

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50212323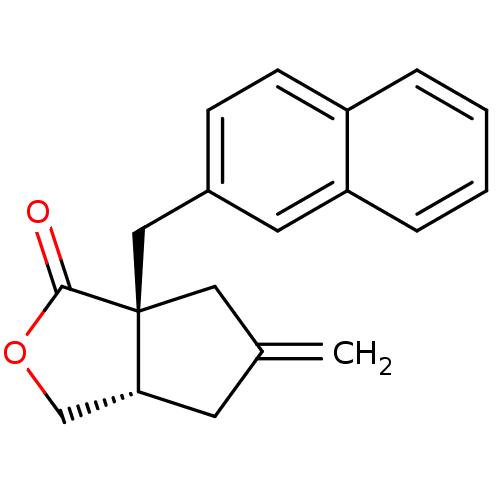 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 Curated by PDSP Ki Database | Mol Pharmacol 59: 965-73 (2001) BindingDB Entry DOI: 10.7270/Q2125R7R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50212323 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 Curated by PDSP Ki Database | Mol Pharmacol 59: 965-73 (2001) BindingDB Entry DOI: 10.7270/Q2125R7R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50212323 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 Curated by PDSP Ki Database | Mol Pharmacol 59: 965-73 (2001) BindingDB Entry DOI: 10.7270/Q2125R7R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92912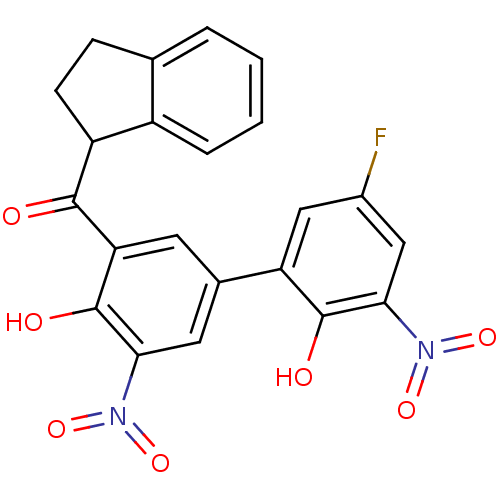 (Aryl 1-indanylketone, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 520 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92915 (Aryl 1-indanylketone, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92914 (Aryl 1-indanylketone, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92915 (Aryl 1-indanylketone, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.42E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.28E+3 | -27.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92914 (Aryl 1-indanylketone, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | -27.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92916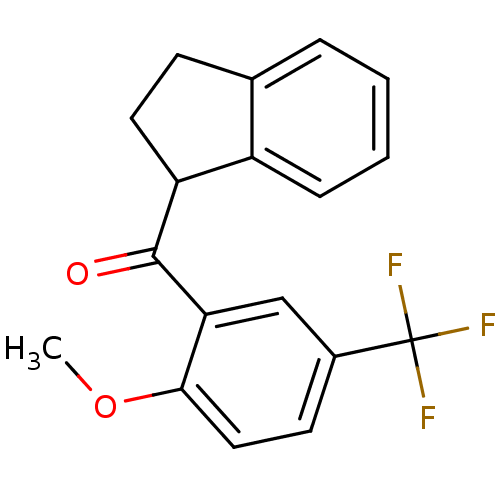 (Aryl 1-indanylketone, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50212323 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 Curated by PDSP Ki Database | Mol Pharmacol 59: 965-73 (2001) BindingDB Entry DOI: 10.7270/Q2125R7R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50212323 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 Curated by PDSP Ki Database | Mol Pharmacol 59: 965-73 (2001) BindingDB Entry DOI: 10.7270/Q2125R7R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50212323 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 Curated by PDSP Ki Database | Mol Pharmacol 59: 965-73 (2001) BindingDB Entry DOI: 10.7270/Q2125R7R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92918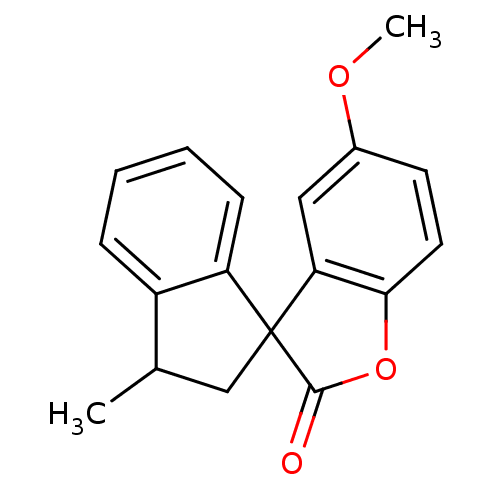 (Benzofuranone, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+4 | -24.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase-like 1 (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92920 (Benzofuranone, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30E+4 | -22.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92916 (Aryl 1-indanylketone, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92917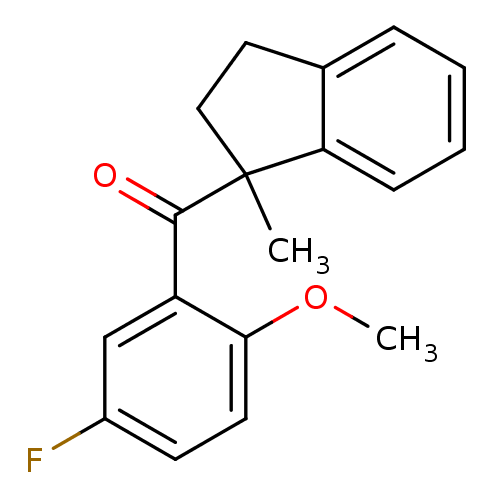 (Aryl 1-indanylketone, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92918 (Benzofuranone, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92919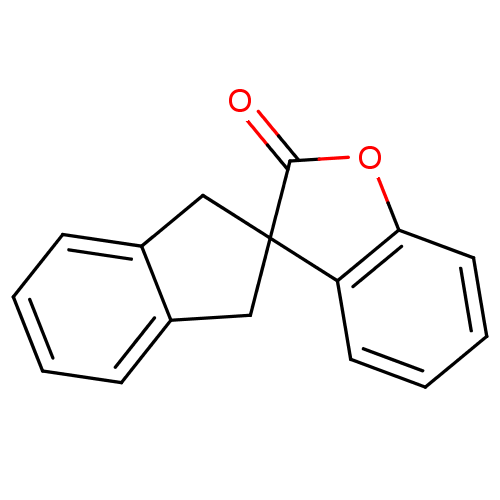 (Benzofuranone, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92920 (Benzofuranone, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase C (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92919 (Benzofuranone, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92917 (Aryl 1-indanylketone, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase H (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase C (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase-like 1 (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase H (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470573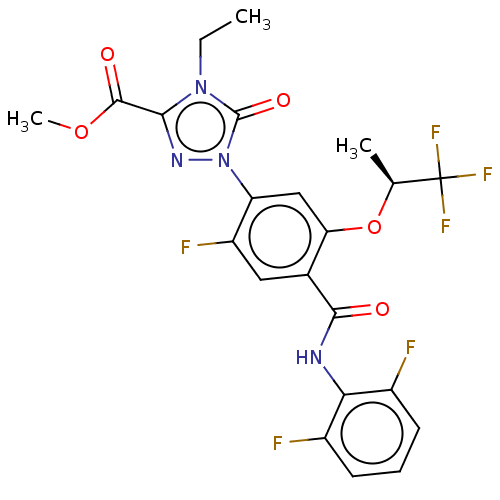 (US10815215, Example 233 | US11130745, Example 233 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50043839 ((E)-6-[2-(4-Fluoro-benzenesulfonylamino)-indan-5-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition test of thromboxane A2 synthetase in human gel-filtered platelets. | J Med Chem 37: 26-39 (1994) BindingDB Entry DOI: 10.7270/Q2SB44T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470358 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470561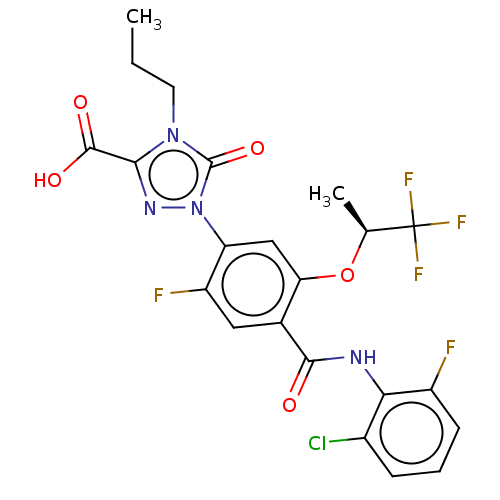 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470358 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470358 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470561 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085135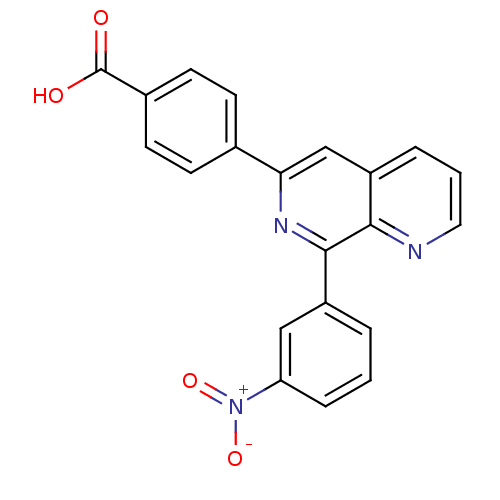 (4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470561 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 9 (Homo sapiens (Human)) | BDBM458963 ((4-{[2-(4-Bromophenyl)imidazo[,2-a]pyridin-3-yl]me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The investigations on the inhibition of the recombinant TASK-1 and TASK-3 channels were carried out using stably transfected CHO cells. Here, the com... | US Patent US10759794 (2020) BindingDB Entry DOI: 10.7270/Q2KK9FW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 9 (Homo sapiens (Human)) | BDBM532051 (3-{[2-(4-Chlorophenyl)imidazo[1,2-a]pyridin-3-yl]m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The investigations on the inhibition of the recombinant TASK-1 and TASK-3 channels were conducted using stably transfected CHO cells. The compounds o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 9 (Homo sapiens (Human)) | BDBM532241 (US11208422, Example 82 | US11208422, Example 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The investigations on the inhibition of the recombinant TASK-1 and TASK-3 channels were conducted using stably transfected CHO cells. The compounds o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470552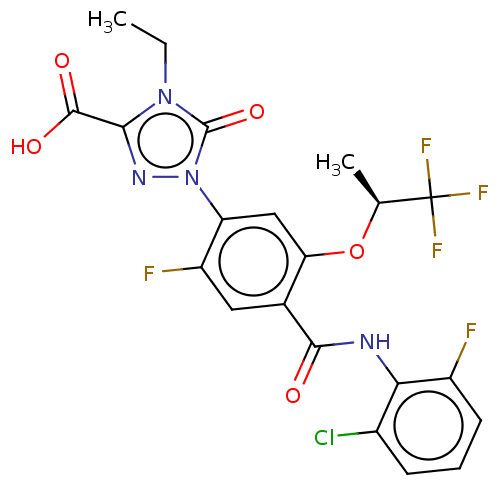 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470552 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 9 (Homo sapiens (Human)) | BDBM515603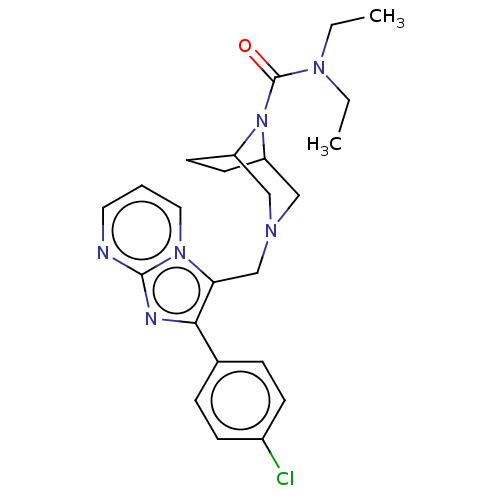 (US11098063, Example 109) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The investigations on the inhibition of the recombinant TASK-1 and TASK-3 channels were conducted using stably transfected CHO cells. The compounds a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470552 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470555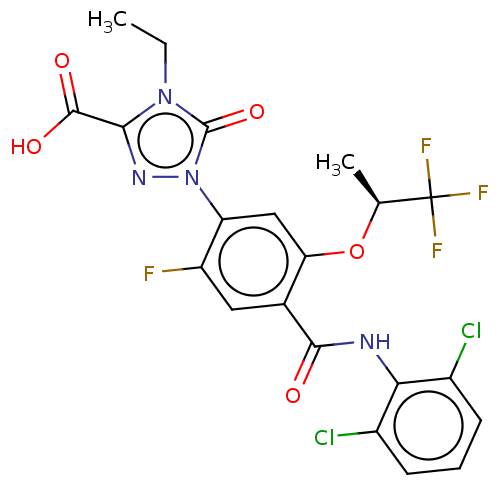 (1-(4-[(2,6-dichlorophenyl)carbamoyl]-2-fluoro-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470555 (1-(4-[(2,6-dichlorophenyl)carbamoyl]-2-fluoro-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2371 total ) | Next | Last >> |