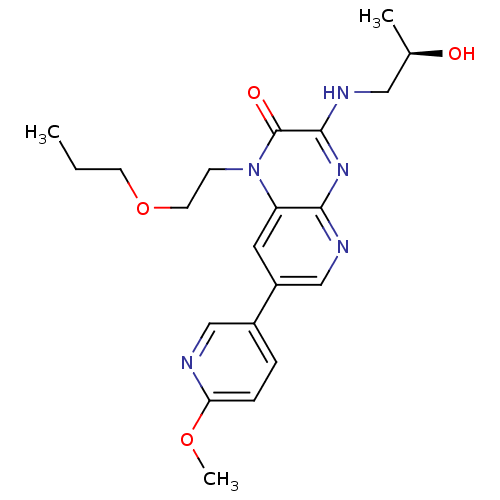
((R)-3-(2-hydroxypropylamino)-7-(6-methoxypyridin-3...)Show SMILES CCCOCCn1c2cc(cnc2nc(NC[C@@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-17-10-16(15-5-6-18(29-3)22-12-15)13-24-19(17)25-20(21(26)28)23-11-14(2)27/h5-6,10,12-14,27H,4,7-9,11H2,1-3H3,(H,23,24,25)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300949
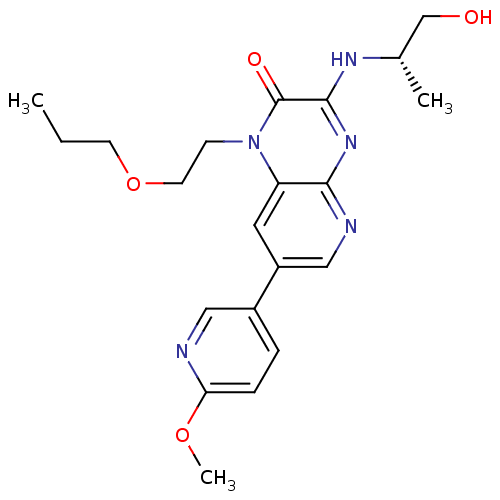
((S)-3-(1-hydroxypropan-2-ylamino)-7-(6-methoxypyri...)Show SMILES CCCOCCn1c2cc(cnc2nc(N[C@@H](C)CO)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-17-10-16(15-5-6-18(29-3)22-11-15)12-23-19(17)25-20(21(26)28)24-14(2)13-27/h5-6,10-12,14,27H,4,7-9,13H2,1-3H3,(H,23,24,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300974
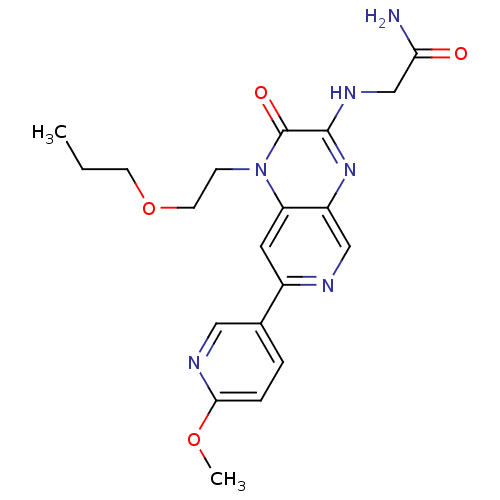
(2-(7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyeth...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(N)=O)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C20H24N6O4/c1-3-7-30-8-6-26-16-9-14(13-4-5-18(29-2)23-10-13)22-11-15(16)25-19(20(26)28)24-12-17(21)27/h4-5,9-11H,3,6-8,12H2,1-2H3,(H2,21,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300968
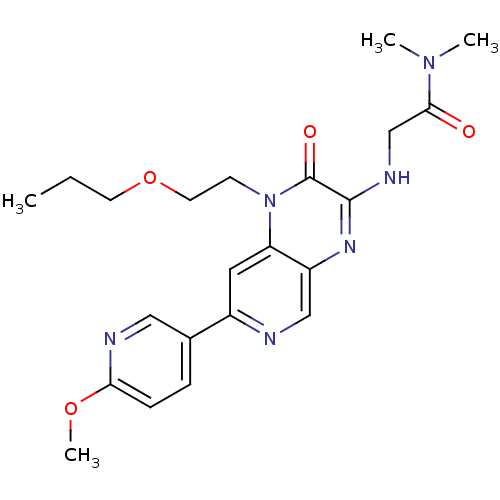
(2-(7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyeth...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N(C)C)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C22H28N6O4/c1-5-9-32-10-8-28-18-11-16(15-6-7-19(31-4)24-12-15)23-13-17(18)26-21(22(28)30)25-14-20(29)27(2)3/h6-7,11-13H,5,8-10,14H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300986
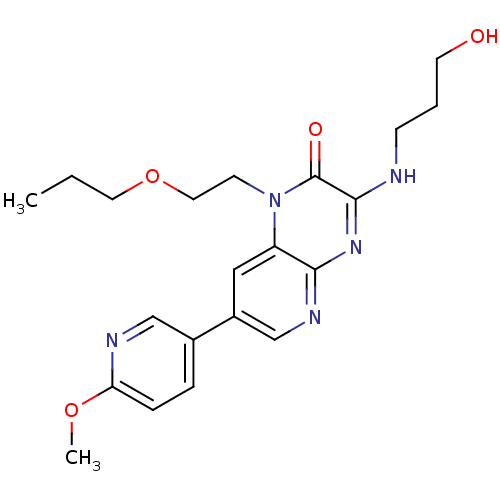
(3-(3-hydroxypropylamino)-7-(6-methoxypyridin-3-yl)...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCCCO)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H27N5O4/c1-3-10-30-11-8-26-17-12-16(15-5-6-18(29-2)23-13-15)14-24-19(17)25-20(21(26)28)22-7-4-9-27/h5-6,12-14,27H,3-4,7-11H2,1-2H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308555

(3-(4-(2-hydroxyethyl)-1,4-diazepan-1-yl)-1-(2-isop...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCCN(CCO)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H34N6O4/c1-3-14-35-15-12-31-22-16-20(19-5-6-23(34-2)27-17-19)26-18-21(22)28-24(25(31)33)30-8-4-7-29(9-10-30)11-13-32/h5-6,16-18,32H,3-4,7-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data