

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 protease (Human immunodeficiency virus) | BDBM586099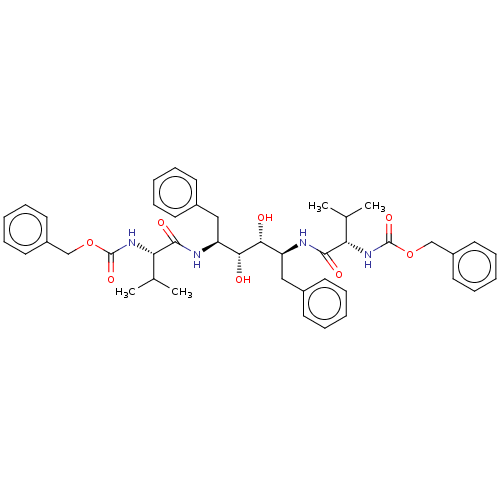 (BDBM50064200 | TL-3) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460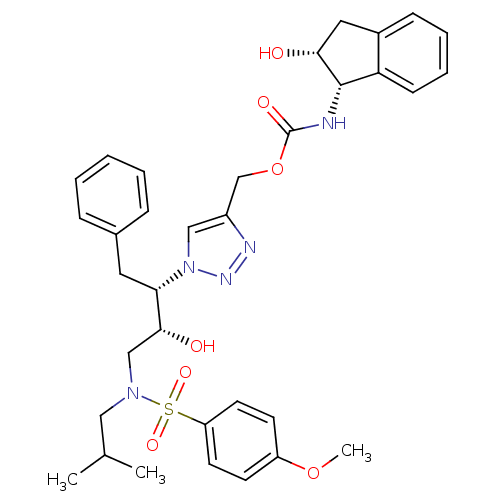 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 1.70 | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM586090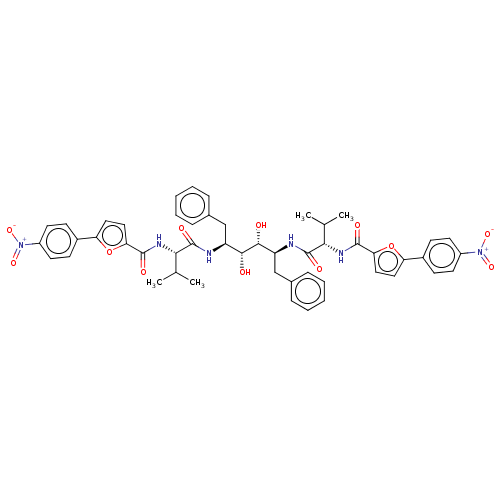 (P3-P3' Entry 8) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 51: 6263-70 (2008) Article DOI: 10.1021/jm800149m BindingDB Entry DOI: 10.7270/Q2MW2KZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84461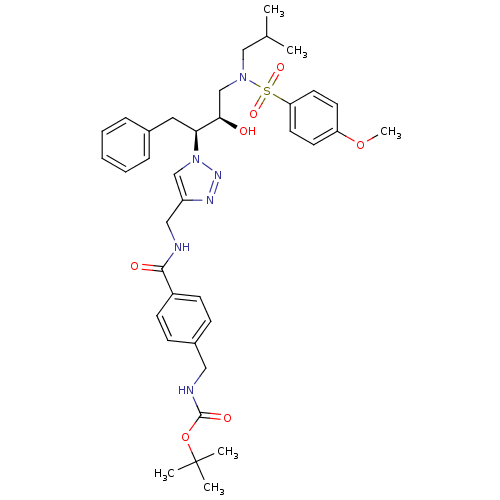 (HIV-1 PR Inhibitor, compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 4 | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200943 (CHEMBL269769 | cyclopentyl (2S,3S)-3-[4-([4-(5-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84461 (HIV-1 PR Inhibitor, compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 9.70 | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 10 | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200945 (CHEMBL262751 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84461 (HIV-1 PR Inhibitor, compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 13 | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200956 (CHEMBL262496 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200944 (CHEMBL407802 | cyclopentyl (2S,3S)-3-(4-((4-(5-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 22 | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200949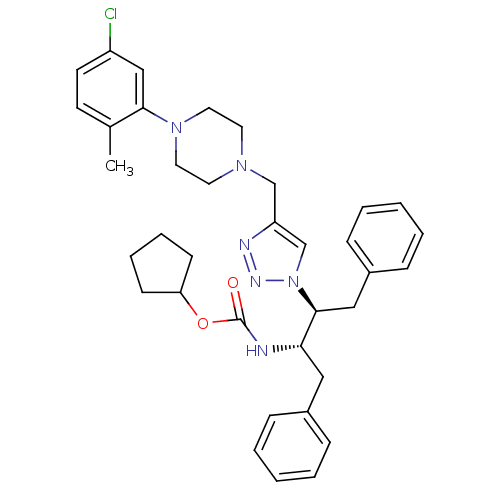 (CHEMBL262738 | cyclopentyl (2S,3S)-3-[4-([4-(5-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200954 (CHEMBL386849 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-D...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 27 | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200951 (CHEMBL267645 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200953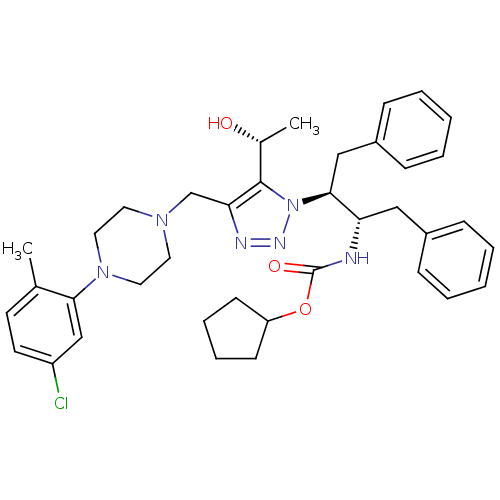 (CHEMBL405393 | cyclopentyl (2S,3S)-3-(4-((4-(5-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84461 (HIV-1 PR Inhibitor, compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 30 | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200948 (CHEMBL386847 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200958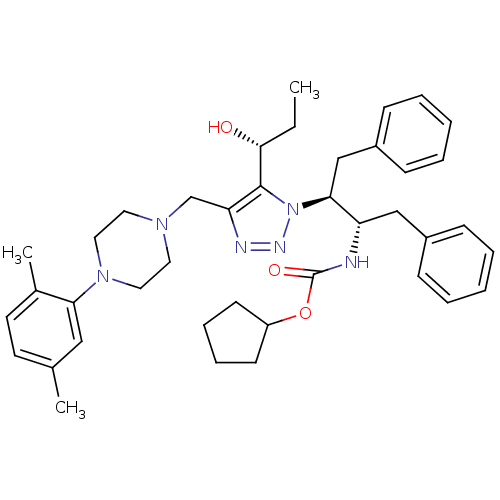 (CHEMBL428624 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438750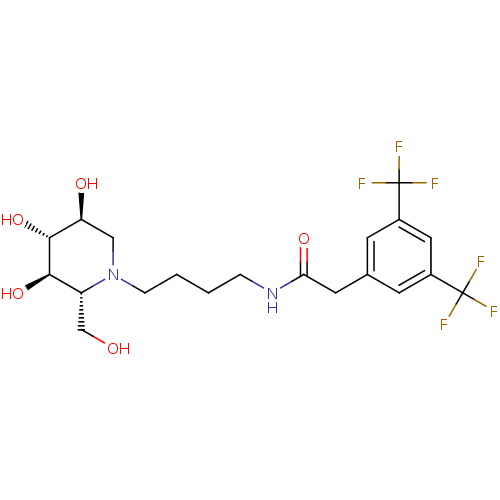 (CHEMBL2414884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200950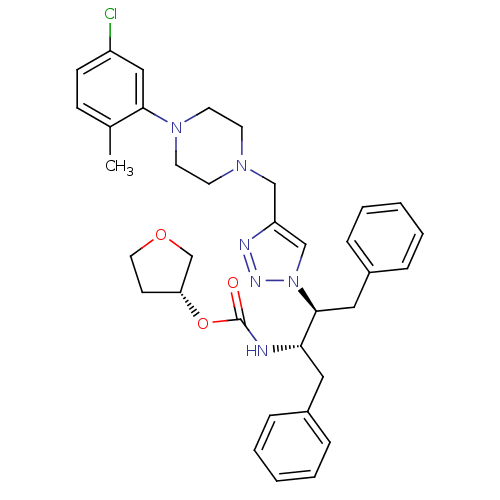 ((R)-tetrahydrofuran-3-yl (2S,3S)-3-(4-((4-(5-chlor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200955 (CHEMBL218359 | cyclopentyl (2S,3S)-5-methyl-1-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200946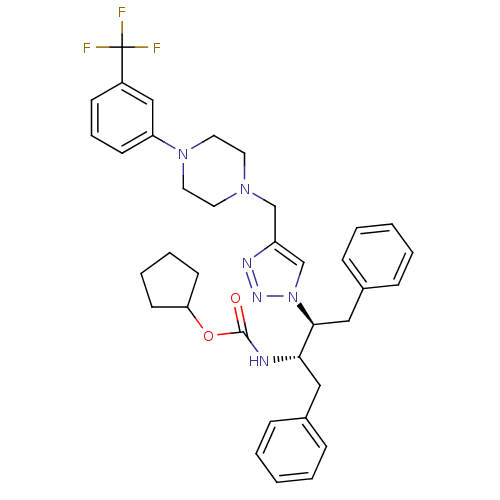 (CHEMBL385816 | cyclopentyl (2S,3S)-1,4-diphenyl-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438752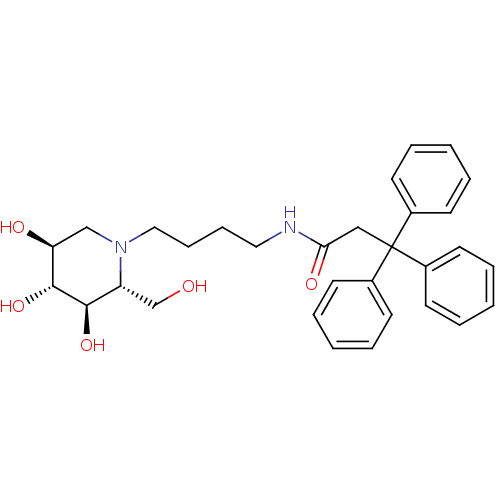 (CHEMBL2414882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438754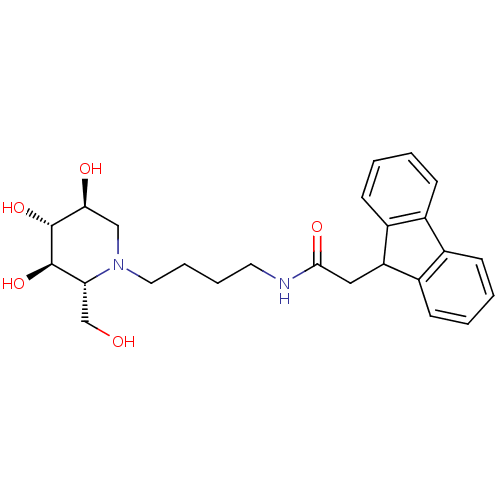 (CHEMBL2414880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438753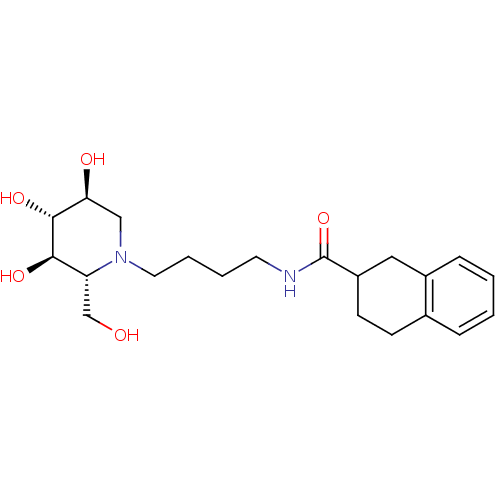 (CHEMBL2414881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438748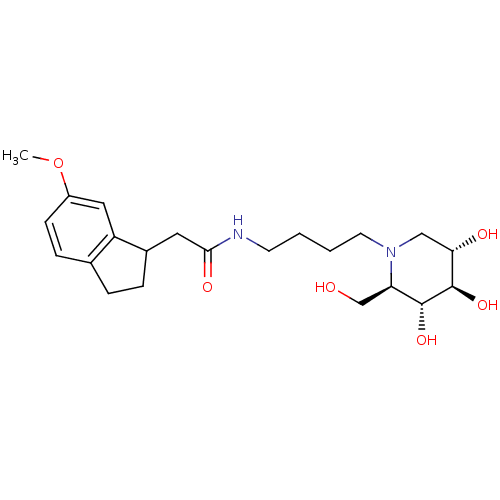 (CHEMBL2414879) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Non-competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358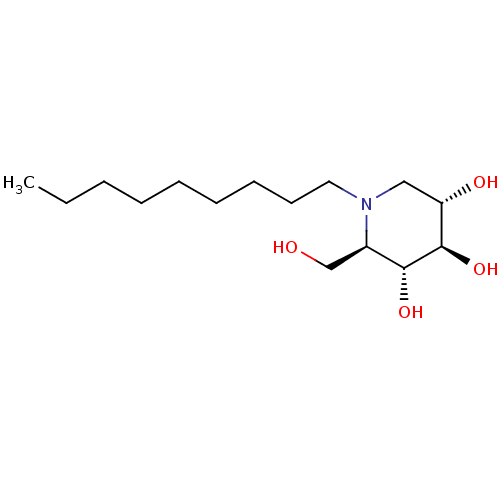 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200952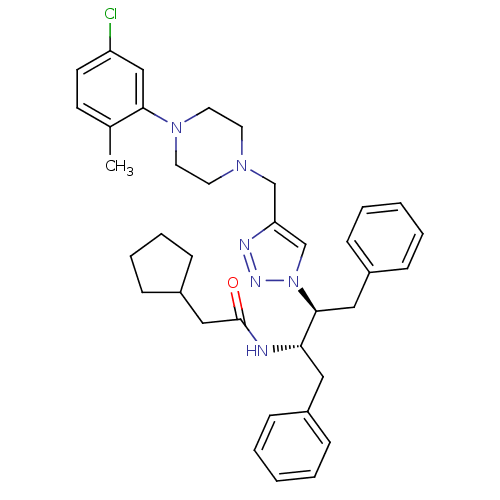 (CHEMBL407996 | N-((2S,3S)-3-(4-((4-(5-chloro-2-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200947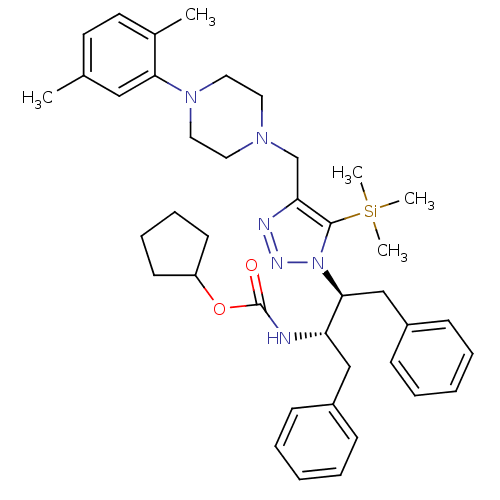 (CHEMBL405122 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438751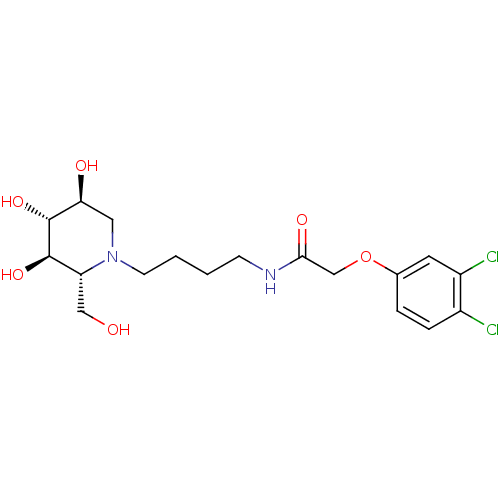 (CHEMBL2414883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438749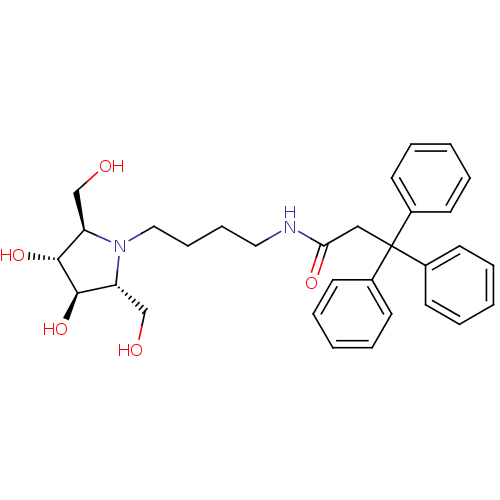 (CHEMBL2414888) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200957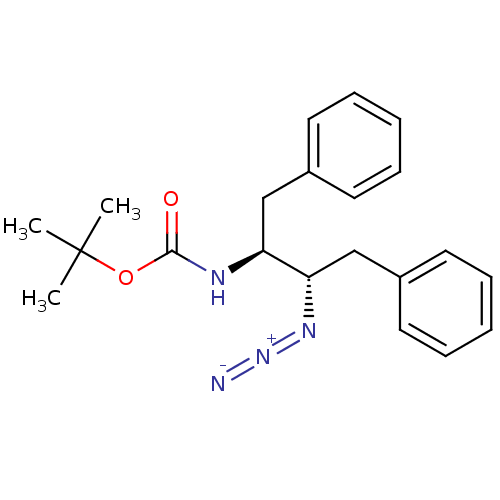 (CHEMBL216912 | [(1S,2S)-2-azido-1,2-dibenzylethyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50096449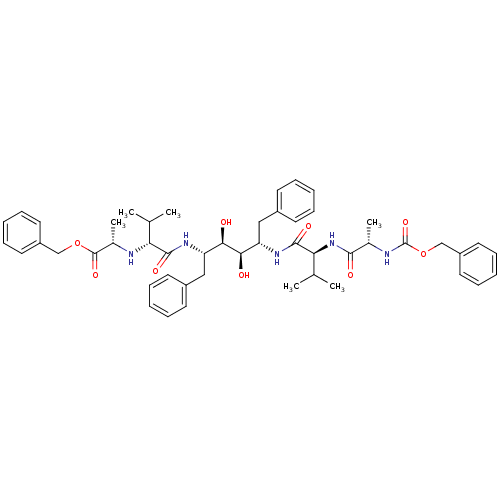 ((S)-2-[(R)-1-((1S,2R,3R,4S)-1-Benzyl-2,3-dihydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against TL3-resistant HIV(V82A) mutant | Bioorg Med Chem Lett 11: 219-22 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 51: 6263-70 (2008) Article DOI: 10.1021/jm800149m BindingDB Entry DOI: 10.7270/Q2MW2KZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM586090 (P3-P3' Entry 8) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50096447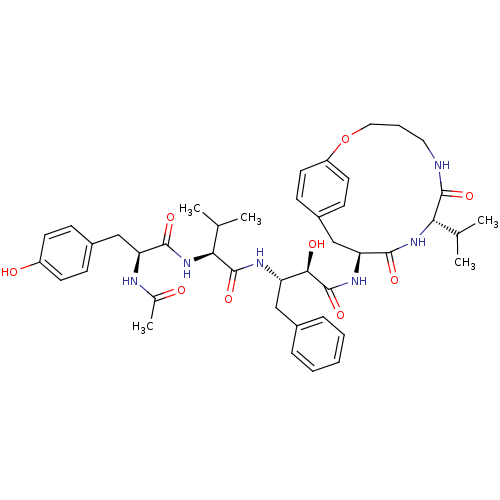 ((2R,3S)-3-{(S)-2-[(S)-2-Acetylamino-3-(4-hydroxy-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against drug-resistant HIV(G48V) mutant protease | Bioorg Med Chem Lett 11: 219-22 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50096451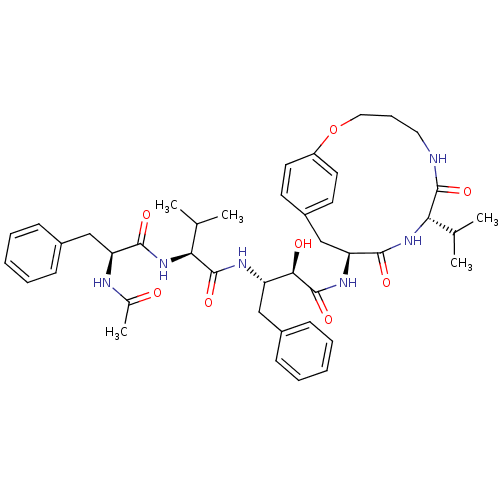 ((2R,3S)-3-[(S)-2-((S)-2-Acetylamino-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against TL3-resistant HIV(L63P) mutant protease | Bioorg Med Chem Lett 11: 219-22 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50096445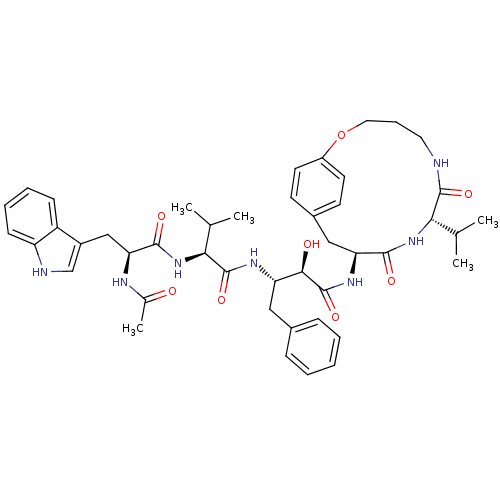 ((2R,3S)-3-{(S)-2-[(S)-2-Acetylamino-3-(1H-indol-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against TL3-resistant HIV(V771) mutant | Bioorg Med Chem Lett 11: 219-22 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM586084 (P3-P3' Entry 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM586088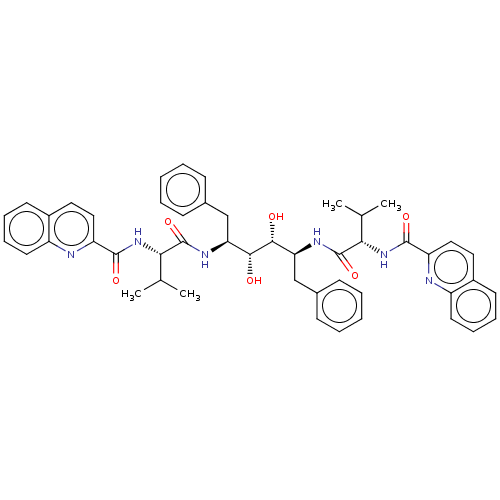 (P3-P3' Entry 6) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM586089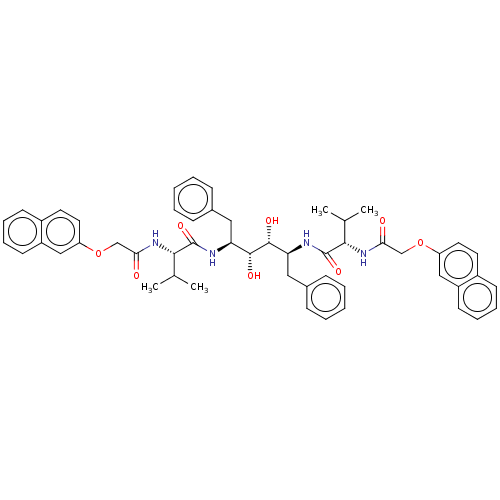 (P3-P3' Entry 7) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM586094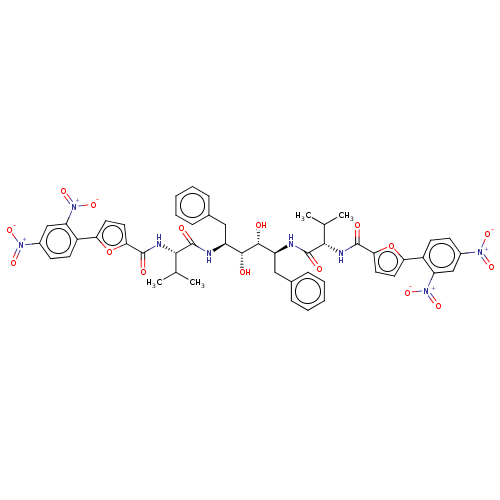 (P3-P3' Entry 12) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM586095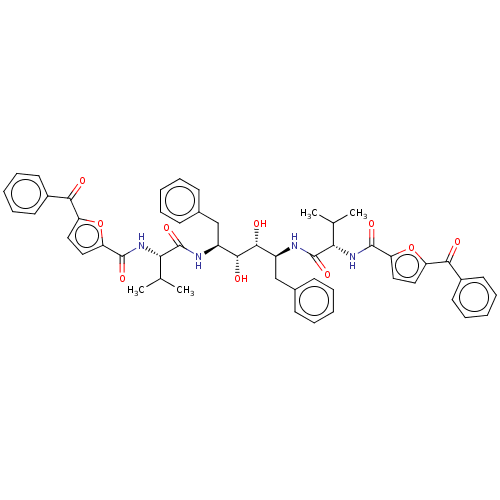 (P3-P3' Entry 13) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM586098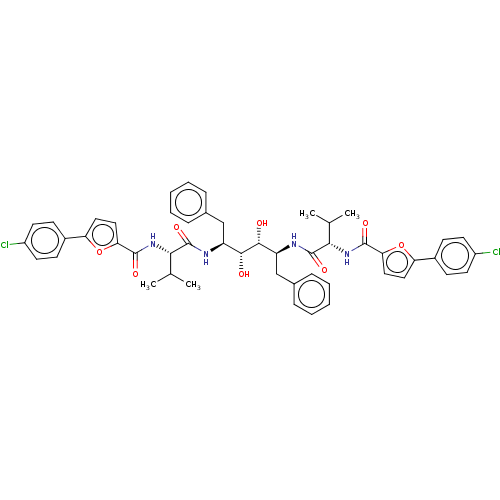 (P3-P3' Entry 16) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute | Assay Description Inhibition of HIV-protease activity for selected acids at P3-P3' positions. | Chem Biol 9: 891-6 (2002) Article DOI: 10.1016/S1074-5521(02)00184-9 BindingDB Entry DOI: 10.7270/Q2GM85QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50096445 ((2R,3S)-3-{(S)-2-[(S)-2-Acetylamino-3-(1H-indol-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against TL3-resistant HIV(V82A) mutant | Bioorg Med Chem Lett 11: 219-22 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50096447 ((2R,3S)-3-{(S)-2-[(S)-2-Acetylamino-3-(4-hydroxy-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against drug-resistant HIV(G48V) mutant | Bioorg Med Chem Lett 11: 219-22 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50096451 ((2R,3S)-3-[(S)-2-((S)-2-Acetylamino-3-phenyl-propi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against TL3-resistant HIV(V82A) mutant | Bioorg Med Chem Lett 11: 219-22 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 133 total ) | Next | Last >> |