

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128532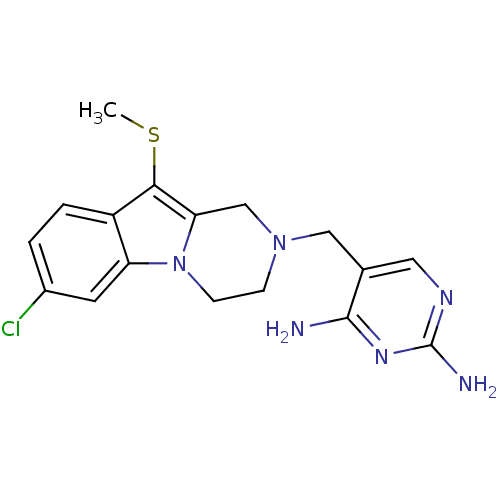 (5-(7-Chloro-10-methylsulfanyl-3,4-dihydro-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128535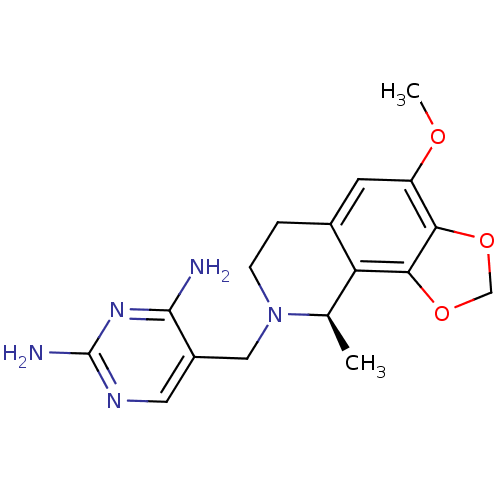 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128534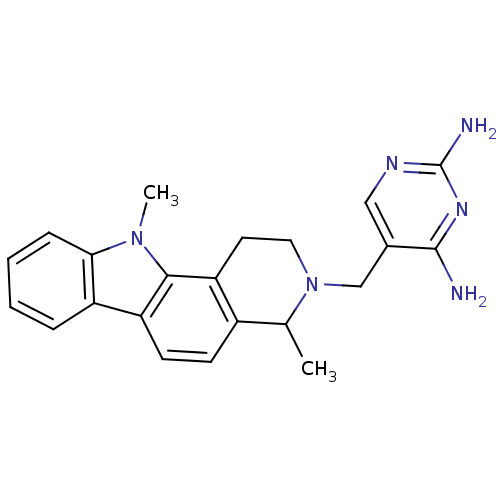 (5-(4,11-Dimethyl-1,2,4,11-tetrahydro-pyrido[4,3-a]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128540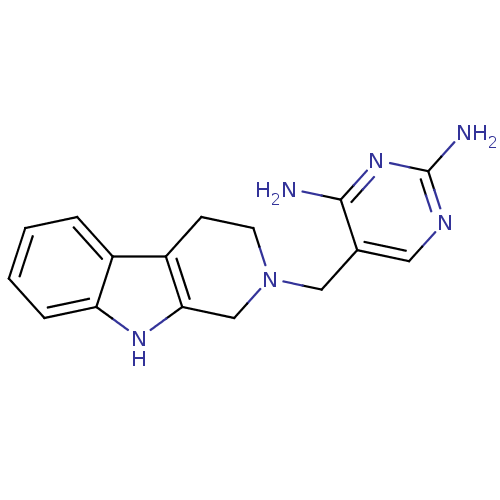 (5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089194 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128538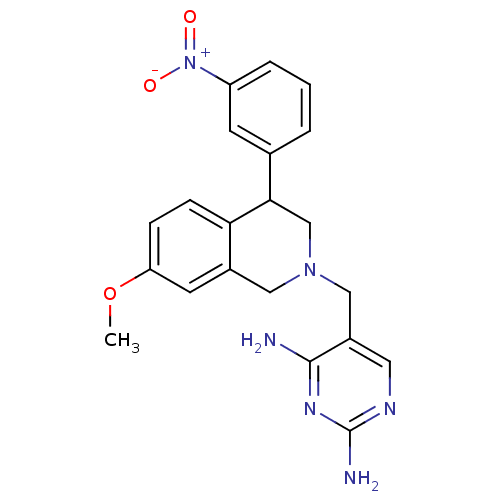 (5-[7-Methoxy-4-(3-nitro-phenyl)-3,4-dihydro-1H-iso...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128533 (5-[4-(2,4-Dichloro-phenyl)-6,7-dimethoxy-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50089199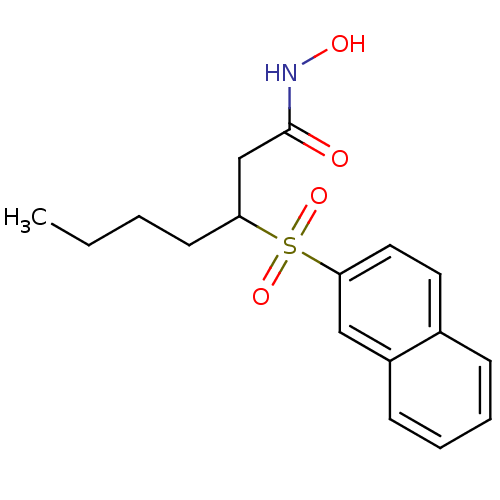 (3-(Naphthalene-2-sulfonyl)-heptanoic acid hydroxya...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50408913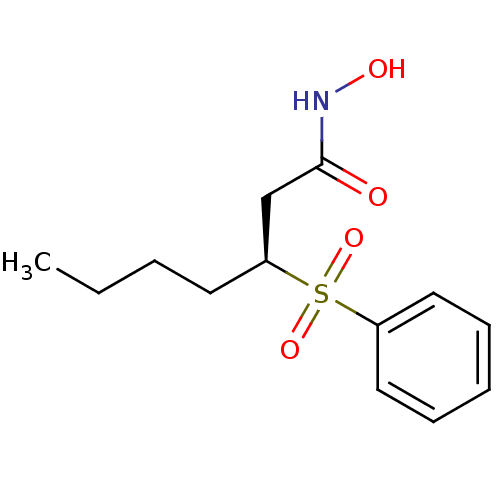 (CHEMBL2092878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128535 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128534 (5-(4,11-Dimethyl-1,2,4,11-tetrahydro-pyrido[4,3-a]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128536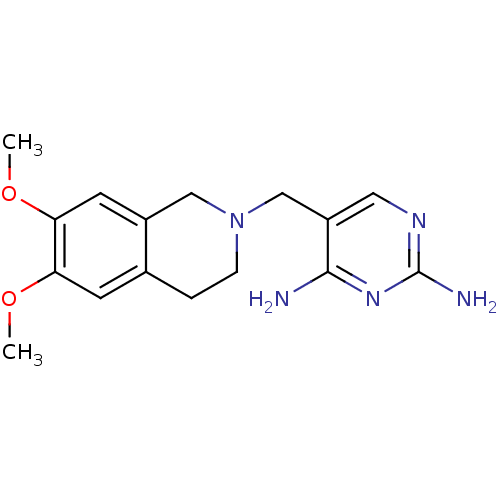 (5-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089201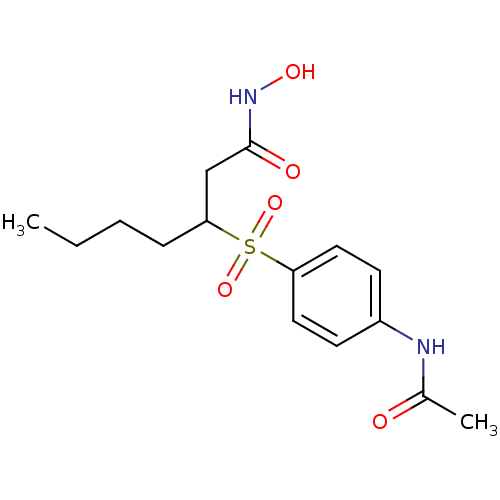 (3-(4-Acetylamino-benzenesulfonyl)-heptanoic acid h...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50089196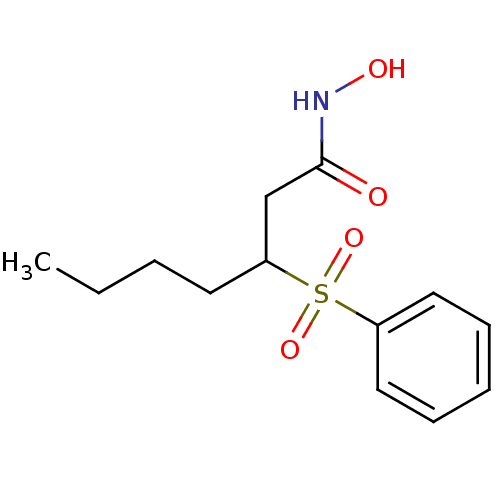 (3-Benzenesulfonyl-heptanoic acid hydroxyamide | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128537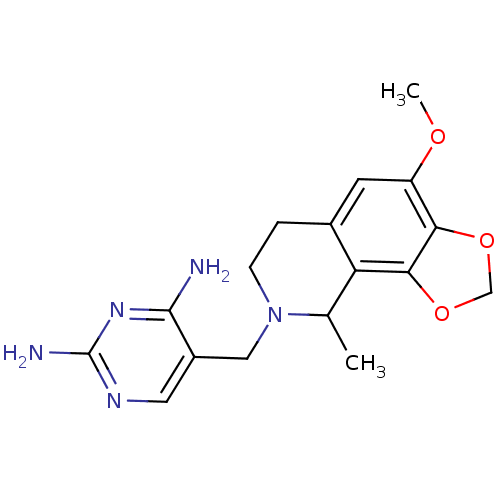 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50089199 (3-(Naphthalene-2-sulfonyl)-heptanoic acid hydroxya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50089198 (3-Cyclohexanesulfonyl-heptanoic acid hydroxyamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50369525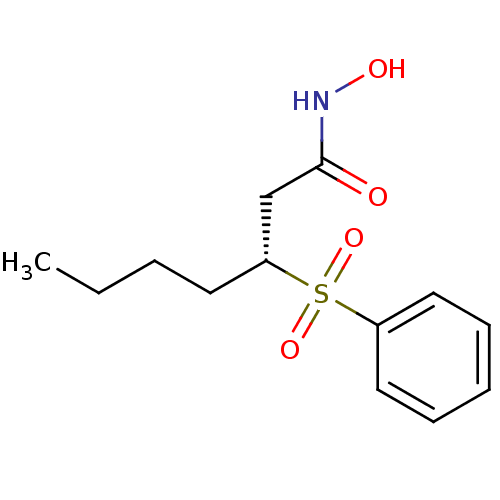 (CHEMBL1788203) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128529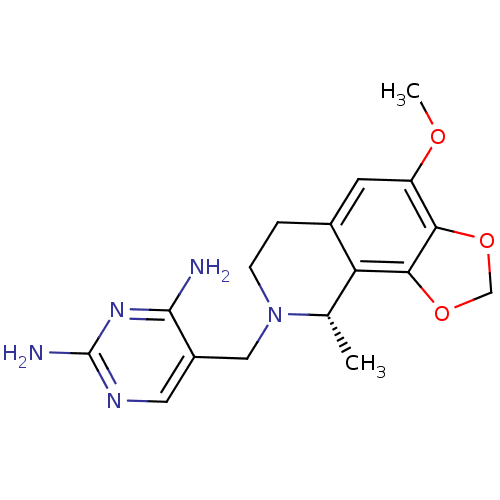 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50089196 (3-Benzenesulfonyl-heptanoic acid hydroxyamide | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089199 (3-(Naphthalene-2-sulfonyl)-heptanoic acid hydroxya...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128537 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-Resistant Dihydrofolate reductase from Staphylococcus aureus 157/4696 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128529 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50395398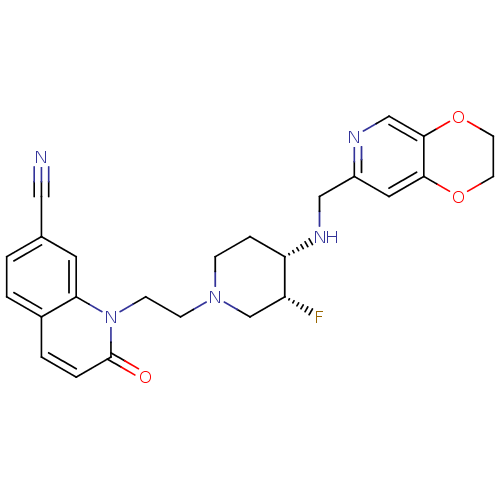 (CHEMBL2165064) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440321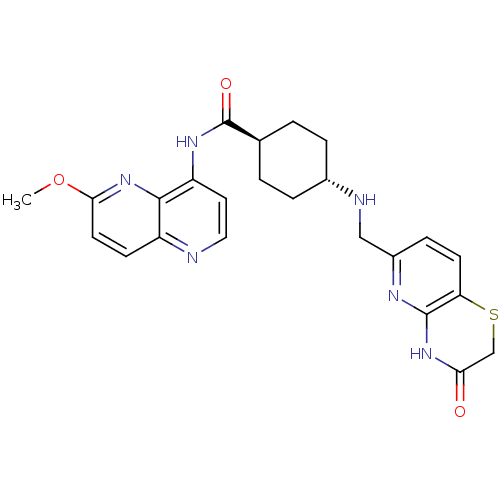 (CHEMBL2424893) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440327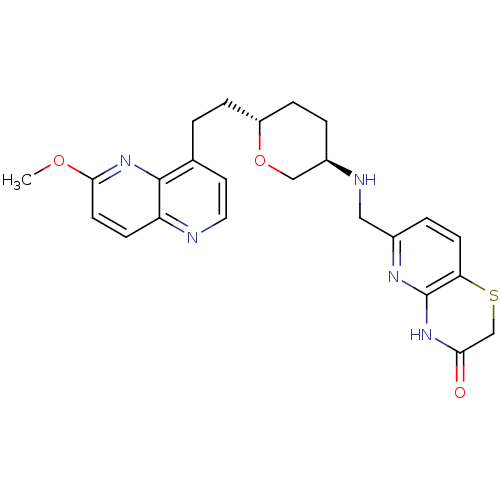 (CHEMBL2424883) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440326 (CHEMBL2424886) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440309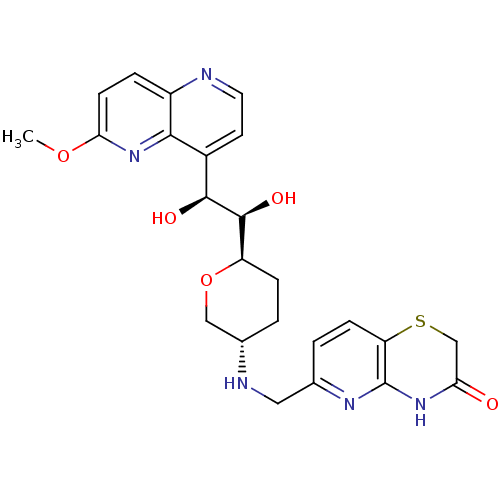 (CHEMBL2424889) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440325 (CHEMBL2424890) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440324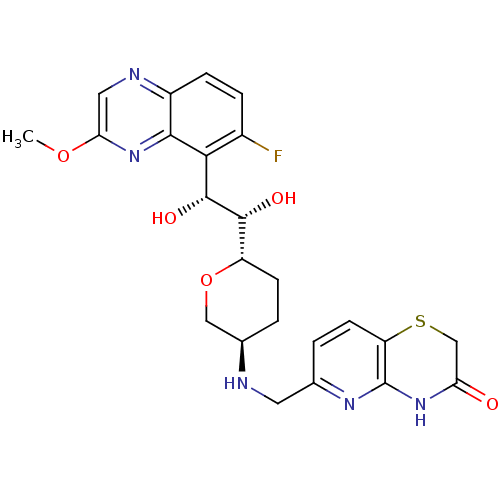 (CHEMBL2424892) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440323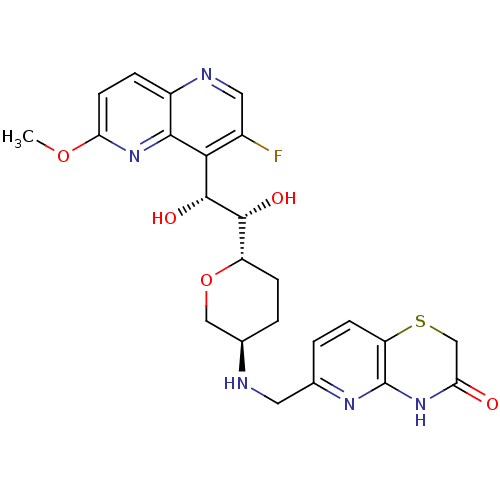 (CHEMBL2424833) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440322 (CHEMBL2424836) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440304 (CHEMBL2424874) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440303 (CHEMBL2424876) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50440302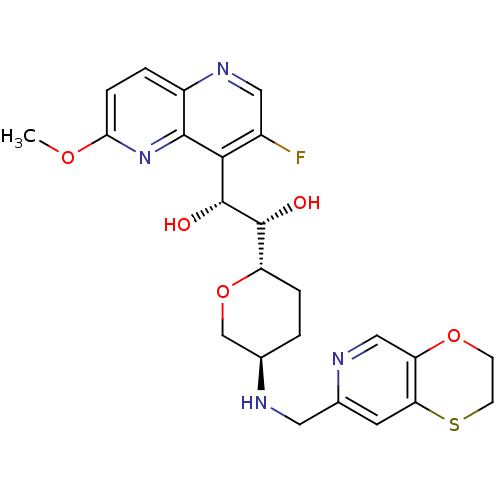 (CHEMBL2424877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus DNA gyrase subunit 2GyrA/2GyrB assessed as pBR322 supercoiling after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128532 (5-(7-Chloro-10-methylsulfanyl-3,4-dihydro-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128533 (5-[4-(2,4-Dichloro-phenyl)-6,7-dimethoxy-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089209 (3-(4-Methoxy-benzenesulfonyl)-heptanoic acid hydro...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089196 (3-Benzenesulfonyl-heptanoic acid hydroxyamide | CH...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus (strain MW2)) | BDBM50128531 (5-(7-Benzyl-1-methyl-6-phenyl-3,4-dihydro-1H-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-Resistance Dihydrofolate reductase from Staphylococcus aureus 157/4696 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50369525 (CHEMBL1788203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128530 (5-(6,7-Dimethoxy-1-methyl-3,4-dihydro-1H-isoquinol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128540 (5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli (strain K12)) | BDBM50100105 (2-(5-Bromo-2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of the isolated native E. coli peptide deformylase (PDF) | J Med Chem 44: 1847-52 (2001) BindingDB Entry DOI: 10.7270/Q2ZW1K6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128530 (5-(6,7-Dimethoxy-1-methyl-3,4-dihydro-1H-isoquinol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50089198 (3-Cyclohexanesulfonyl-heptanoic acid hydroxyamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-1 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128538 (5-[7-Methoxy-4-(3-nitro-phenyl)-3,4-dihydro-1H-iso...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089204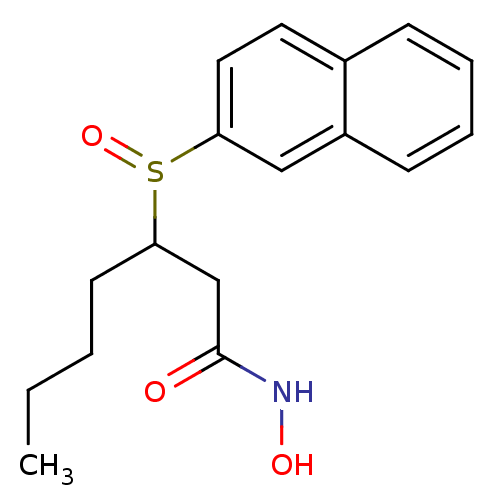 (3-(Naphthalene-2-sulfinyl)-heptanoic acid hydroxya...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli (strain K12)) | BDBM50100101 (2-(5-Bromo-1-cyclopropylmethyl-2,2-dioxo-1,4-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of the isolated native E. coli peptide deformylase (PDF) | J Med Chem 44: 1847-52 (2001) BindingDB Entry DOI: 10.7270/Q2ZW1K6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 245 total ) | Next | Last >> |