 Found 212 hits with Last Name = 'luu' and Initial = 'c'
Found 212 hits with Last Name = 'luu' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Translocator protein
(Homo sapiens (Human)) | BDBM98172
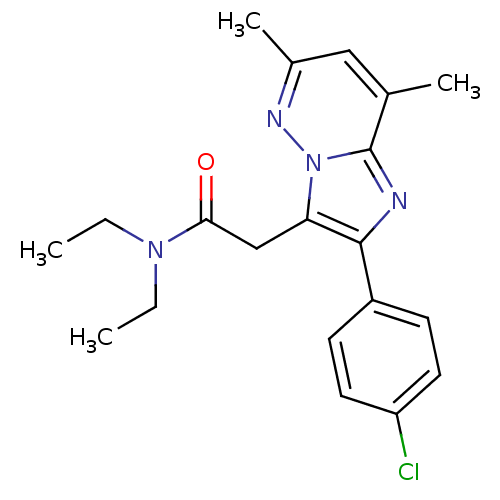
(US8492379, 28)Show SMILES CCN(CC)C(=O)Cc1c(nc2c(C)cc(C)nn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClN4O/c1-5-24(6-2)18(26)12-17-19(15-7-9-16(21)10-8-15)22-20-13(3)11-14(4)23-25(17)20/h7-11H,5-6,12H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98171

(US8492379, 27)Show SMILES CCN(CC)C(=O)Cc1c(nc2c(C)cc(C)nn12)-c1ccc(C)cc1 Show InChI InChI=1S/C21H26N4O/c1-6-24(7-2)19(26)13-18-20(17-10-8-14(3)9-11-17)22-21-15(4)12-16(5)23-25(18)21/h8-12H,6-7,13H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98173
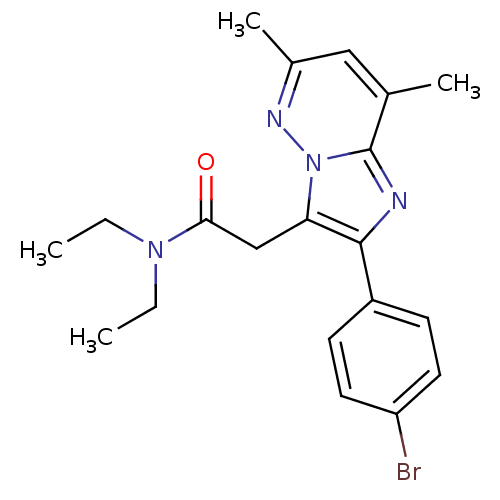
(US8492379, 29)Show SMILES CCN(CC)C(=O)Cc1c(nc2c(C)cc(C)nn12)-c1ccc(Br)cc1 Show InChI InChI=1S/C20H23BrN4O/c1-5-24(6-2)18(26)12-17-19(15-7-9-16(21)10-8-15)22-20-13(3)11-14(4)23-25(17)20/h7-11H,5-6,12H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98176

(US8492379, 53)Show SMILES CCN(CC)C(=O)Cc1c(nc2c(C)cc(C)nn12)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C22H27FN4O2/c1-5-26(6-2)20(28)14-19-21(17-7-9-18(10-8-17)29-12-11-23)24-22-15(3)13-16(4)25-27(19)22/h7-10,13H,5-6,11-12,14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98174
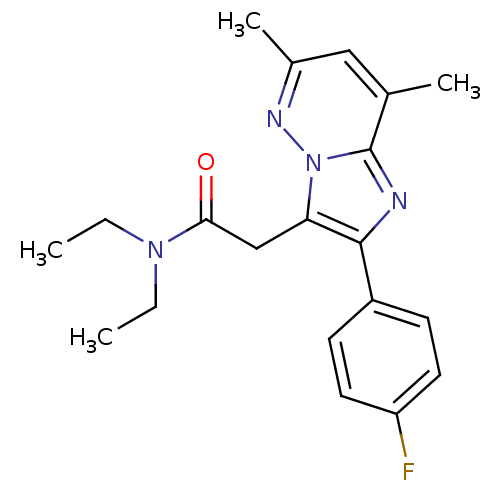
(US8492379, 30)Show SMILES CCN(CC)C(=O)Cc1c(nc2c(C)cc(C)nn12)-c1ccc(F)cc1 Show InChI InChI=1S/C20H23FN4O/c1-5-24(6-2)18(26)12-17-19(15-7-9-16(21)10-8-15)22-20-13(3)11-14(4)23-25(17)20/h7-11H,5-6,12H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98170
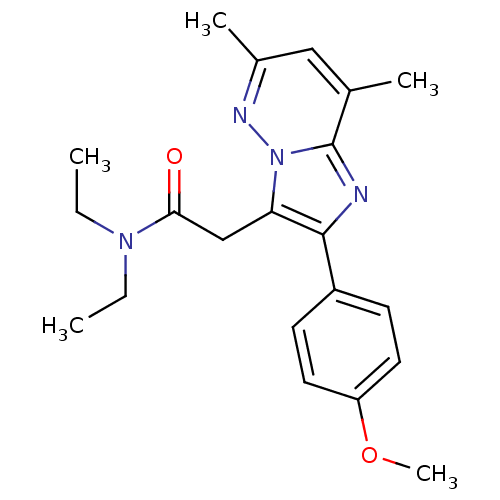
(US8492379, 26)Show SMILES CCN(CC)C(=O)Cc1c(nc2c(C)cc(C)nn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H26N4O2/c1-6-24(7-2)19(26)13-18-20(16-8-10-17(27-5)11-9-16)22-21-14(3)12-15(4)23-25(18)21/h8-12H,6-7,13H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98177

(US8492379, 54)Show SMILES CCN(CC)C(=O)C(Oc1cccnc1-n1cccc1)c1ccc(OC)cc1 Show InChI InChI=1S/C22H25N3O3/c1-4-24(5-2)22(26)20(17-10-12-18(27-3)13-11-17)28-19-9-8-14-23-21(19)25-15-6-7-16-25/h6-16,20H,4-5H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98178

(US8492379, 55)Show SMILES CCN(CC)C(=O)C(Oc1ccccc1-n1cccc1)c1ccc(OC)cc1 Show InChI InChI=1S/C23H26N2O3/c1-4-24(5-2)23(26)22(18-12-14-19(27-3)15-13-18)28-21-11-7-6-10-20(21)25-16-8-9-17-25/h6-17,22H,4-5H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98179
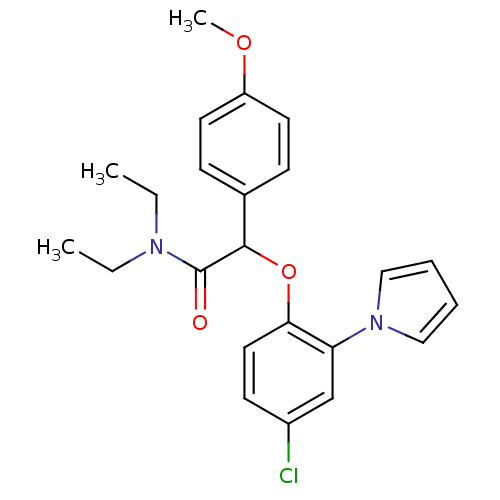
(US8492379, 56)Show SMILES CCN(CC)C(=O)C(Oc1ccc(Cl)cc1-n1cccc1)c1ccc(OC)cc1 Show InChI InChI=1S/C23H25ClN2O3/c1-4-25(5-2)23(27)22(17-8-11-19(28-3)12-9-17)29-21-13-10-18(24)16-20(21)26-14-6-7-15-26/h6-16,22H,4-5H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM98175
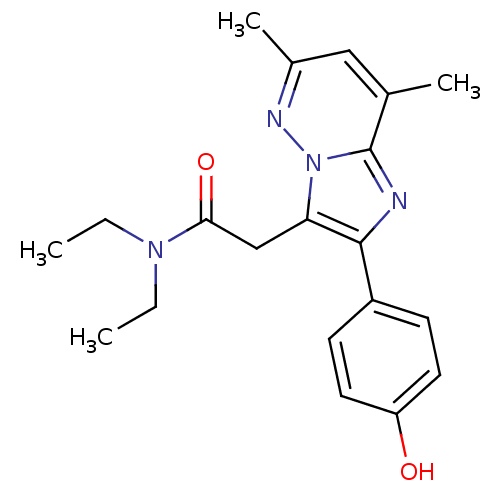
(US8492379, 52)Show SMILES CCN(CC)C(=O)Cc1c(nc2c(C)cc(C)nn12)-c1ccc(O)cc1 Show InChI InChI=1S/C20H24N4O2/c1-5-23(6-2)18(26)12-17-19(15-7-9-16(25)10-8-15)21-20-13(3)11-14(4)22-24(17)20/h7-11,25H,5-6,12H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 11.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
US Patent
| Assay Description
In vitro binding activity of TSPO. |
US Patent US8492379 (2013)
BindingDB Entry DOI: 10.7270/Q25T3J40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M]
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4817

(N-[2-(dimethylamino)ethyl]-5-{[(3Z)-5-fluoro-2-oxo...)Show SMILES CN(C)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C20H23FN4O2/c1-11-17(23-12(2)18(11)20(27)22-7-8-25(3)4)10-15-14-9-13(21)5-6-16(14)24-19(15)26/h5-6,9-10,23H,7-8H2,1-4H3,(H,22,27)(H,24,26)/b15-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M]
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase [531-875]
(Homo sapiens (Human)) | BDBM14776

(2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...)Show SMILES CCCc1nc(C)c2n1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4818

(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NCCN1CCCC1 Show InChI InChI=1S/C22H25FN4O2/c1-13-19(12-17-16-11-15(23)5-6-18(16)26-21(17)28)25-14(2)20(13)22(29)24-7-10-27-8-3-4-9-27/h5-6,11-12,25H,3-4,7-10H2,1-2H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4821
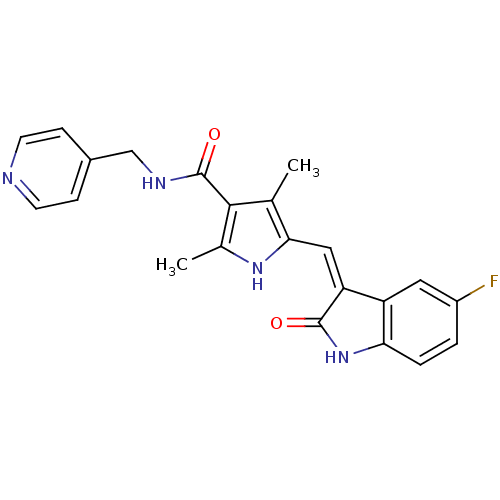
(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NCc1ccncc1 Show InChI InChI=1S/C22H19FN4O2/c1-12-19(10-17-16-9-15(23)3-4-18(16)27-21(17)28)26-13(2)20(12)22(29)25-11-14-5-7-24-8-6-14/h3-10,26H,11H2,1-2H3,(H,25,29)(H,27,28)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase [531-875]
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4820
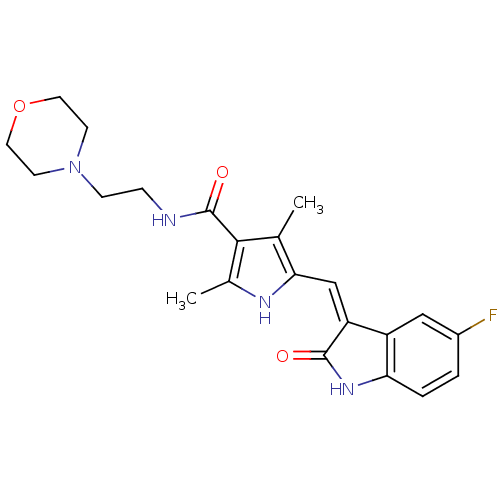
(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C22H25FN4O3/c1-13-19(12-17-16-11-15(23)3-4-18(16)26-21(17)28)25-14(2)20(13)22(29)24-5-6-27-7-9-30-10-8-27/h3-4,11-12,25H,5-10H2,1-2H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase [531-875]
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4815
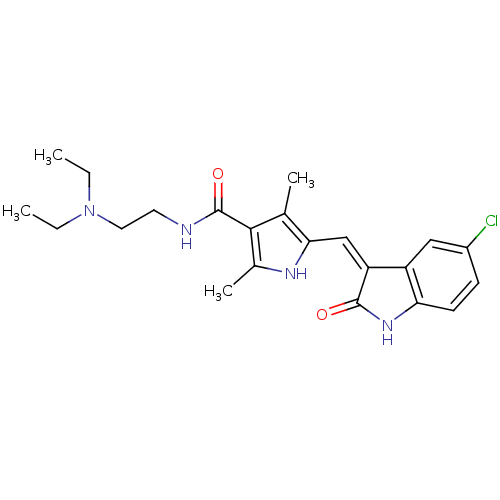
(5-{[(3Z)-5-chloro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(Cl)cc23)c1C Show InChI InChI=1S/C22H27ClN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4819

(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES CN1CCC(CNC(=O)c2c(C)[nH]c(\C=C3/C(=O)Nc4ccc(F)cc34)c2C)CC1 Show InChI InChI=1S/C23H27FN4O2/c1-13-20(11-18-17-10-16(24)4-5-19(17)27-22(18)29)26-14(2)21(13)23(30)25-12-15-6-8-28(3)9-7-15/h4-5,10-11,15,26H,6-9,12H2,1-3H3,(H,25,30)(H,27,29)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Probable global transcription activator SNF2L2
(Homo sapiens (Human)) | BDBM50469320
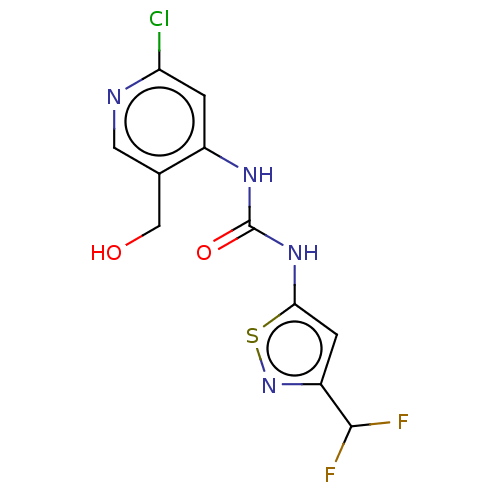
(CHEMBL4293567)Show InChI InChI=1S/C11H9ClF2N4O2S/c12-8-1-6(5(4-19)3-15-8)16-11(20)17-9-2-7(10(13)14)18-21-9/h1-3,10,19H,4H2,(H2,15,16,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Probable global transcription activator SNF2L2
(Homo sapiens (Human)) | BDBM50469324
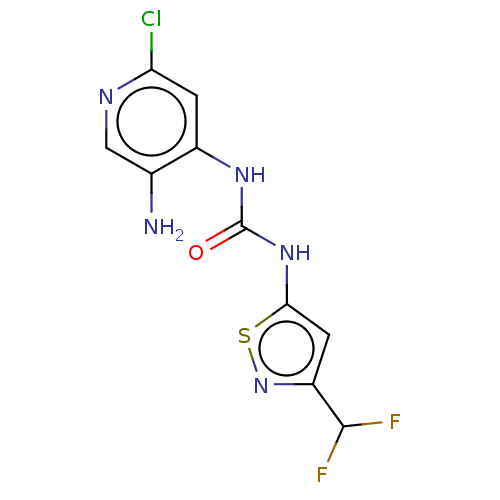
(CHEMBL4286345)Show InChI InChI=1S/C10H8ClF2N5OS/c11-7-1-5(4(14)3-15-7)16-10(19)17-8-2-6(9(12)13)18-20-8/h1-3,9H,14H2,(H2,15,16,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50469330
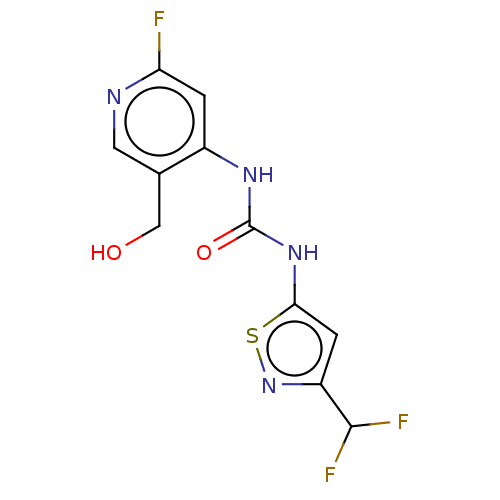
(CHEMBL4295096)Show InChI InChI=1S/C11H9F3N4O2S/c12-8-1-6(5(4-19)3-15-8)16-11(20)17-9-2-7(10(13)14)18-21-9/h1-3,10,19H,4H2,(H2,15,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50469324

(CHEMBL4286345)Show InChI InChI=1S/C10H8ClF2N5OS/c11-7-1-5(4(14)3-15-7)16-10(19)17-8-2-6(9(12)13)18-20-8/h1-3,9H,14H2,(H2,15,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Probable global transcription activator SNF2L2
(Homo sapiens (Human)) | BDBM50469331
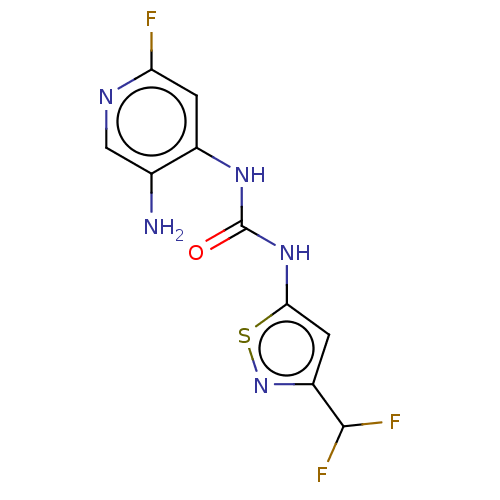
(CHEMBL4282980)Show InChI InChI=1S/C10H8F3N5OS/c11-7-1-5(4(14)3-15-7)16-10(19)17-8-2-6(9(12)13)18-20-8/h1-3,9H,14H2,(H2,15,16,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Probable global transcription activator SNF2L2
(Homo sapiens (Human)) | BDBM50469330

(CHEMBL4295096)Show InChI InChI=1S/C11H9F3N4O2S/c12-8-1-6(5(4-19)3-15-8)16-11(20)17-9-2-7(10(13)14)18-21-9/h1-3,10,19H,4H2,(H2,15,16,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Probable global transcription activator SNF2L2
(Homo sapiens (Human)) | BDBM50469329
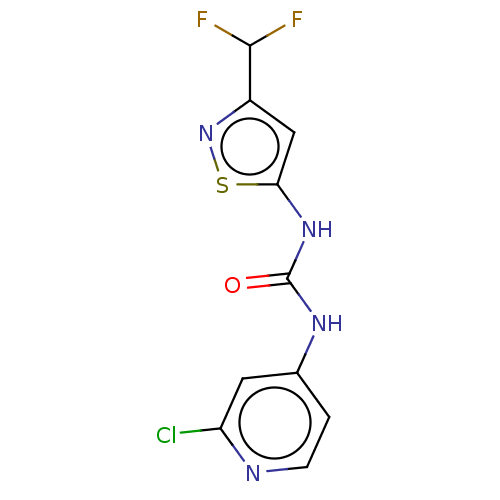
(CHEMBL4278436)Show InChI InChI=1S/C10H7ClF2N4OS/c11-7-3-5(1-2-14-7)15-10(18)16-8-4-6(9(12)13)17-19-8/h1-4,9H,(H2,14,15,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50469331

(CHEMBL4282980)Show InChI InChI=1S/C10H8F3N5OS/c11-7-1-5(4(14)3-15-7)16-10(19)17-8-2-6(9(12)13)18-20-8/h1-3,9H,14H2,(H2,15,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4816
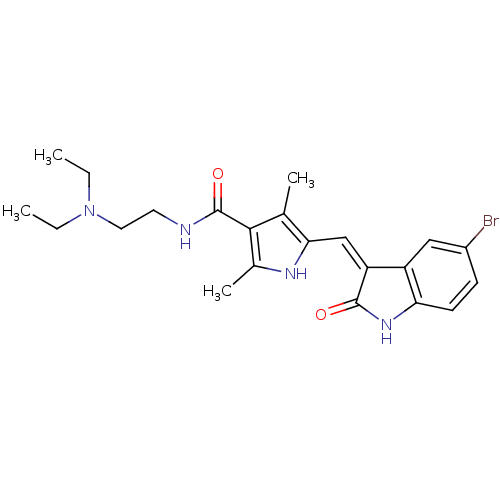
(5-{[(3Z)-5-bromo-2-oxo-2,3-dihydro-1H-indol-3-ylid...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(Br)cc23)c1C Show InChI InChI=1S/C22H27BrN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50469320

(CHEMBL4293567)Show InChI InChI=1S/C11H9ClF2N4O2S/c12-8-1-6(5(4-19)3-15-8)16-11(20)17-9-2-7(10(13)14)18-21-9/h1-3,10,19H,4H2,(H2,15,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50469329

(CHEMBL4278436)Show InChI InChI=1S/C10H7ClF2N4OS/c11-7-3-5(1-2-14-7)15-10(18)16-8-4-6(9(12)13)17-19-8/h1-4,9H,(H2,14,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Probable global transcription activator SNF2L2
(Homo sapiens (Human)) | BDBM50469325
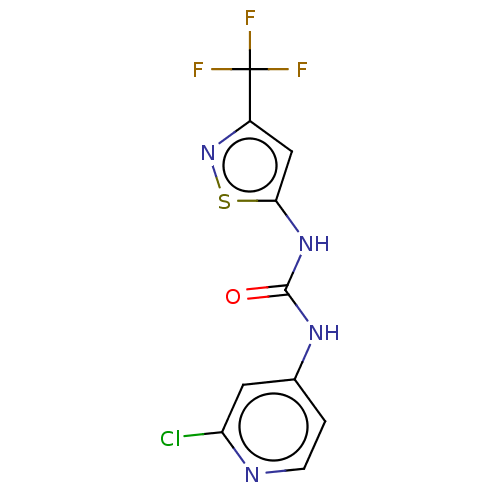
(CHEMBL4294655)Show InChI InChI=1S/C10H6ClF3N4OS/c11-7-3-5(1-2-15-7)16-9(19)17-8-4-6(18-20-8)10(12,13)14/h1-4H,(H2,15,16,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50469325

(CHEMBL4294655)Show InChI InChI=1S/C10H6ClF3N4OS/c11-7-3-5(1-2-15-7)16-9(19)17-8-4-6(18-20-8)10(12,13)14/h1-4H,(H2,15,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4822
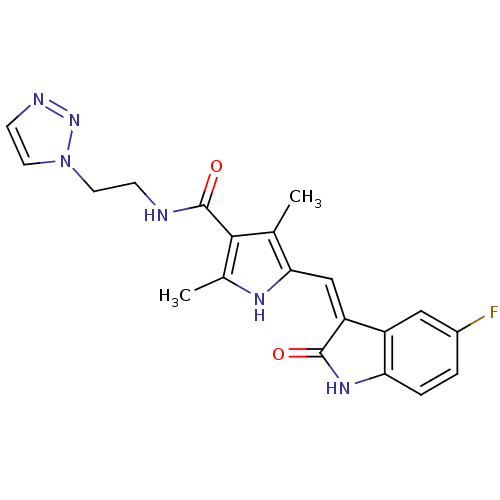
(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NCCn1ccnn1 Show InChI InChI=1S/C20H19FN6O2/c1-11-17(10-15-14-9-13(21)3-4-16(14)25-19(15)28)24-12(2)18(11)20(29)22-5-7-27-8-6-23-26-27/h3-4,6,8-10,24H,5,7H2,1-2H3,(H,22,29)(H,25,28)/b15-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Isoform 4 of Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (PDE11A1) 35-489]
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)
(Homo sapiens (Human)) | BDBM14773
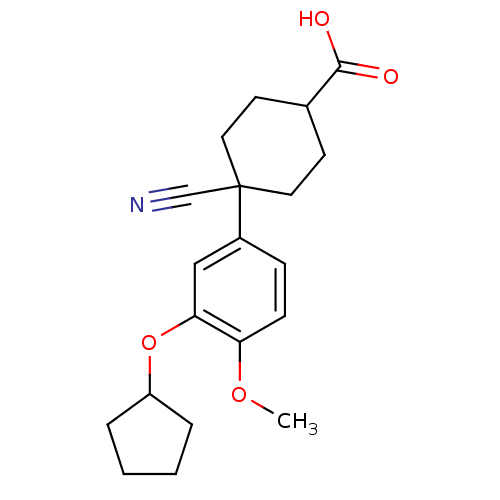
(4-cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)cyclo...)Show SMILES COc1ccc(cc1OC1CCCC1)C1(CCC(CC1)C(O)=O)C#N |(1.4,-2.1,;1.4,-.56,;.07,.21,;-1.27,-.56,;-2.6,.21,;-2.6,1.75,;-1.27,2.52,;.07,1.75,;1.4,2.52,;1.4,4.06,;2.65,4.96,;2.17,6.43,;.63,6.43,;.15,4.96,;-3.93,2.52,;-4.44,3.97,;-5.95,4.26,;-6.96,3.1,;-6.46,1.65,;-4.94,1.35,;-8.47,3.39,;-9.48,2.23,;-8.97,4.85,;-3.16,3.85,;-2.39,5.18,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM25617
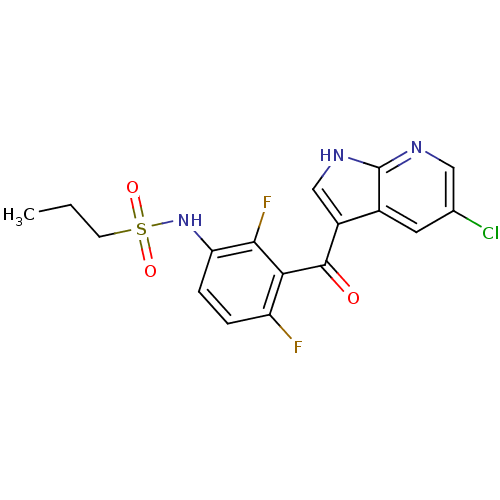
(N-[3-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}carb...)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(C(=O)c2c[nH]c3ncc(Cl)cc23)c1F Show InChI InChI=1S/C17H14ClF2N3O3S/c1-2-5-27(25,26)23-13-4-3-12(19)14(15(13)20)16(24)11-8-22-17-10(11)6-9(18)7-21-17/h3-4,6-8,23H,2,5H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Plexxikon
| Assay Description
The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ... |
Proc Natl Acad Sci U S A 105: 3041-6 (2008)
Article DOI: 10.1073/pnas.0711741105
BindingDB Entry DOI: 10.7270/Q2SB441T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4813

(N-[2-(diethylamino)ethyl]-2,4-dimethyl-5-{[(3Z)-2-...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccccc23)c1C Show InChI InChI=1S/C22H28N4O2/c1-5-26(6-2)12-11-23-22(28)20-14(3)19(24-15(20)4)13-17-16-9-7-8-10-18(16)25-21(17)27/h7-10,13,24H,5-6,11-12H2,1-4H3,(H,23,28)(H,25,27)/b17-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M]
(Homo sapiens (Human)) | BDBM14773

(4-cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)cyclo...)Show SMILES COc1ccc(cc1OC1CCCC1)C1(CCC(CC1)C(O)=O)C#N |(1.4,-2.1,;1.4,-.56,;.07,.21,;-1.27,-.56,;-2.6,.21,;-2.6,1.75,;-1.27,2.52,;.07,1.75,;1.4,2.52,;1.4,4.06,;2.65,4.96,;2.17,6.43,;.63,6.43,;.15,4.96,;-3.93,2.52,;-4.44,3.97,;-5.95,4.26,;-6.96,3.1,;-6.46,1.65,;-4.94,1.35,;-8.47,3.39,;-9.48,2.23,;-8.97,4.85,;-3.16,3.85,;-2.39,5.18,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4819

(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES CN1CCC(CNC(=O)c2c(C)[nH]c(\C=C3/C(=O)Nc4ccc(F)cc34)c2C)CC1 Show InChI InChI=1S/C23H27FN4O2/c1-13-20(11-18-17-10-16(24)4-5-19(17)27-22(18)29)26-14(2)21(13)23(30)25-12-15-6-8-28(3)9-7-15/h4-5,10-11,15,26H,6-9,12H2,1-3H3,(H,25,30)(H,27,29)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | 22 |
SUGEN, Inc.
| Assay Description
The assays were performed in 96-well microtiter plates that had been coated with a polyGluTyr peptide. Negative control wells received buffer alone w... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4815

(5-{[(3Z)-5-chloro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(Cl)cc23)c1C Show InChI InChI=1S/C22H27ClN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
SUGEN, Inc.
| Assay Description
The assays were performed in 96-well microtiter plates that had been coated with a polyGluTyr peptide. Negative control wells received buffer alone w... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50469333
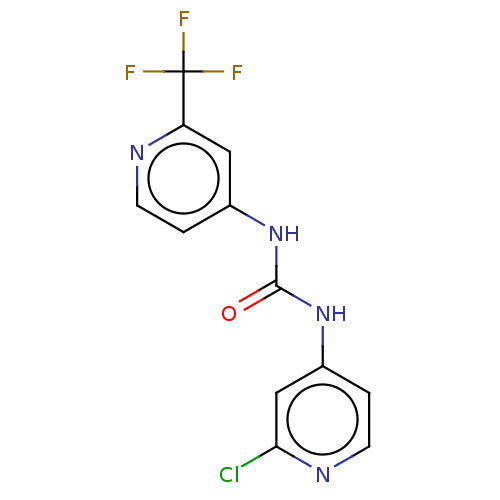
(CHEMBL4290175)Show InChI InChI=1S/C12H8ClF3N4O/c13-10-6-8(2-4-18-10)20-11(21)19-7-1-3-17-9(5-7)12(14,15)16/h1-6H,(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50469321
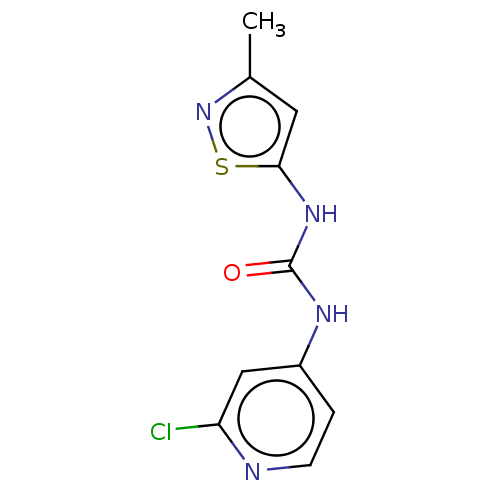
(CHEMBL4286757)Show InChI InChI=1S/C10H9ClN4OS/c1-6-4-9(17-15-6)14-10(16)13-7-2-3-12-8(11)5-7/h2-5H,1H3,(H2,12,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4816

(5-{[(3Z)-5-bromo-2-oxo-2,3-dihydro-1H-indol-3-ylid...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(Br)cc23)c1C Show InChI InChI=1S/C22H27BrN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | 22 |
SUGEN, Inc.
| Assay Description
The assays were performed in 96-well microtiter plates that had been coated with a polyGluTyr peptide. Negative control wells received buffer alone w... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Probable global transcription activator SNF2L2
(Homo sapiens (Human)) | BDBM50469321

(CHEMBL4286757)Show InChI InChI=1S/C10H9ClN4OS/c1-6-4-9(17-15-6)14-10(16)13-7-2-3-12-8(11)5-7/h2-5H,1H3,(H2,12,13,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... |
J Med Chem 61: 10155-10172 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01318
BindingDB Entry DOI: 10.7270/Q2TH8QDB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4813

(N-[2-(diethylamino)ethyl]-2,4-dimethyl-5-{[(3Z)-2-...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccccc23)c1C Show InChI InChI=1S/C22H28N4O2/c1-5-26(6-2)12-11-23-22(28)20-14(3)19(24-15(20)4)13-17-16-9-7-8-10-18(16)25-21(17)27/h7-10,13,24H,5-6,11-12H2,1-4H3,(H,23,28)(H,25,27)/b17-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 22 |
SUGEN, Inc.
| Assay Description
The assays were performed in 96-well microtiter plates that had been coated with a polyGluTyr peptide. Negative control wells received buffer alone w... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data