

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090035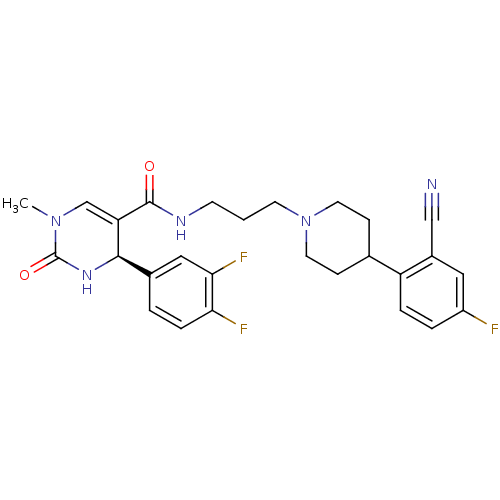 (4-(3,4-Difluoro-phenyl)-1-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090023 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090010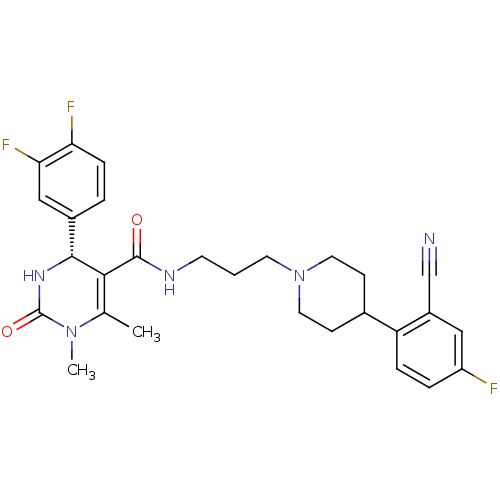 (4-(3,4-Difluoro-phenyl)-1,6-dimethyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090032 ((R)-4-(3,4-Difluoro-phenyl)-1-methyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM85335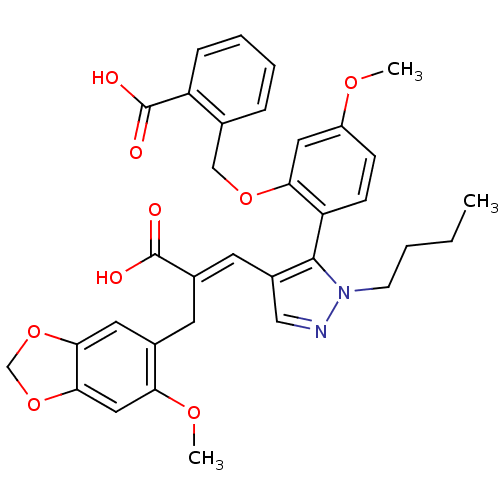 (SB 234551 | SB-234551) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 286: 650-6 (1998) BindingDB Entry DOI: 10.7270/Q2V40SSK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090018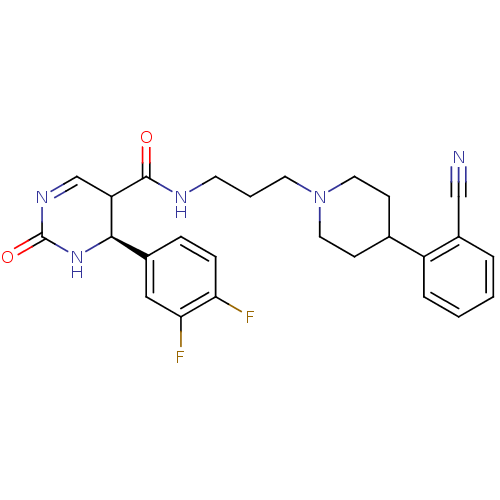 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090042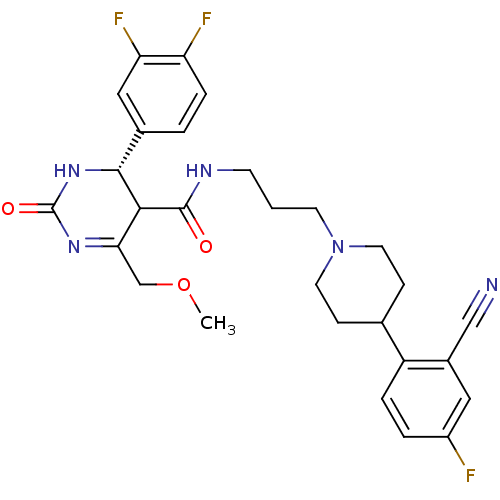 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090019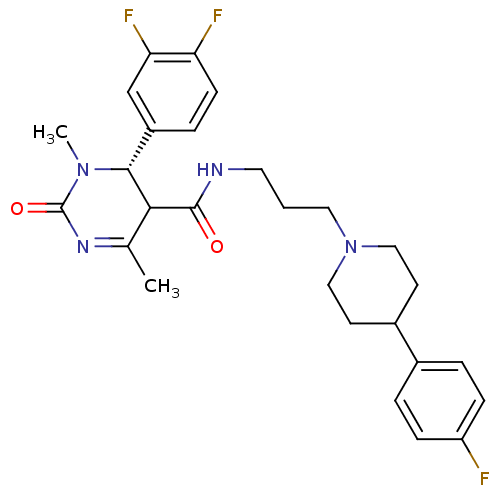 (4-(3,4-Difluoro-phenyl)-3,6-dimethyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090036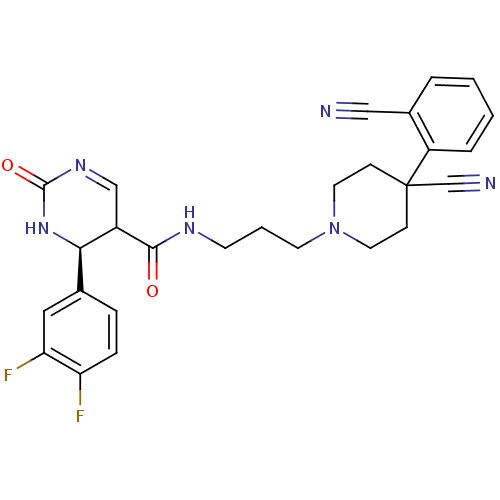 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090031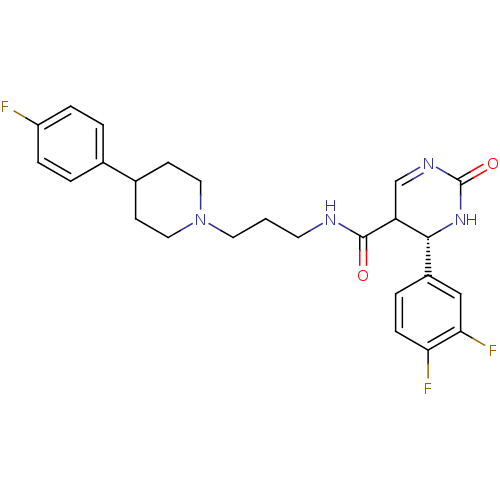 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090025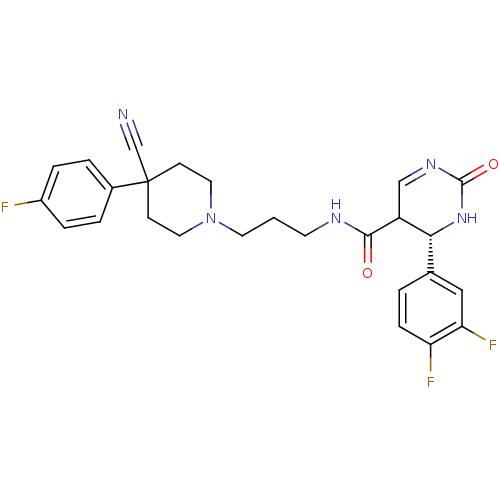 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090015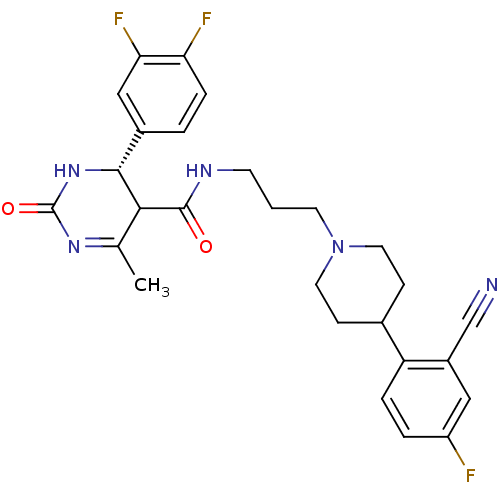 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090016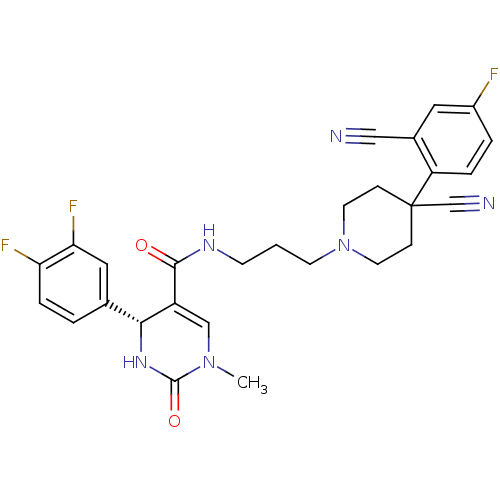 (4-(3,4-Difluoro-phenyl)-1-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090027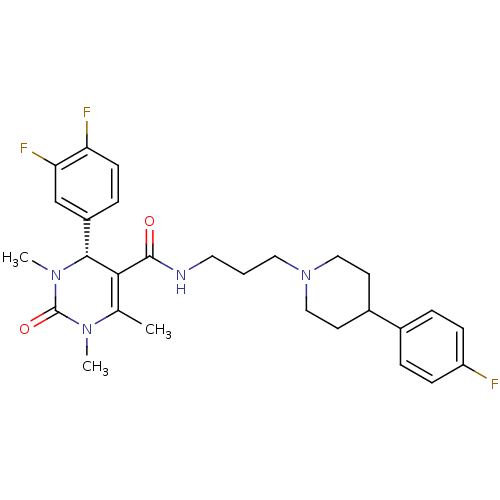 ((R)-4-(3,4-Difluoro-phenyl)-1,3,6-trimethyl-2-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090017 ((R)-4-(3,4-Difluoro-phenyl)-1,6-dimethyl-2-oxo-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156774 (CHEMBL3792888) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442142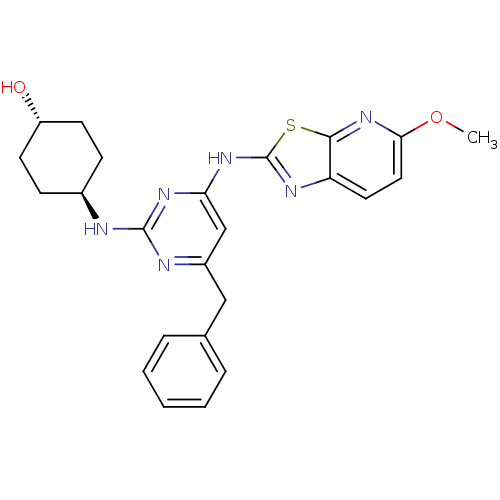 (CHEMBL2441275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50306153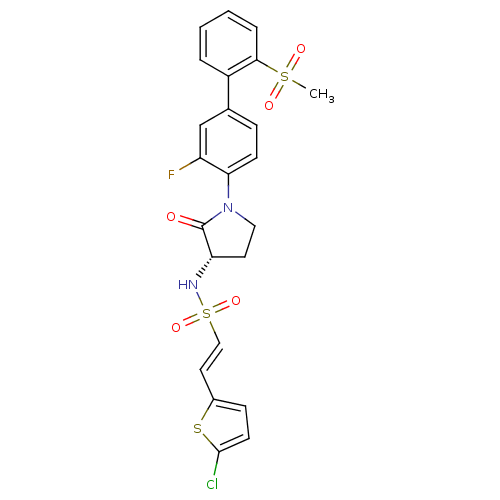 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(3-fluoro-2'-(m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human factor 10a by fluorescence assay | Bioorg Med Chem Lett 20: 618-22 (2010) Article DOI: 10.1016/j.bmcl.2009.11.077 BindingDB Entry DOI: 10.7270/Q24M94NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50228676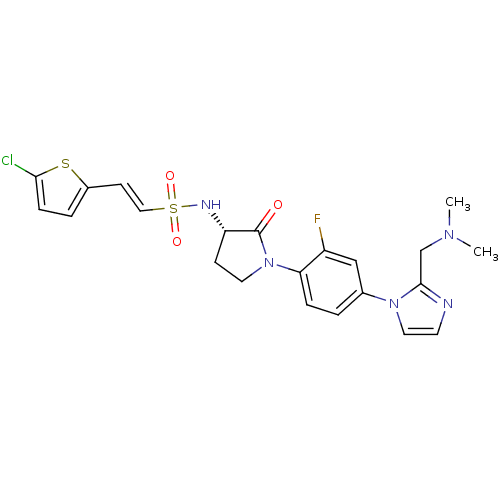 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(4-(2-((dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human factor 10a by fluorescence assay | Bioorg Med Chem Lett 20: 618-22 (2010) Article DOI: 10.1016/j.bmcl.2009.11.077 BindingDB Entry DOI: 10.7270/Q24M94NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090043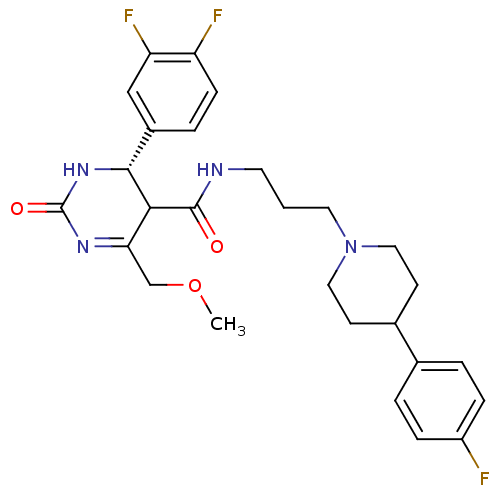 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090014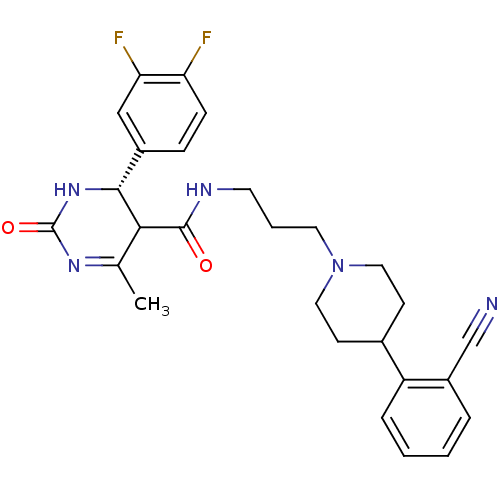 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442141 (CHEMBL2441276) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442143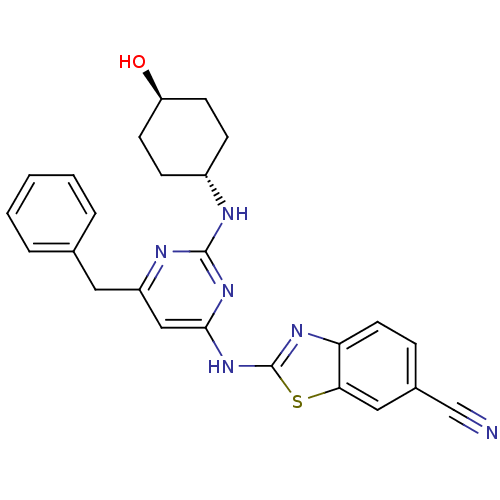 (CHEMBL2441274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494235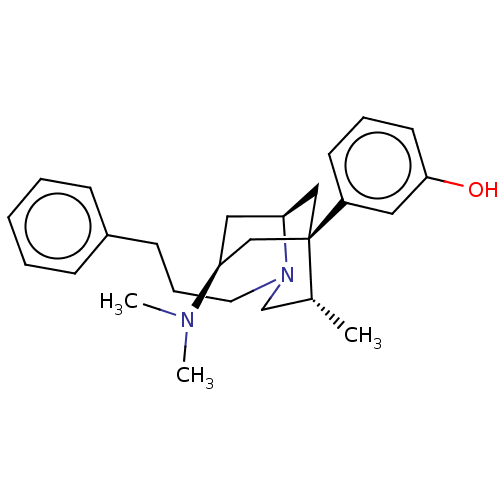 (CHEMBL3216613) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 56: 8826-33 (2013) Article DOI: 10.1021/jm401250s BindingDB Entry DOI: 10.7270/Q2V127SP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090012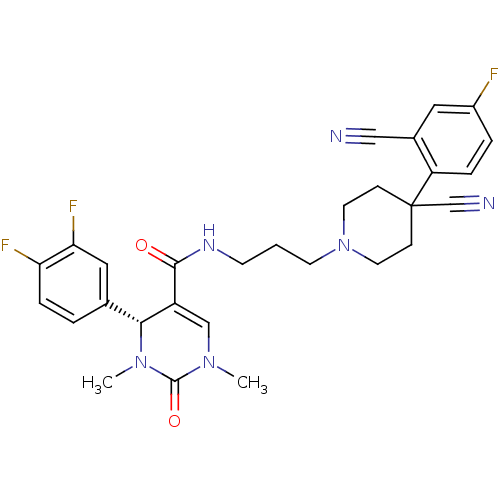 (4-(3,4-Difluoro-phenyl)-1,3-dimethyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50338686 ((R/S)-3-chloro-N-((3S)-1-(1-(methylamino)-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1582-7 (2011) Article DOI: 10.1016/j.bmcl.2011.01.131 BindingDB Entry DOI: 10.7270/Q28052WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442145 (CHEMBL2441271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494231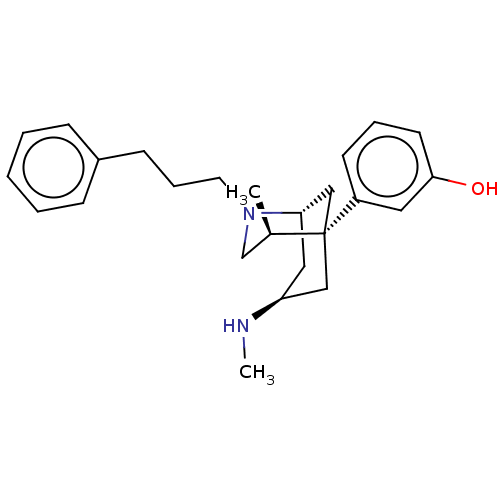 (CHEMBL3217272) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.371 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 56: 8826-33 (2013) Article DOI: 10.1021/jm401250s BindingDB Entry DOI: 10.7270/Q2V127SP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090020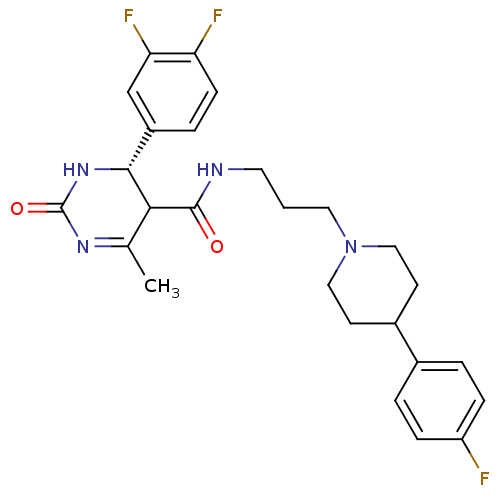 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090029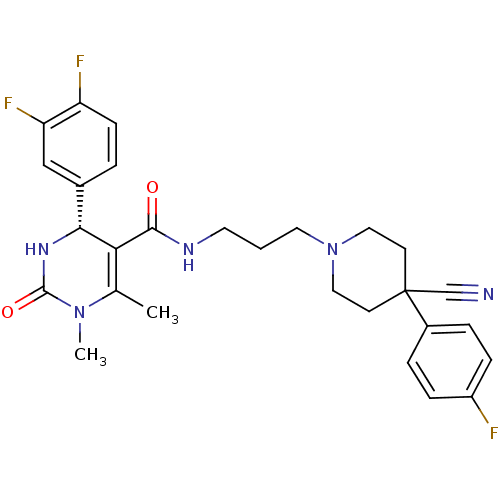 (4-(3,4-Difluoro-phenyl)-1,6-dimethyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442146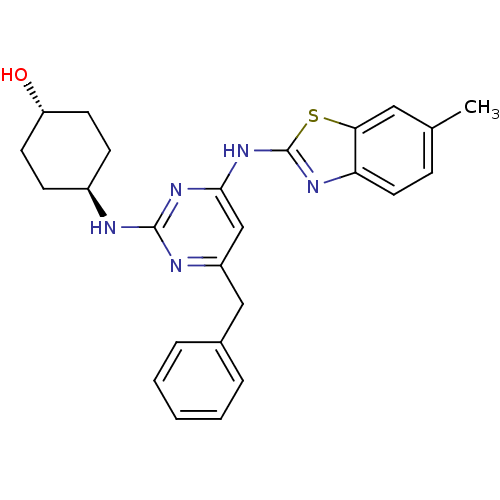 (CHEMBL2441270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156788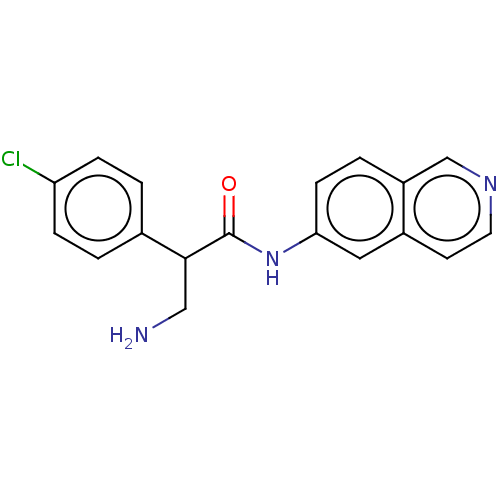 (CHEMBL3794104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156799 (CHEMBL3793004) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156784 (CHEMBL3792673) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50041617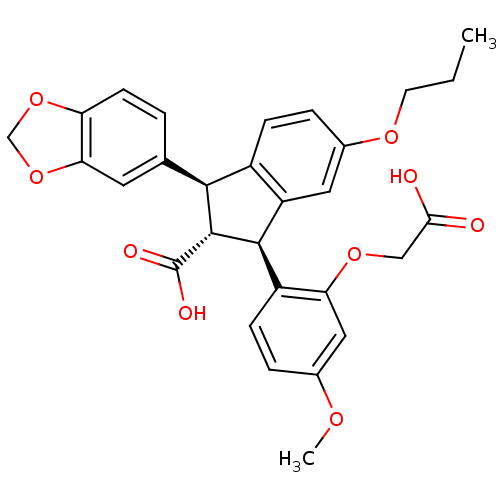 ((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 286: 650-6 (1998) BindingDB Entry DOI: 10.7270/Q2V40SSK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090037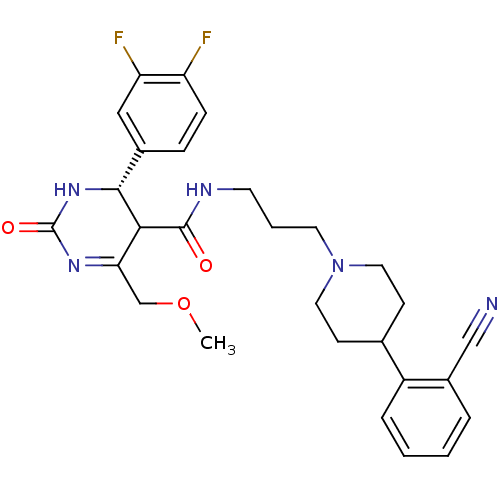 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50041617 ((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to cloned human ET-A receptor | J Med Chem 37: 1553-7 (1994) BindingDB Entry DOI: 10.7270/Q2154G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494228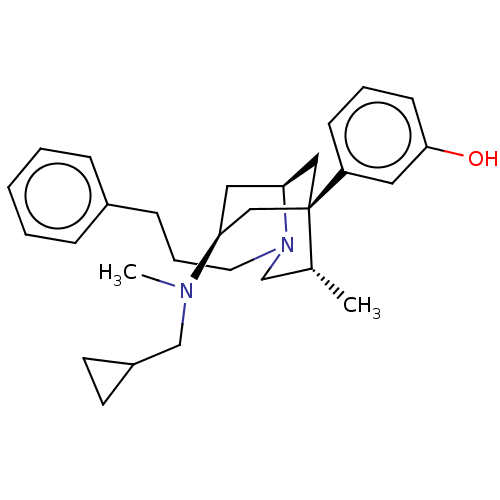 (CHEMBL3217271) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 56: 8826-33 (2013) Article DOI: 10.1021/jm401250s BindingDB Entry DOI: 10.7270/Q2V127SP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090013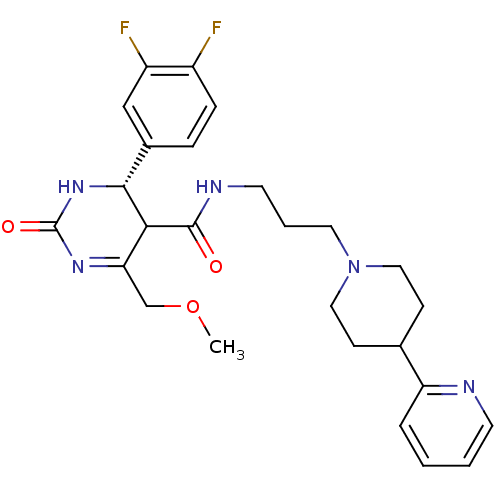 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50228078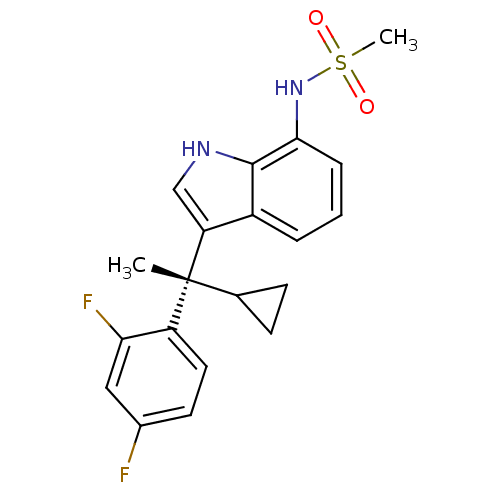 ((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.494 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human mineralocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50442149 (CHEMBL2441267) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Aurora-B (unknown origin) using 5FAM-PKAtide as substrate after 120 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50326709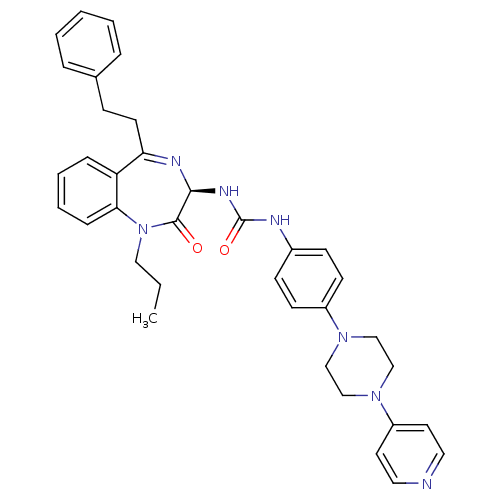 ((R)-1-(2-oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-b...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human bradykinin B1 receptor | J Med Chem 46: 1803-6 (2003) Article DOI: 10.1021/jm034020y BindingDB Entry DOI: 10.7270/Q29P32C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090028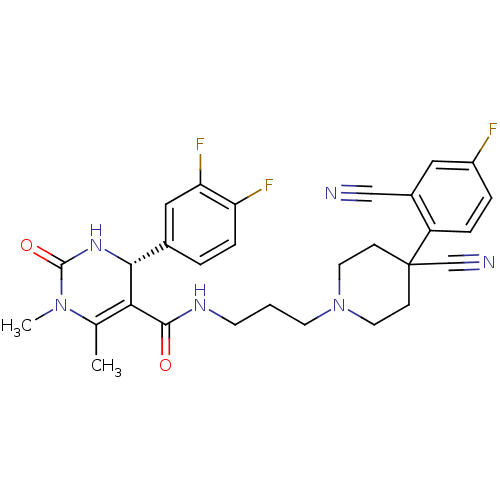 (4-(3,4-Difluoro-phenyl)-1,6-dimethyl-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339718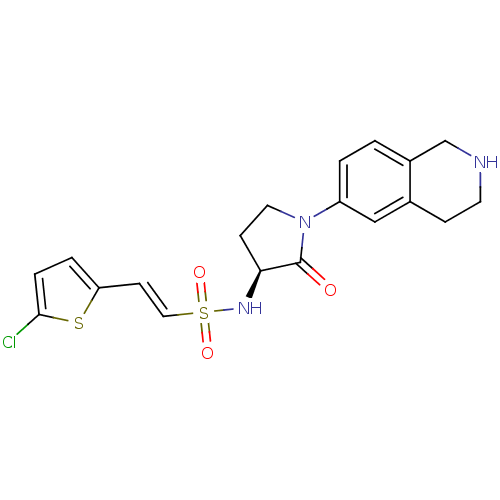 ((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156782 (CHEMBL3792663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339708 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(5-fluoro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442139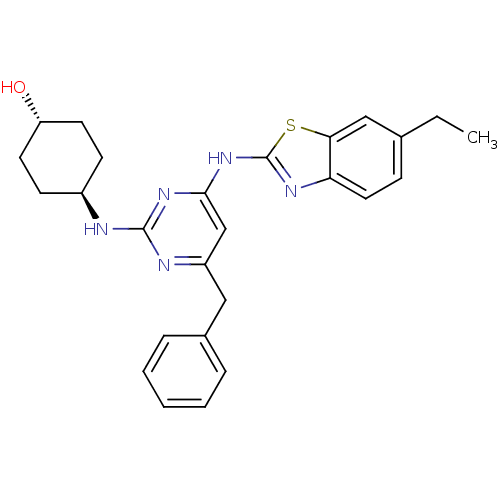 (CHEMBL2441273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156683 (CHEMBL3793788) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156732 (CHEMBL3793497) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090041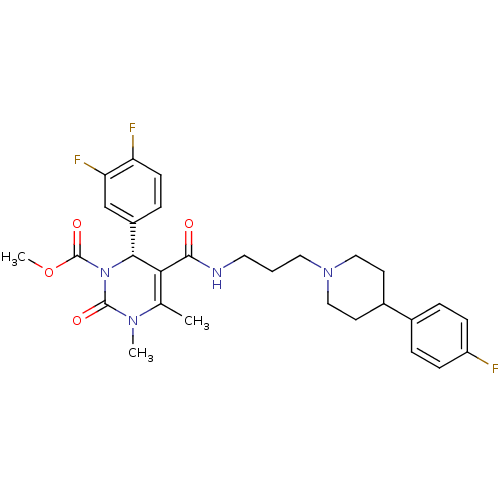 (6-(3,4-Difluoro-phenyl)-5-{3-[4-(4-fluoro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3077 total ) | Next | Last >> |