

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165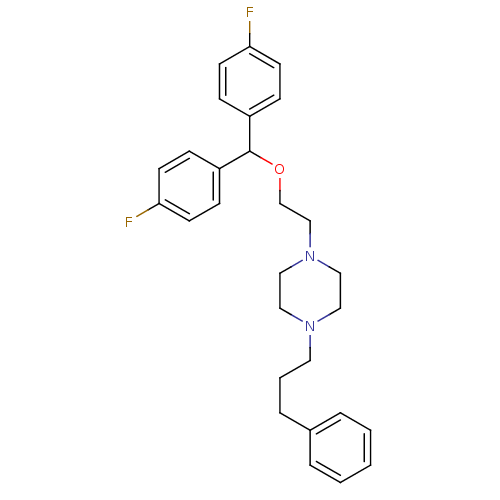 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine transporter in rat striatal membranes by [3H]GBR-12395 displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048558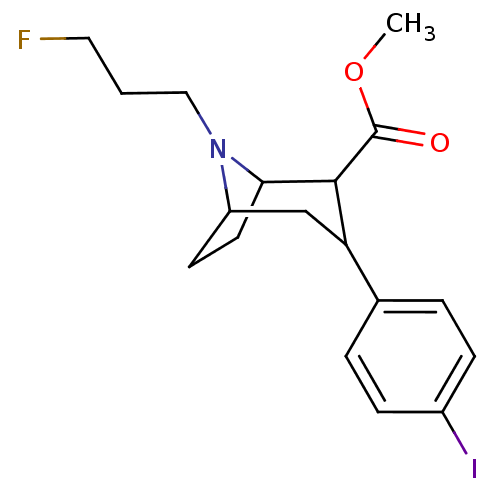 (8-(3-Fluoro-propyl)-3-(4-iodo-phenyl)-8-aza-bicycl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048561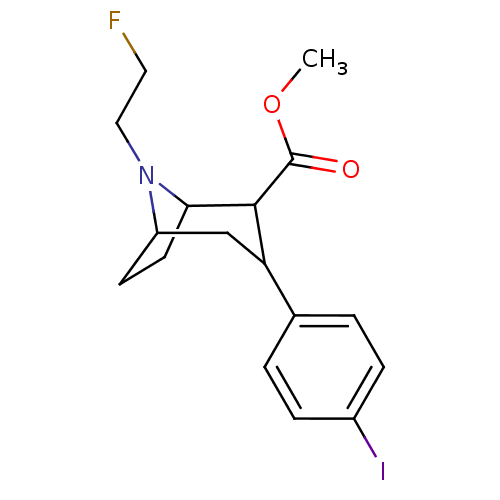 (8-(2-Fluoro-ethyl)-3-(4-iodo-phenyl)-8-aza-bicyclo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235631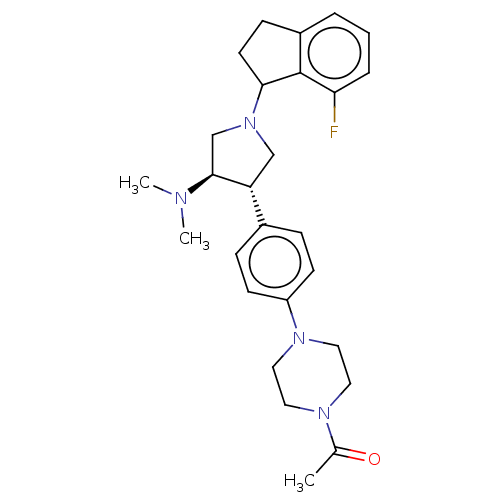 (CHEMBL4060827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235630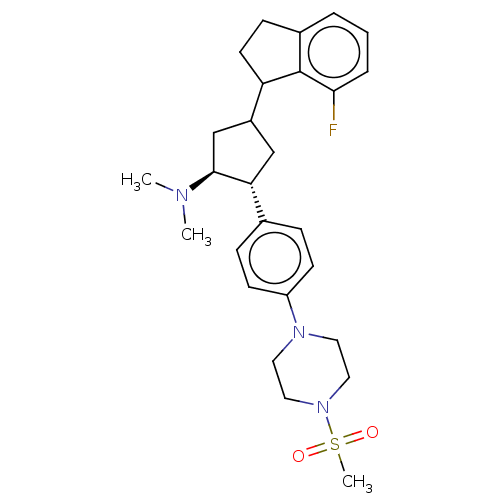 (CHEMBL4093096) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048566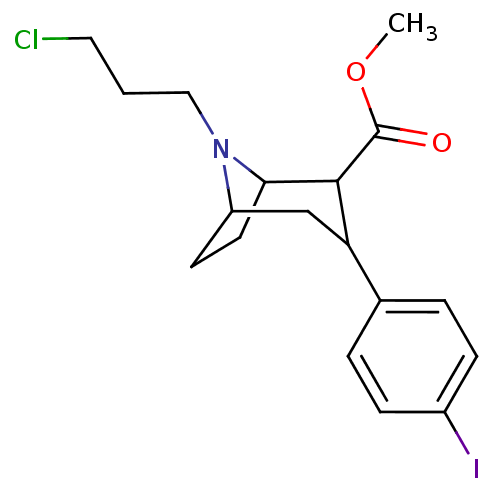 (8-(3-Chloro-propyl)-3-(4-iodo-phenyl)-8-aza-bicycl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50409919 (CHEMBL12282) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048563 (8-(3-Bromo-propyl)-3-(4-iodo-phenyl)-8-aza-bicyclo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235643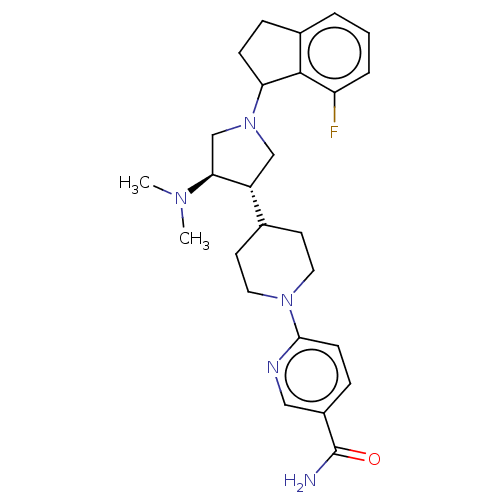 (CHEMBL4076017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50010195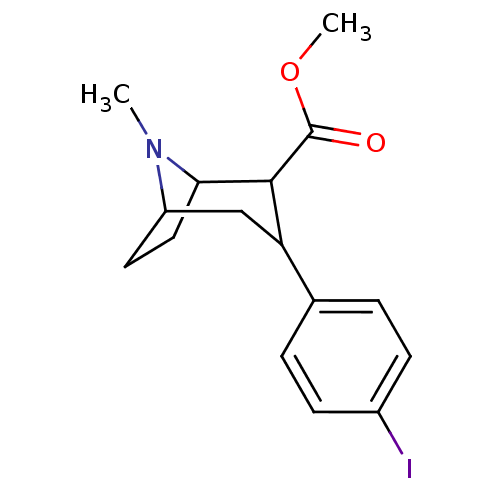 ((3S,5R)-methyl 3-(4-iodophenyl)-8-methyl-8-aza-bic...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217096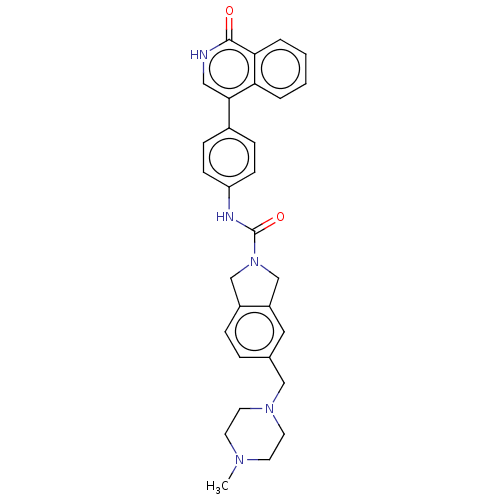 (US9302989, 391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50450533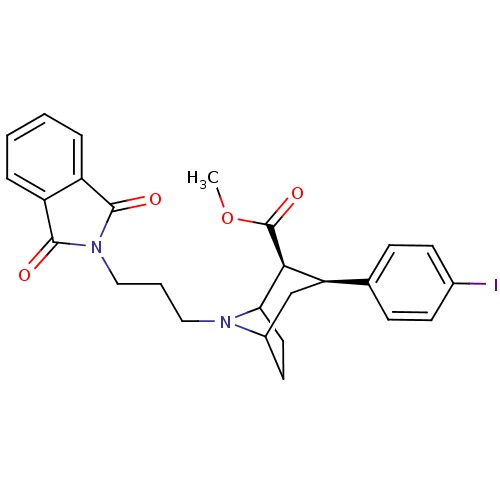 (CHEMBL2112550) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125339 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235658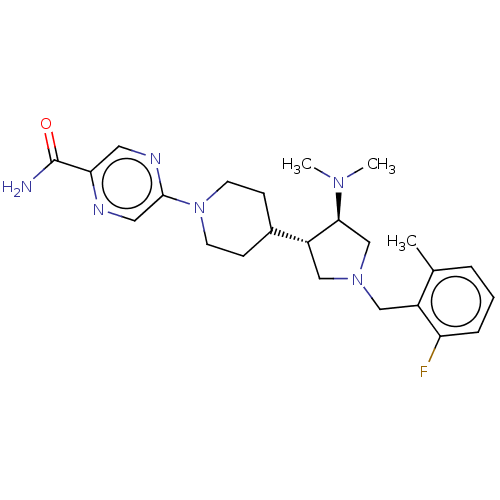 (CHEMBL4073166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235644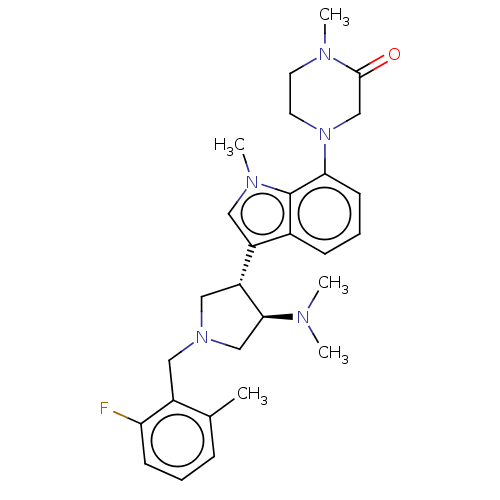 (CHEMBL4065766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048560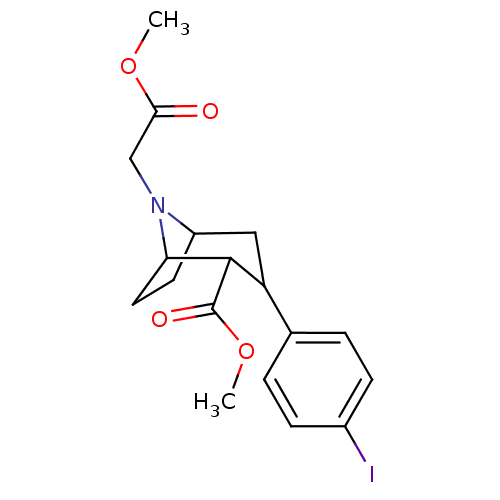 (3-(4-Iodo-phenyl)-8-methoxycarbonylmethyl-8-aza-bi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224090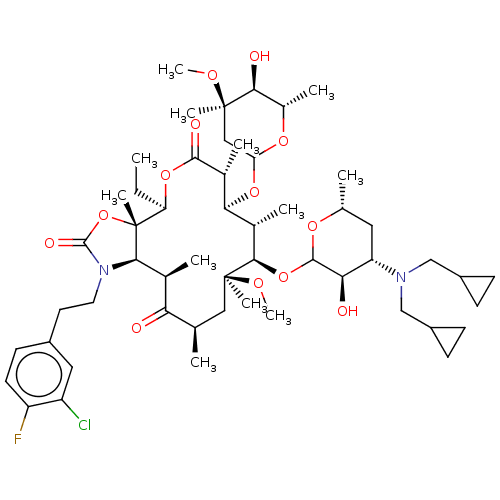 (CHEMBL410940) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235632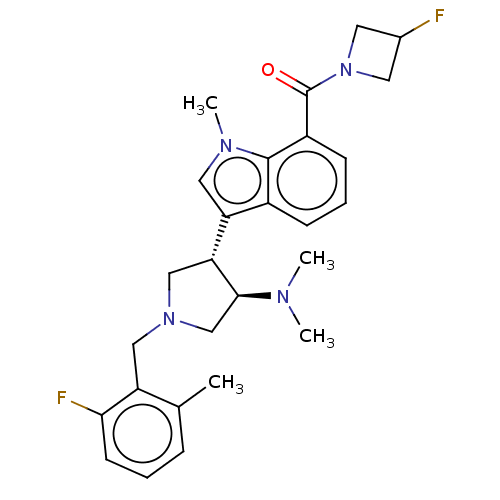 (CHEMBL4077363) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125343 (2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PknB (Mycobacterium tuberculosis) | BDBM50279715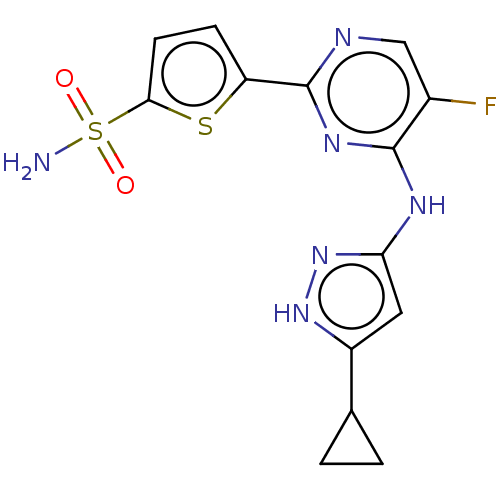 (CHEMBL4162544) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis PknB (M1 to G279 residues) expressed in Escherichia coli BL21 (DE3) cells using [T-33P]ATP as substrate afte... | ACS Med Chem Lett 8: 1224-1229 (2017) Article DOI: 10.1021/acsmedchemlett.7b00239 BindingDB Entry DOI: 10.7270/Q2183910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217112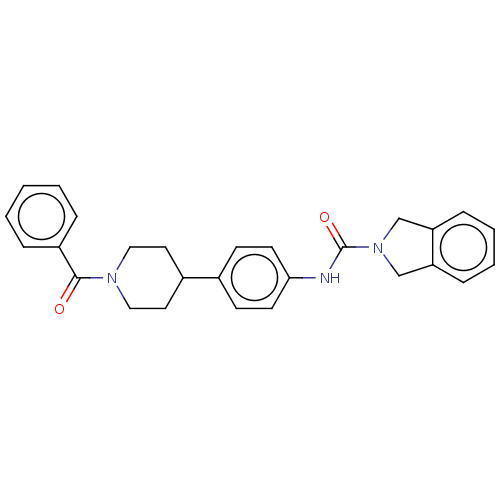 (US9302989, 407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PknB (Mycobacterium tuberculosis) | BDBM50279757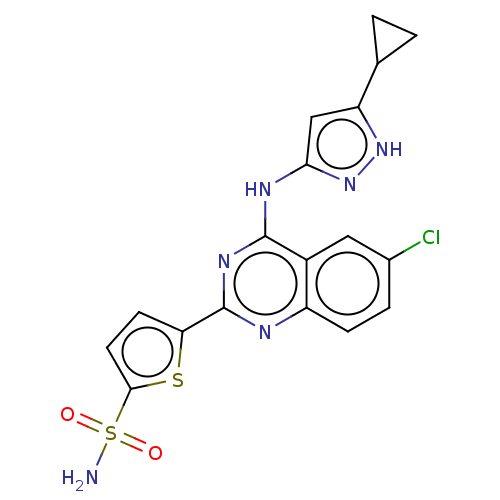 (CHEMBL4166160) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis PknB (M1 to G279 residues) expressed in Escherichia coli BL21 (DE3) cells using [T-33P]ATP as substrate afte... | ACS Med Chem Lett 8: 1224-1229 (2017) Article DOI: 10.1021/acsmedchemlett.7b00239 BindingDB Entry DOI: 10.7270/Q2183910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50041628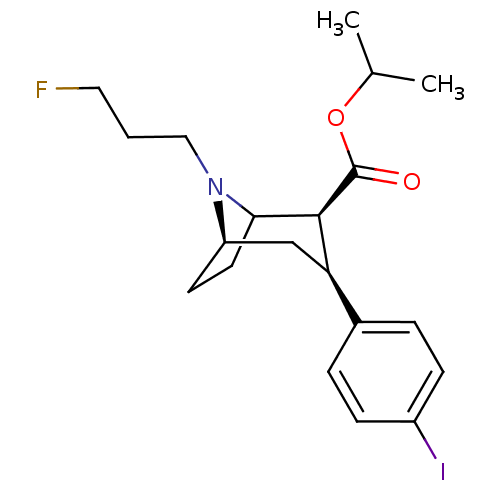 ((2S,3S,5R)-8-(3-Fluoro-propyl)-3-(4-iodo-phenyl)-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description Binding affinity for dopamine transporters in rat brain tissue. | J Med Chem 37: 1558-61 (1994) BindingDB Entry DOI: 10.7270/Q2P55P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50048569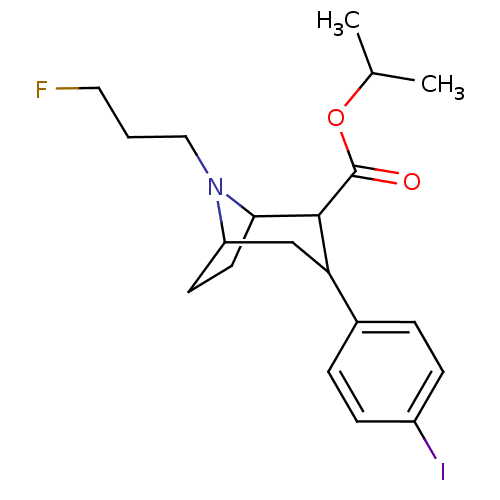 (8-(3-Fluoro-propyl)-3-(4-iodo-phenyl)-8-aza-bicycl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine transporter in rat striatal membranes by [3H]GBR-12395 displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PknB (Mycobacterium tuberculosis) | BDBM50279763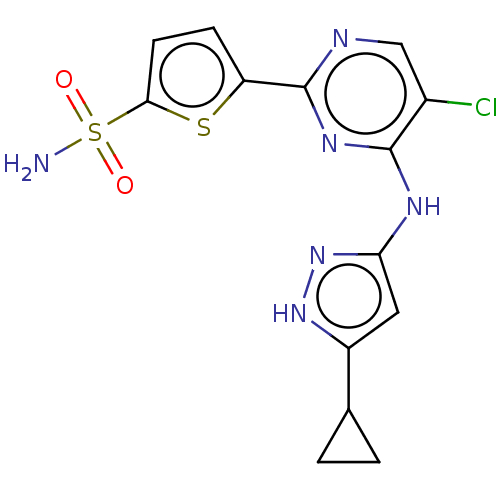 (CHEMBL4162308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis PknB (M1 to G279 residues) expressed in Escherichia coli BL21 (DE3) cells using [T-33P]ATP as substrate afte... | ACS Med Chem Lett 8: 1224-1229 (2017) Article DOI: 10.1021/acsmedchemlett.7b00239 BindingDB Entry DOI: 10.7270/Q2183910 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048567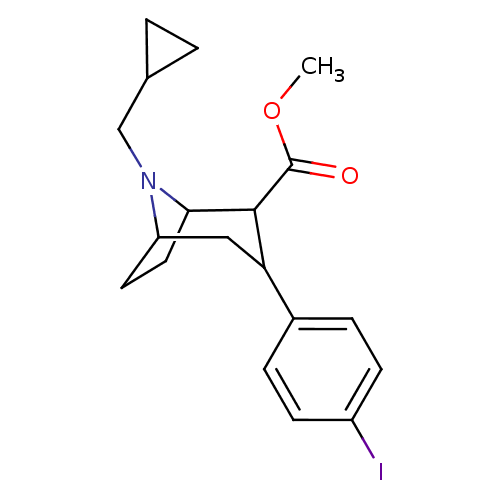 (8-Cyclopropylmethyl-3-(4-iodo-phenyl)-8-aza-bicycl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125362 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224093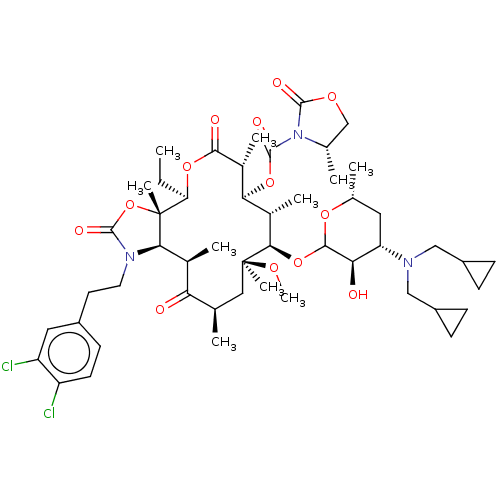 (CHEMBL408925) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217092 (US9302989, 387) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50041625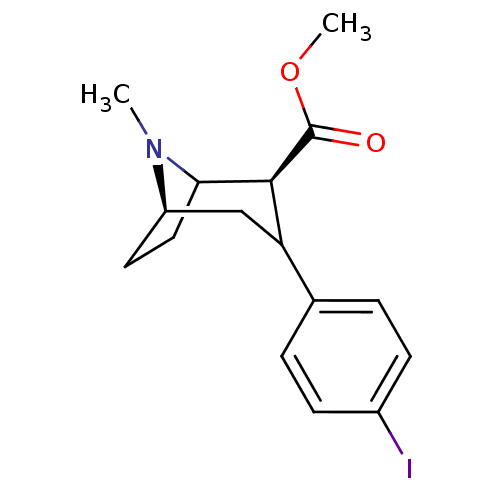 ((2S,5R)-3-(4-Iodo-phenyl)-8-methyl-8-aza-bicyclo[3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description Binding affinity for dopamine transporters in rat brain tissue. | J Med Chem 37: 1558-61 (1994) BindingDB Entry DOI: 10.7270/Q2P55P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50010195 ((3S,5R)-methyl 3-(4-iodophenyl)-8-methyl-8-aza-bic...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine transporter in rat striatal membranes by [3H]GBR-12395 displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224095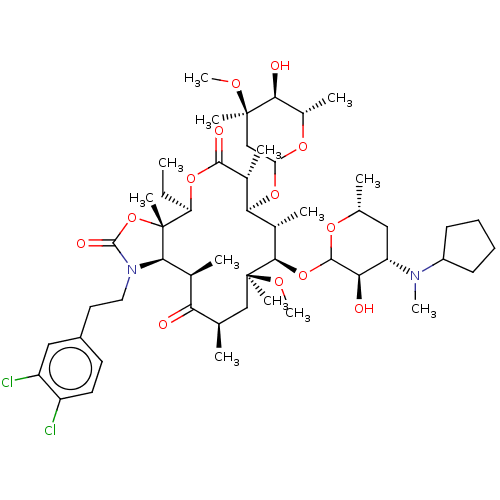 (CHEMBL264043) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254198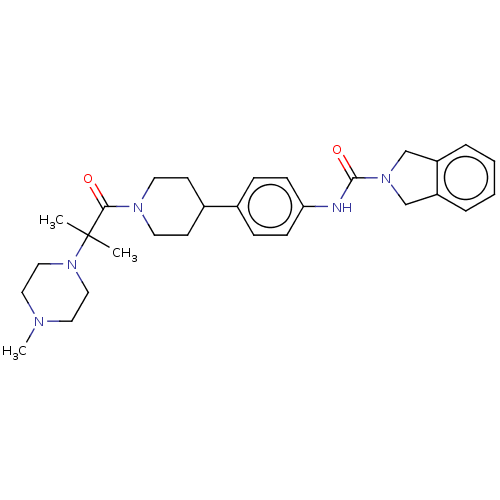 (CHEMBL4060799) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217057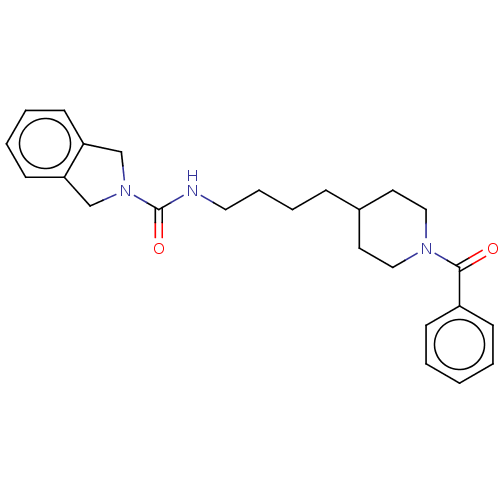 (US9302989, 349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048564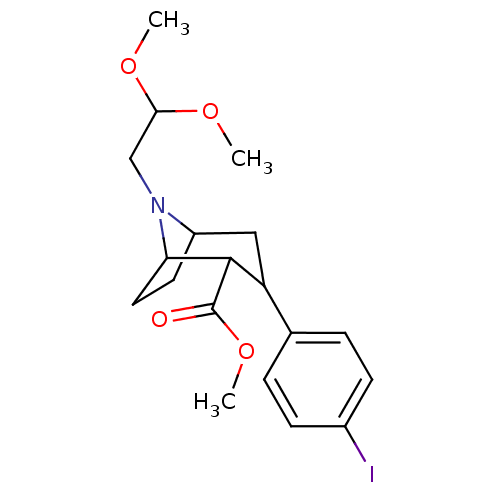 (8-(2,2-Dimethoxy-ethyl)-3-(4-iodo-phenyl)-8-aza-bi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254199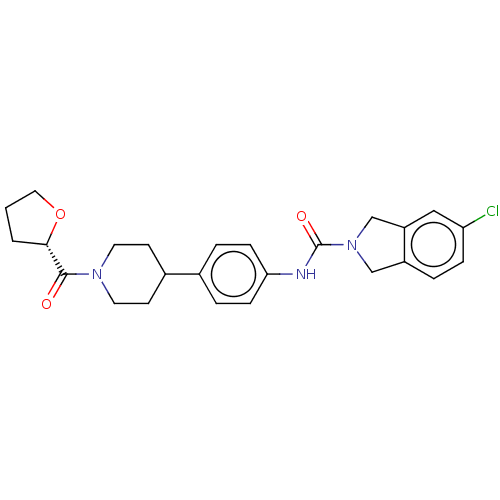 (CHEMBL4083505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217094 (US9302989, 389) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117910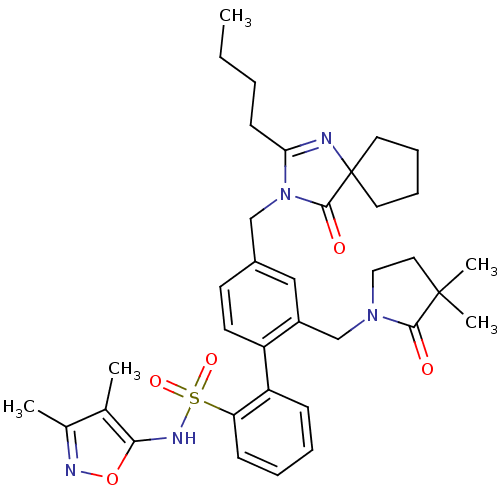 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50423641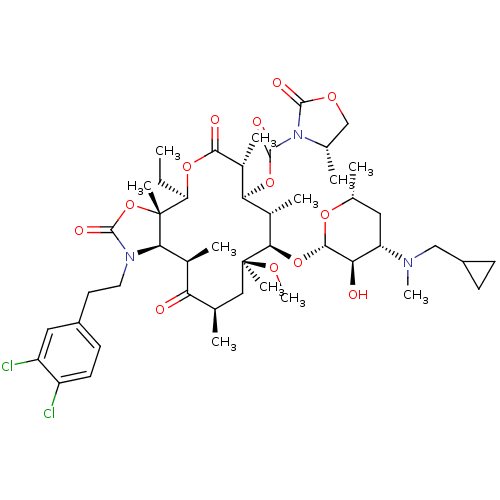 (A-198401 | CHEMBL303274) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254216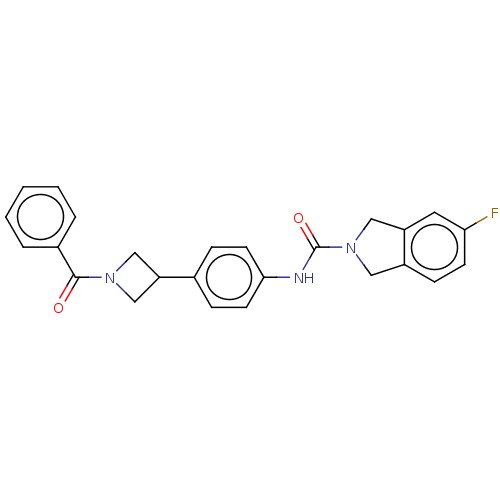 (CHEMBL4096471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50202094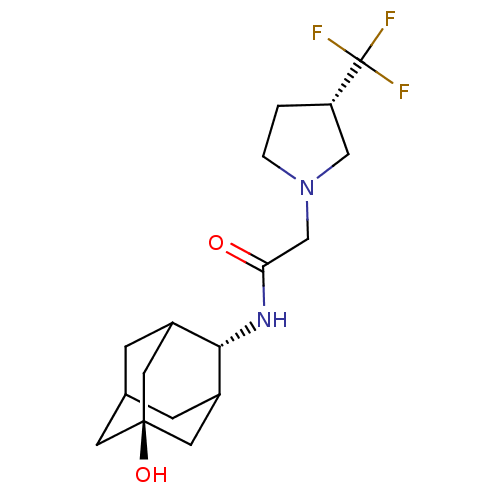 (CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA | J Med Chem 50: 149-64 (2007) Article DOI: 10.1021/jm0609364 BindingDB Entry DOI: 10.7270/Q2Z60NQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PknB (Mycobacterium tuberculosis) | BDBM50279744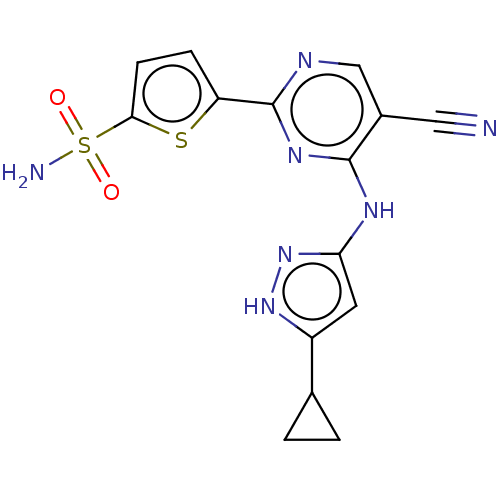 (CHEMBL4165984) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of hog kidney renin at 10e-9 M concentration | ACS Med Chem Lett 8: 1224-1229 (2017) Article DOI: 10.1021/acsmedchemlett.7b00239 BindingDB Entry DOI: 10.7270/Q2183910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125352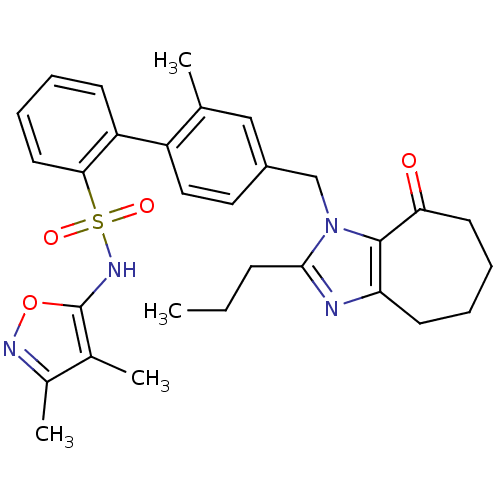 (2'-Methyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahydro-4H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3917 total ) | Next | Last >> |