

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora A kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora A (1 to 403 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora B kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora C kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora C (1 to 309 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50280297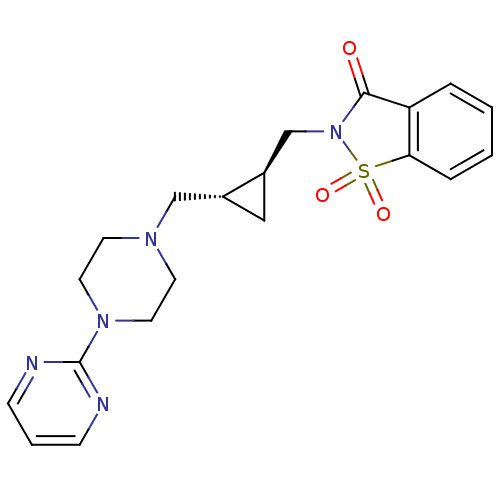 (1,1-Dioxo-2-[(1S,2S)-2-(4-pyrimidin-2-yl-piperazin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity to 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as a radioligand | Bioorg Med Chem Lett 2: 1703-1706 (1992) Article DOI: 10.1016/S0960-894X(00)80460-6 BindingDB Entry DOI: 10.7270/Q25M65N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50005127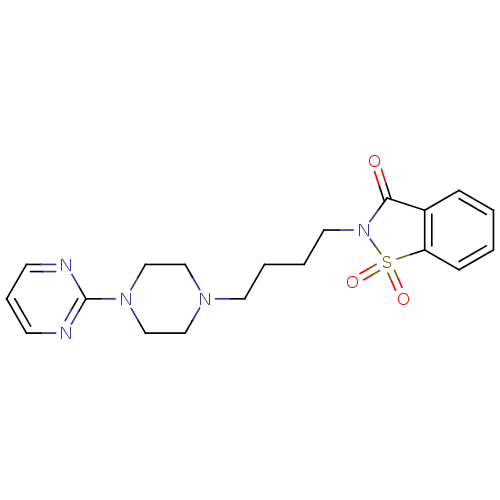 (1,1-Dioxo-2-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity to 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as a radioligand | Bioorg Med Chem Lett 2: 1703-1706 (1992) Article DOI: 10.1016/S0960-894X(00)80460-6 BindingDB Entry DOI: 10.7270/Q25M65N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12985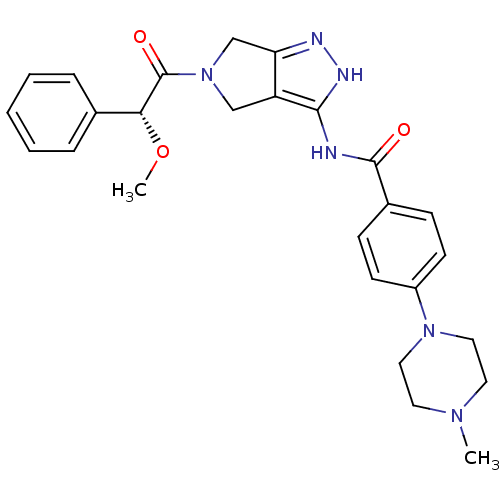 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora A kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora B (62 to 344 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50280296 (1,1-Dioxo-2-[(1R,2S)-2-(4-pyrimidin-2-yl-piperazin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity to 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as a radioligand | Bioorg Med Chem Lett 2: 1703-1706 (1992) Article DOI: 10.1016/S0960-894X(00)80460-6 BindingDB Entry DOI: 10.7270/Q25M65N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM12985 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora C kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12985 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora B kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D820Y single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50401401 (CHEMBL2206459) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/reassortant/NIBRG-14(Viet Nam/1194/2004 x Puerto Rico/8/1934)(H5N1)) neuraminidase in virus-infected allantoic flu... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623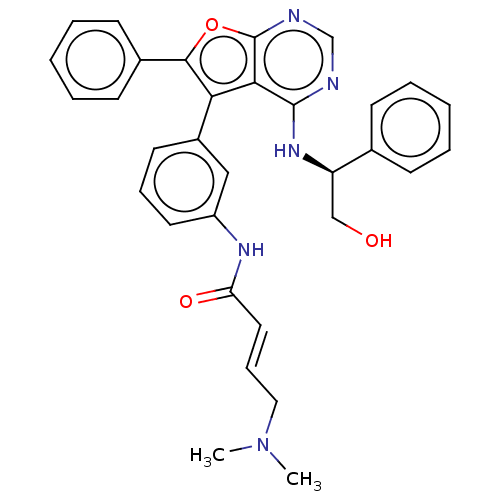 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50175305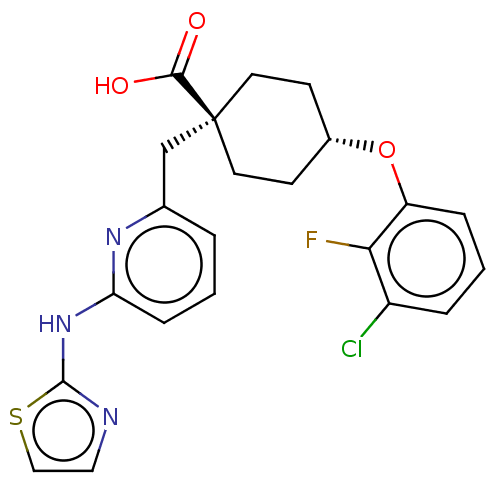 (CHEMBL3600873) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Aurora A expressed in Escherichia coli using RRR(GLRRASLG)4R-NH2 as substrate after 40 mins in presence of... | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50507492 (Loxo-195 | Selitrectinib | US10966985, Compound 33...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247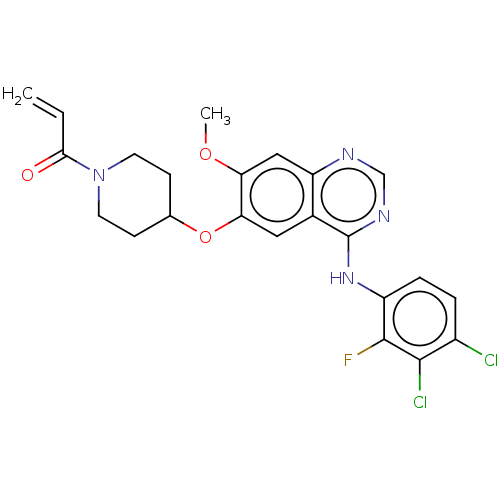 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50401401 (CHEMBL2206459) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of wild type Influenza A virus A/WSN/1933(H1N1) neuraminidase in virus-infected allantoic fluid using 2'-(4-methylumbelliferyl)-alpha-D-N-... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 phosphorylation at 0.1 to 1000 nM after 1 hr by... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 D835Y mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 D835Y phosphorylation at 0.1 to 10... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50206389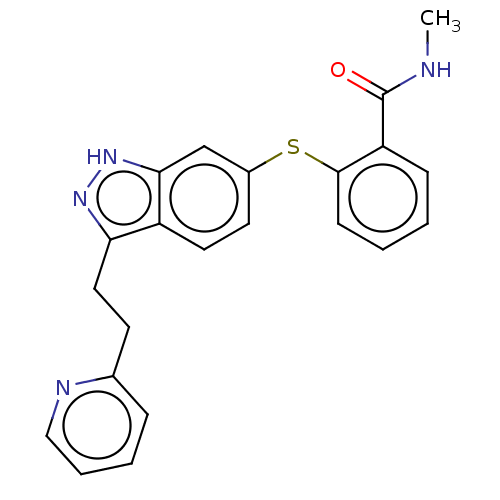 (CHEMBL3939307) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR3 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 ITD mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 ITD phosphorylation at 0.1 to 1000 n... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50206389 (CHEMBL3939307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR1 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM374727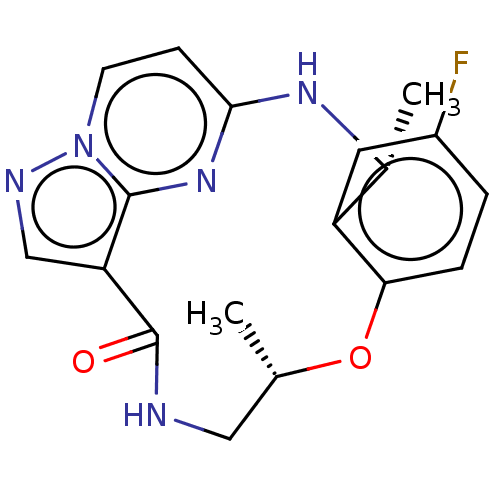 ((7S,13R)-11-fluoro-7,13-dimethyl-6,7,13,14- tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 Y823D single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618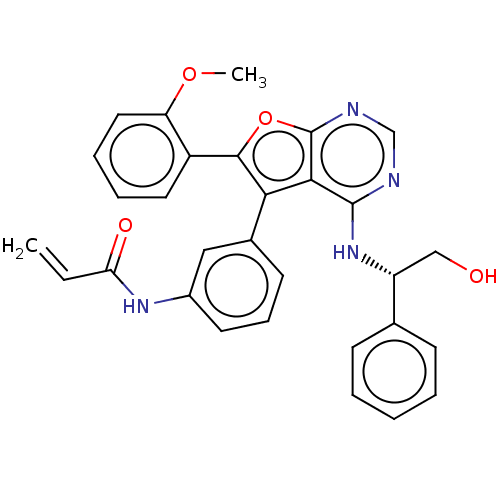 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 11/17 V560G/N822K double mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot k... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50206389 (CHEMBL3939307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50579500 (CHEMBL4864729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKC (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM50401401 (CHEMBL2206459) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Udorn/1972(H3N2) neuraminidase in virus-infected allantoic fluid using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylne... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50241089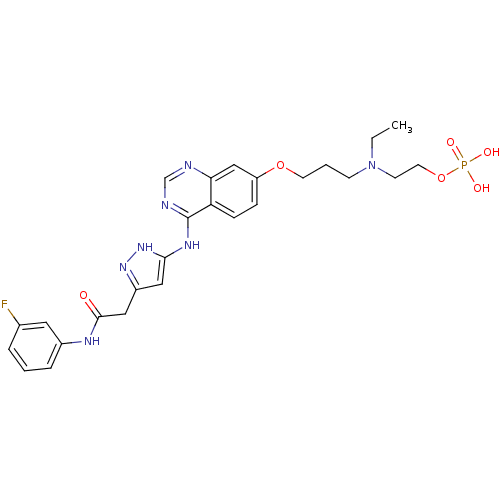 (2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant GST-tagged N-terminal truncated human Aurora A (123 to 401 residues) expressed in Sf9 insect cell using tetra-LRRASLG pepti... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01806 BindingDB Entry DOI: 10.7270/Q2Q2444S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50401399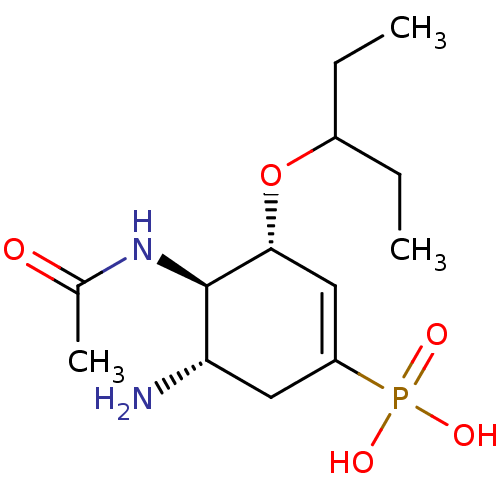 (CHEMBL2206456) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/reassortant/NIBRG-14(Viet Nam/1194/2004 x Puerto Rico/8/1934)(H5N1)) neuraminidase in virus-infected allantoic flu... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50579499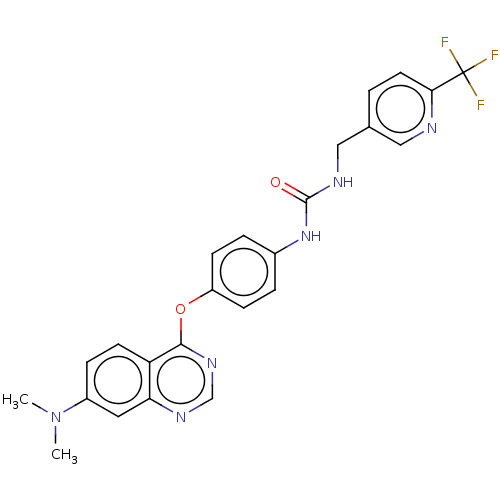 (CHEMBL4847875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human partial length CSF1R (I564 to S939 residues) expressed in bacterial expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01006 BindingDB Entry DOI: 10.7270/Q2W3815D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D816H single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/reassortant/NIBRG-14(Viet Nam/1194/2004 x Puerto Rico/8/1934)(H5N1)) neuraminidase in virus-infected allantoic flu... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D820E single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1420 total ) | Next | Last >> |