

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (Rattus norvegicus (rat)) | BDBM364425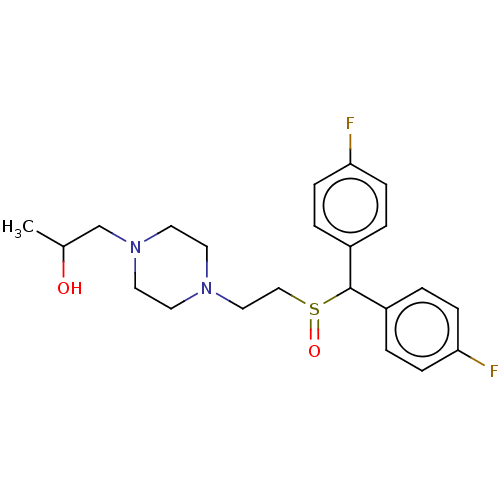 (US10913711, Compound 10d | US11555013, Compound 10...) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Norepinephrine Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, fron... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7TRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM364427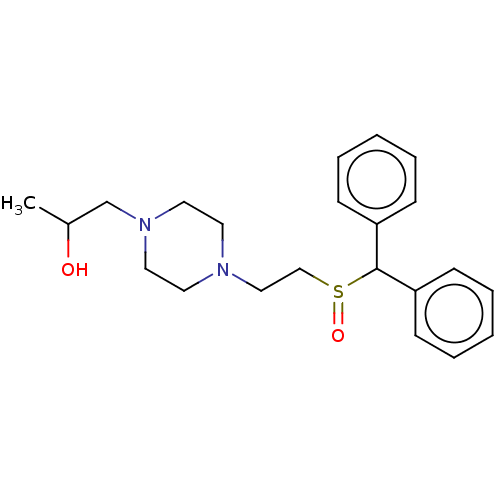 (US10913711, Compound 10a | US11555013, Compound 10...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serotonin Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, midbrain ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7TRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus (rat)) | BDBM364427 (US10913711, Compound 10a | US11555013, Compound 10...) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Norepinephrine Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, fron... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7TRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176988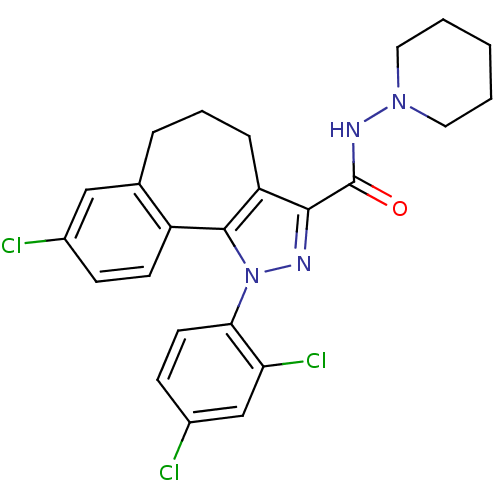 (8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | J Med Chem 51: 3526-39 (2008) Article DOI: 10.1021/jm8000778 BindingDB Entry DOI: 10.7270/Q29K4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50111903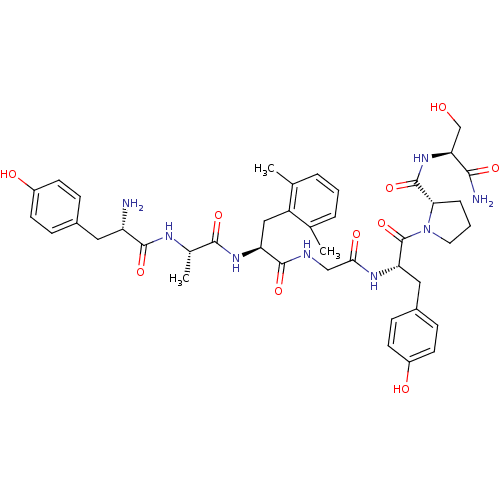 ((S)-1-[(S)-2-{2-[(S)-2-{(S)-2-[(S)-2-Amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.000540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor mu 1 using [3H]-DAMGO in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423789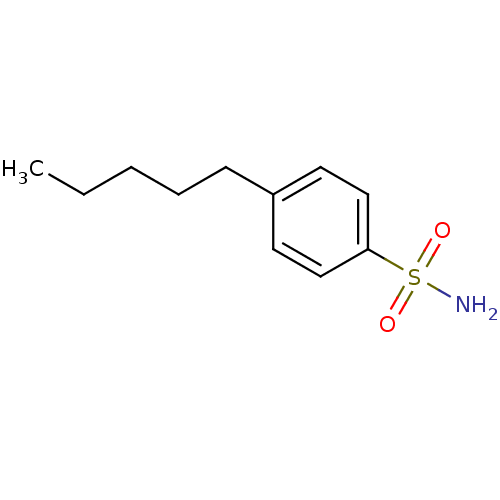 (4-Pentyl-Benzenesulfonamide | 4-Pentylbenzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423789 (4-Pentyl-Benzenesulfonamide | 4-Pentylbenzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50111905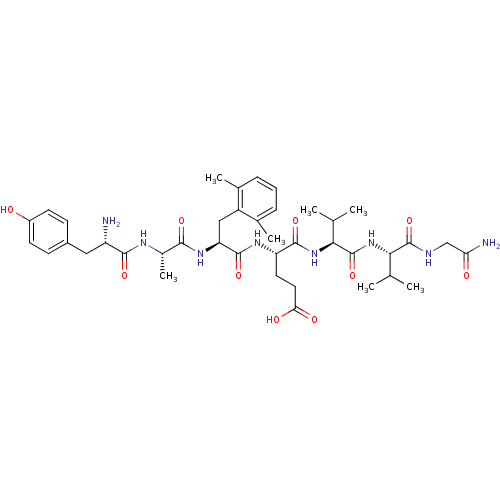 ((S)-4-[(S)-2-{(S)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 using [3H]-DT in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50295070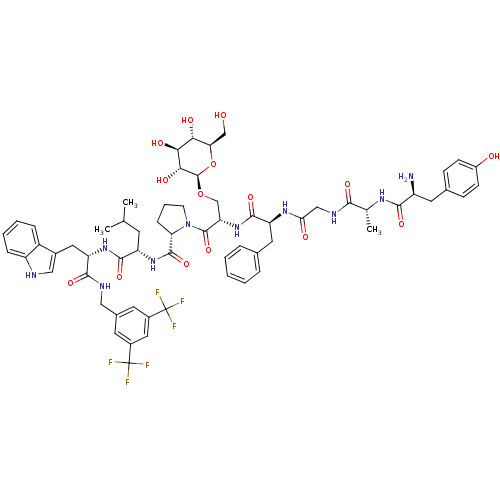 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane | J Med Chem 52: 5164-75 (2010) Article DOI: 10.1021/jm900473p BindingDB Entry DOI: 10.7270/Q2MC90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50295070 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane | J Med Chem 52: 5164-75 (2010) Article DOI: 10.1021/jm900473p BindingDB Entry DOI: 10.7270/Q2MC90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50180775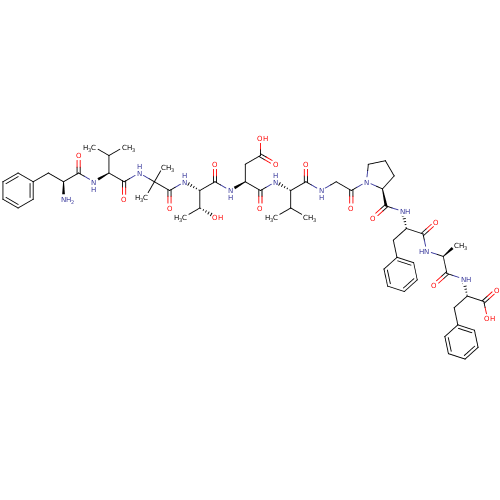 (CHEMBL386763 | FV-Aib-TDVGPFAF | [Aib29,Asp31,Pro3...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells | J Med Chem 49: 616-24 (2006) Article DOI: 10.1021/jm050613s BindingDB Entry DOI: 10.7270/Q280526H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930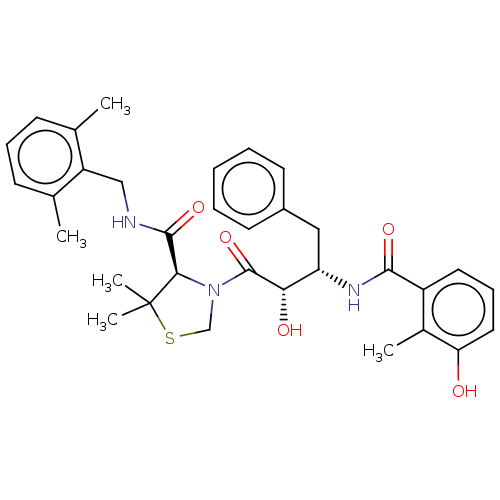 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468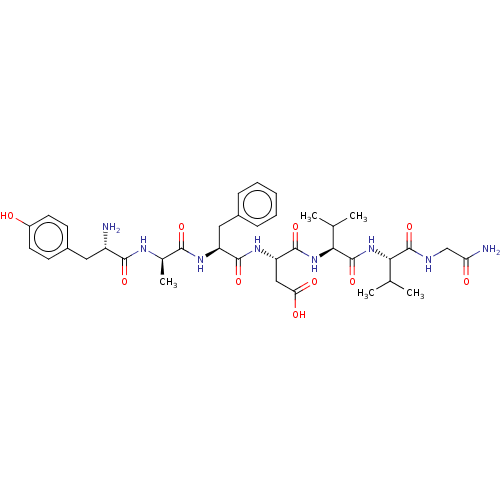 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 using [3H]-DT in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50295069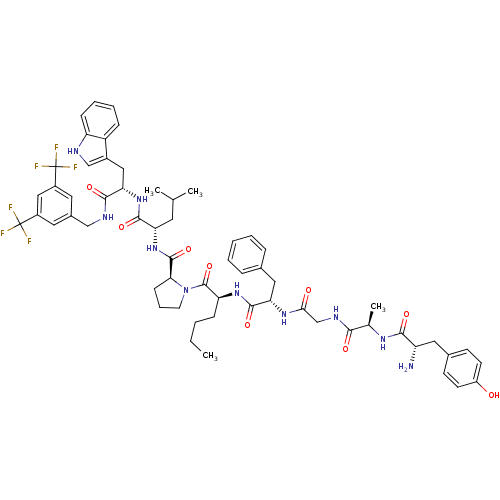 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane | J Med Chem 52: 5164-75 (2010) Article DOI: 10.1021/jm900473p BindingDB Entry DOI: 10.7270/Q2MC90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50295069 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane | J Med Chem 52: 5164-75 (2010) Article DOI: 10.1021/jm900473p BindingDB Entry DOI: 10.7270/Q2MC90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50105033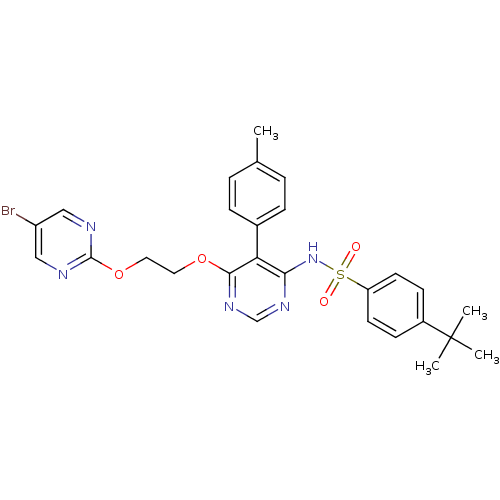 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human CD73 | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423788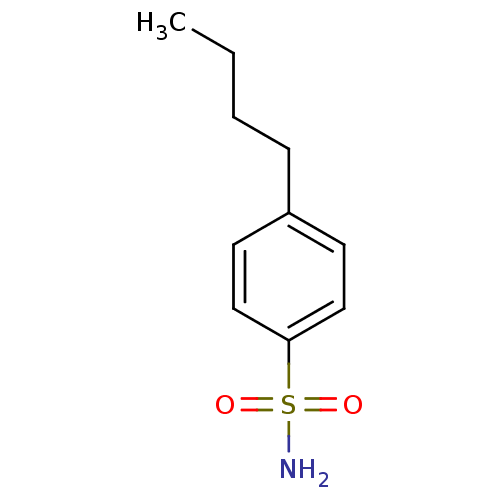 (4-Butyl-Benzenesulfonamide | 4-Butylbenzenesulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423788 (4-Butyl-Benzenesulfonamide | 4-Butylbenzenesulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50323869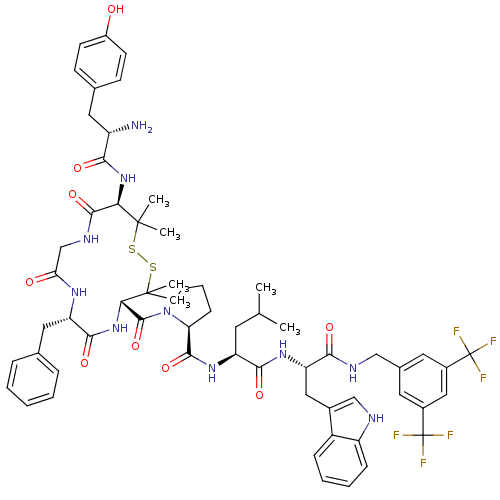 ((S)-1-((4S,7S,13R)-13-((S)-2-amino-3-(4-hydroxyphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P frome human NK1 receptor | J Med Chem 53: 5491-501 (2010) Article DOI: 10.1021/jm100157m BindingDB Entry DOI: 10.7270/Q21N81BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50323869 ((S)-1-((4S,7S,13R)-13-((S)-2-amino-3-(4-hydroxyphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P frome human NK1 receptor | J Med Chem 53: 5491-501 (2010) Article DOI: 10.1021/jm100157m BindingDB Entry DOI: 10.7270/Q21N81BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50180778 (CHEMBL2371891 | FV-Hyp-TDVGPFAF) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells | J Med Chem 49: 616-24 (2006) Article DOI: 10.1021/jm050613s BindingDB Entry DOI: 10.7270/Q280526H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50264406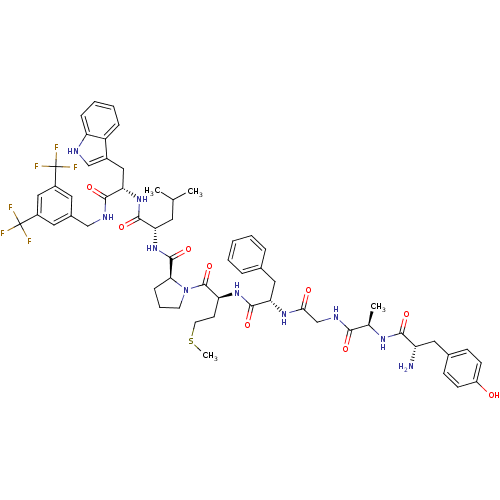 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P frome human NK1 receptor | J Med Chem 53: 5491-501 (2010) Article DOI: 10.1021/jm100157m BindingDB Entry DOI: 10.7270/Q21N81BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50049912 (7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase enzyme from Candida albicans | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50341318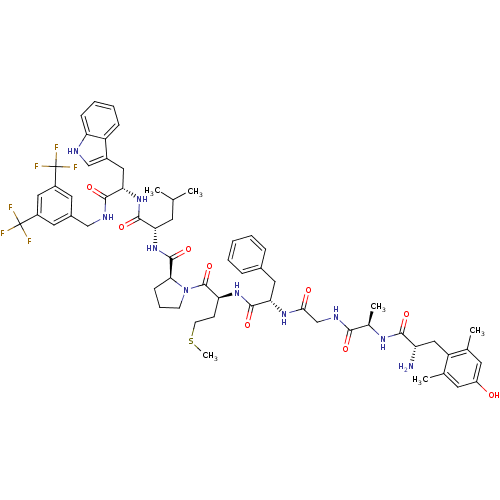 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | J Med Chem 54: 2029-38 (2011) Article DOI: 10.1021/jm101023r BindingDB Entry DOI: 10.7270/Q2862GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50341318 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | J Med Chem 54: 2029-38 (2011) Article DOI: 10.1021/jm101023r BindingDB Entry DOI: 10.7270/Q2862GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114773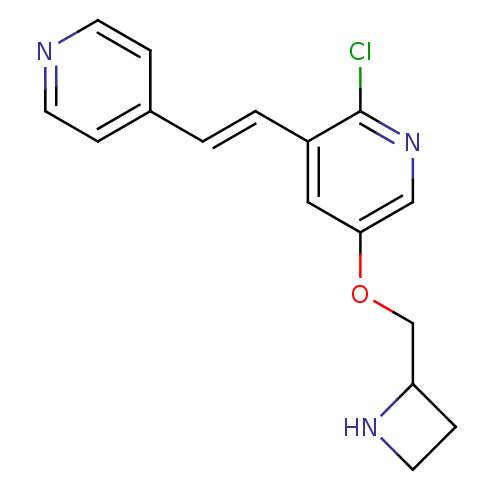 (5-(Azetidin-2-ylmethoxy)-2-chloro-3-(2-pyridin-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Drosophila | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170654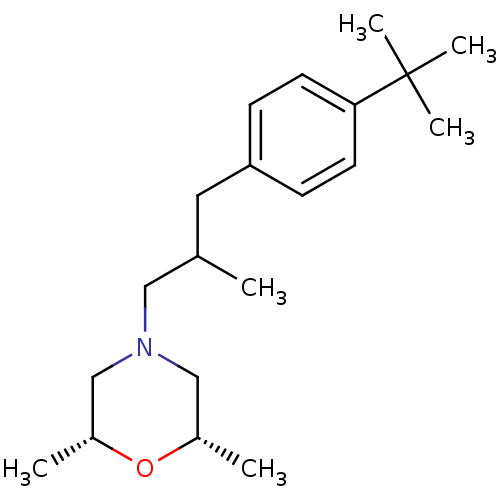 ((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243536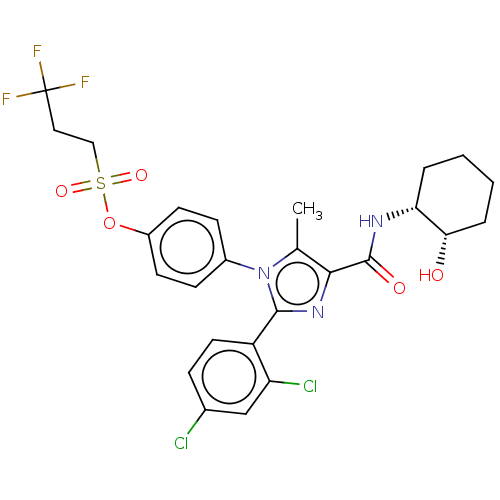 (CHEMBL4062749) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243536 (CHEMBL4062749) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Curated by PDSP Ki Database | Synapse 38: 438-49 (2000) Article DOI: 10.1002/1098-2396(20001215)38:4 BindingDB Entry DOI: 10.7270/Q2SN07HX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Myzus persicae | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114771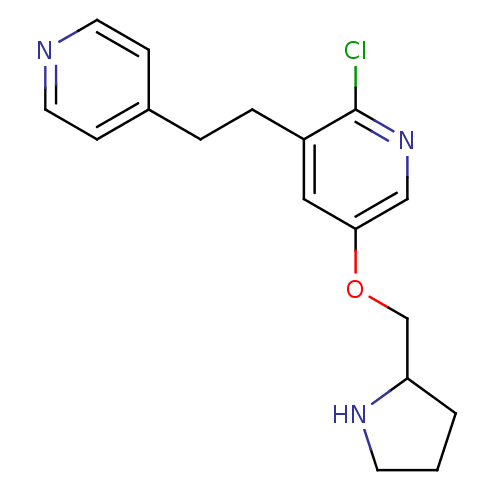 (2-Chloro-3-(2-pyridin-4-yl-ethyl)-5-(pyrrolidin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029704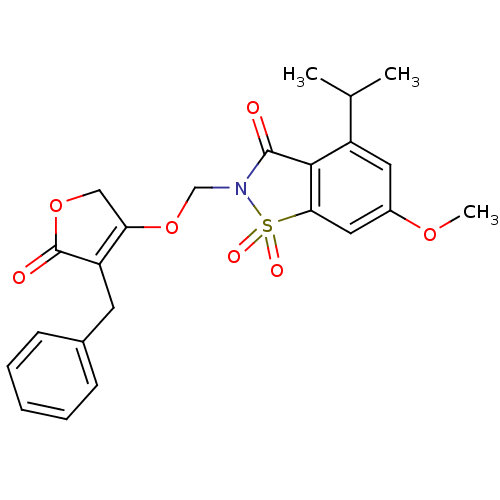 (2-(4-Benzyl-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114774 (2-Chloro-3-(2-pyridin-4-yl-vinyl)-5-(pyrrolidin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Drosophila | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50369953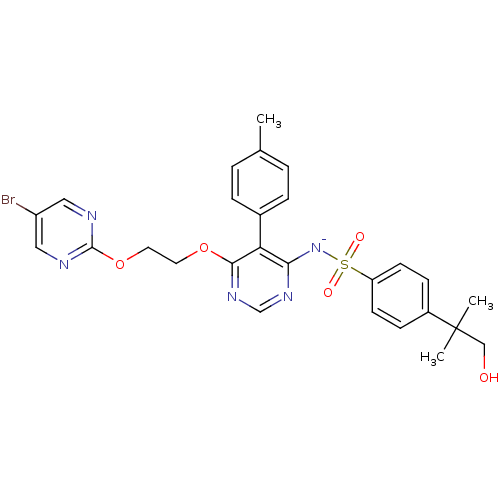 (CHEMBL1627022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029709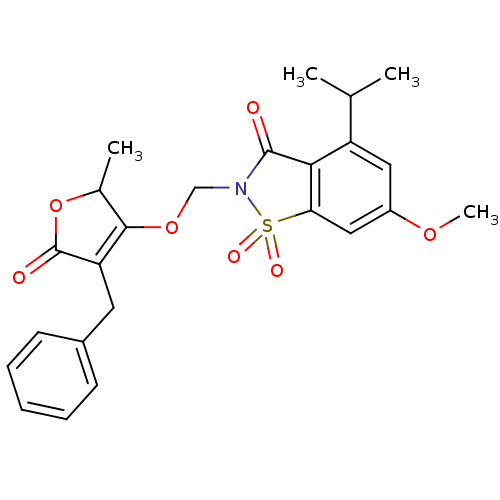 (2-(4-Benzyl-2-methyl-5-oxo-2,5-dihydro-furan-3-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368742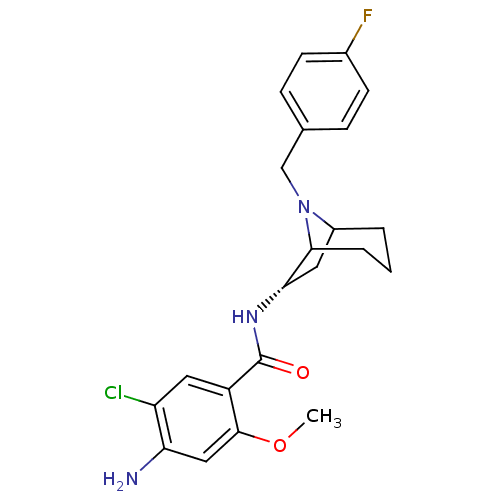 (CHEMBL1169525) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021919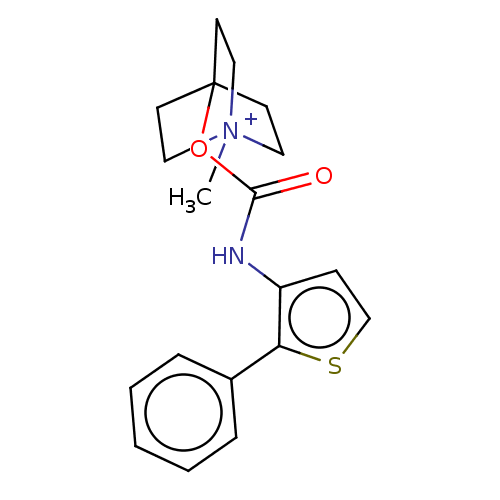 (CHEMBL3298595) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50021919 (CHEMBL3298595) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50062162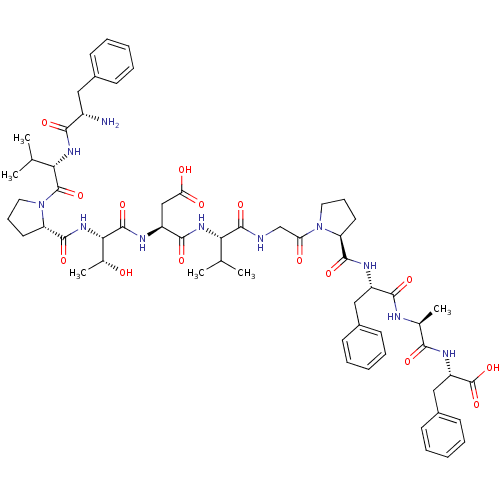 (CHEMBL264010 | FVPTDVGPFAF) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells | J Med Chem 49: 616-24 (2006) Article DOI: 10.1021/jm050613s BindingDB Entry DOI: 10.7270/Q280526H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50180781 (CHEMBL2371890 | FV-Tic-TDVGPFAF) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells | J Med Chem 49: 616-24 (2006) Article DOI: 10.1021/jm050613s BindingDB Entry DOI: 10.7270/Q280526H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50042730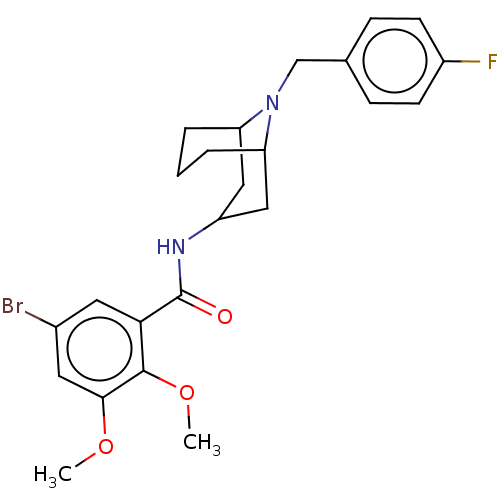 (4-Amino-5-chloro-N-[5-(4-fluoro-benzyl)-octahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029710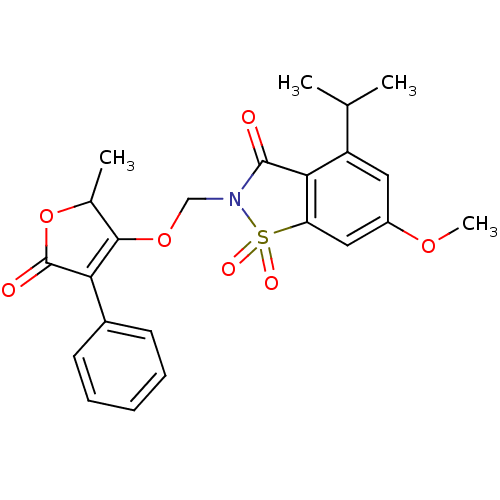 (4-Isopropyl-6-methoxy-2-(2-methyl-5-oxo-4-phenyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126000 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114195 total ) | Next | Last >> |