 Found 968 hits with Last Name = 'ma' and Initial = 'tw'
Found 968 hits with Last Name = 'ma' and Initial = 'tw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic receptor M1
(Bos taurus) | BDBM50055976
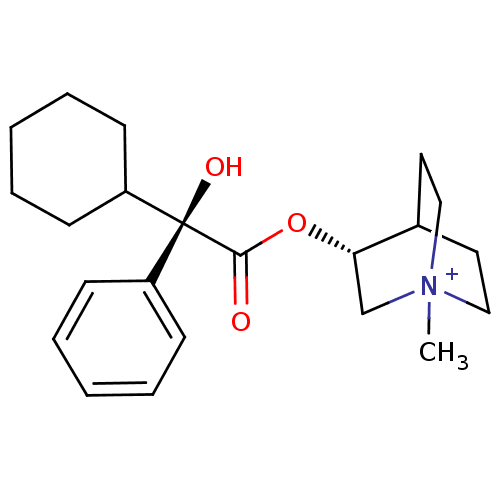
((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 |wU:12.14,wD:12.13,7.10,TLB:9:7:2.3:6.5,(13.94,-4.53,;13.94,-2.99,;14.71,-1.66,;13.24,-1.26,;14.01,.06,;15.34,-.72,;15.34,-2.26,;12.68,-.71,;12.68,-2.25,;11.35,.06,;10.02,-.71,;10.02,-2.26,;8.66,.09,;9.46,1.42,;7.33,.86,;6,.09,;4.67,.86,;4.67,2.4,;6,3.17,;7.33,2.4,;7.89,-1.27,;6.35,-1.27,;5.58,-2.6,;6.35,-3.94,;7.89,-3.95,;8.66,-2.62,)| Show InChI InChI=1S/C22H32NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2,4-5,8-9,17,19-20,25H,3,6-7,10-16H2,1H3/q+1/t17?,20-,22-,23?/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M1 was determined in calf brain membrane |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50055978
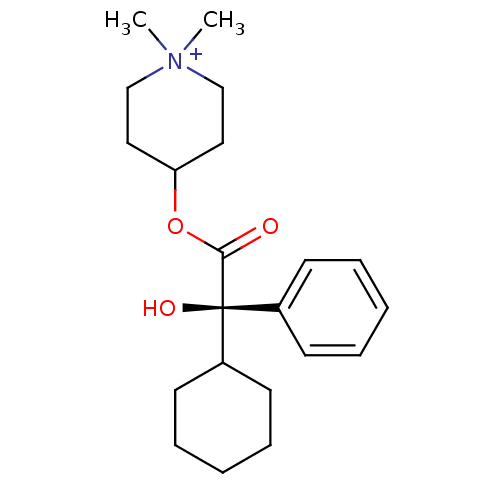
(4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...)Show SMILES C[N+]1(C)CCC(CC1)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 Show InChI InChI=1S/C21H32NO3/c1-22(2)15-13-19(14-16-22)25-20(23)21(24,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3,5-6,9-10,18-19,24H,4,7-8,11-16H2,1-2H3/q+1/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic receptor M1
(Bos taurus) | BDBM50055978

(4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...)Show SMILES C[N+]1(C)CCC(CC1)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 Show InChI InChI=1S/C21H32NO3/c1-22(2)15-13-19(14-16-22)25-20(23)21(24,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3,5-6,9-10,18-19,24H,4,7-8,11-16H2,1-2H3/q+1/t21-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M1 was determined in calf brain membrane |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50055976

((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 |wU:12.14,wD:12.13,7.10,TLB:9:7:2.3:6.5,(13.94,-4.53,;13.94,-2.99,;14.71,-1.66,;13.24,-1.26,;14.01,.06,;15.34,-.72,;15.34,-2.26,;12.68,-.71,;12.68,-2.25,;11.35,.06,;10.02,-.71,;10.02,-2.26,;8.66,.09,;9.46,1.42,;7.33,.86,;6,.09,;4.67,.86,;4.67,2.4,;6,3.17,;7.33,2.4,;7.89,-1.27,;6.35,-1.27,;5.58,-2.6,;6.35,-3.94,;7.89,-3.95,;8.66,-2.62,)| Show InChI InChI=1S/C22H32NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2,4-5,8-9,17,19-20,25H,3,6-7,10-16H2,1H3/q+1/t17?,20-,22-,23?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor
(Cavia porcellus) | BDBM50055978

(4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...)Show SMILES C[N+]1(C)CCC(CC1)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 Show InChI InChI=1S/C21H32NO3/c1-22(2)15-13-19(14-16-22)25-20(23)21(24,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3,5-6,9-10,18-19,24H,4,7-8,11-16H2,1-2H3/q+1/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M3 was determined in guinea pig ileum |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073585
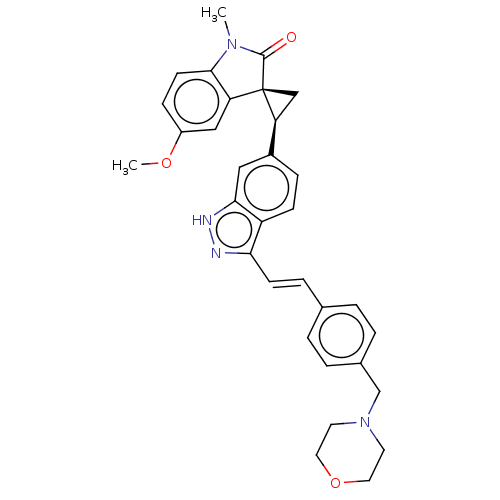
(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50512456
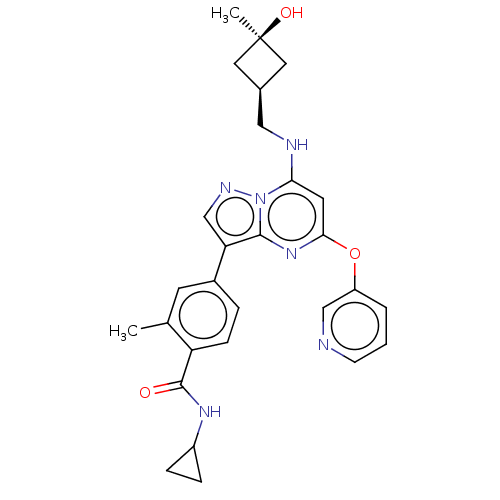
(CHEMBL4469414)Show SMILES Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NC[C@H]3C[C@@](C)(O)C3)cc(Oc3cccnc3)nc12 |r,wU:20.21,22.25,(48.46,-32.37,;49.53,-31.26,;49.1,-29.78,;50.17,-28.67,;51.66,-29.03,;52.09,-30.5,;51.03,-31.63,;51.46,-33.1,;50.39,-34.22,;52.95,-33.47,;54.02,-32.36,;55.49,-31.92,;54.38,-30.85,;49.74,-27.2,;50.69,-25.98,;49.82,-24.7,;48.34,-25.14,;47.04,-24.33,;47.09,-22.79,;45.78,-21.98,;44.42,-22.71,;43.98,-24.18,;42.51,-23.74,;41.01,-23.33,;41.41,-24.82,;42.95,-22.26,;45.69,-25.06,;45.64,-26.6,;44.29,-27.33,;44.25,-28.87,;42.89,-29.59,;42.85,-31.13,;44.16,-31.94,;45.52,-31.2,;45.56,-29.66,;46.95,-27.4,;48.29,-26.68,)| Show InChI InChI=1S/C28H30N6O3/c1-17-10-19(5-8-22(17)27(35)32-20-6-7-20)23-16-31-34-24(30-14-18-12-28(2,36)13-18)11-25(33-26(23)34)37-21-4-3-9-29-15-21/h3-5,8-11,15-16,18,20,30,36H,6-7,12-14H2,1-2H3,(H,32,35)/t18-,28+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... |
ACS Med Chem Lett 7: 671-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00485
BindingDB Entry DOI: 10.7270/Q2K35Z46 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50512456

(CHEMBL4469414)Show SMILES Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NC[C@H]3C[C@@](C)(O)C3)cc(Oc3cccnc3)nc12 |r,wU:20.21,22.25,(48.46,-32.37,;49.53,-31.26,;49.1,-29.78,;50.17,-28.67,;51.66,-29.03,;52.09,-30.5,;51.03,-31.63,;51.46,-33.1,;50.39,-34.22,;52.95,-33.47,;54.02,-32.36,;55.49,-31.92,;54.38,-30.85,;49.74,-27.2,;50.69,-25.98,;49.82,-24.7,;48.34,-25.14,;47.04,-24.33,;47.09,-22.79,;45.78,-21.98,;44.42,-22.71,;43.98,-24.18,;42.51,-23.74,;41.01,-23.33,;41.41,-24.82,;42.95,-22.26,;45.69,-25.06,;45.64,-26.6,;44.29,-27.33,;44.25,-28.87,;42.89,-29.59,;42.85,-31.13,;44.16,-31.94,;45.52,-31.2,;45.56,-29.66,;46.95,-27.4,;48.29,-26.68,)| Show InChI InChI=1S/C28H30N6O3/c1-17-10-19(5-8-22(17)27(35)32-20-6-7-20)23-16-31-34-24(30-14-18-12-28(2,36)13-18)11-25(33-26(23)34)37-21-4-3-9-29-15-21/h3-5,8-11,15-16,18,20,30,36H,6-7,12-14H2,1-2H3,(H,32,35)/t18-,28+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... |
ACS Med Chem Lett 7: 671-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00485
BindingDB Entry DOI: 10.7270/Q2K35Z46 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50055976

((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 |wU:12.14,wD:12.13,7.10,TLB:9:7:2.3:6.5,(13.94,-4.53,;13.94,-2.99,;14.71,-1.66,;13.24,-1.26,;14.01,.06,;15.34,-.72,;15.34,-2.26,;12.68,-.71,;12.68,-2.25,;11.35,.06,;10.02,-.71,;10.02,-2.26,;8.66,.09,;9.46,1.42,;7.33,.86,;6,.09,;4.67,.86,;4.67,2.4,;6,3.17,;7.33,2.4,;7.89,-1.27,;6.35,-1.27,;5.58,-2.6,;6.35,-3.94,;7.89,-3.95,;8.66,-2.62,)| Show InChI InChI=1S/C22H32NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2,4-5,8-9,17,19-20,25H,3,6-7,10-16H2,1H3/q+1/t17?,20-,22-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor
(Cavia porcellus) | BDBM50055976

((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 |wU:12.14,wD:12.13,7.10,TLB:9:7:2.3:6.5,(13.94,-4.53,;13.94,-2.99,;14.71,-1.66,;13.24,-1.26,;14.01,.06,;15.34,-.72,;15.34,-2.26,;12.68,-.71,;12.68,-2.25,;11.35,.06,;10.02,-.71,;10.02,-2.26,;8.66,.09,;9.46,1.42,;7.33,.86,;6,.09,;4.67,.86,;4.67,2.4,;6,3.17,;7.33,2.4,;7.89,-1.27,;6.35,-1.27,;5.58,-2.6,;6.35,-3.94,;7.89,-3.95,;8.66,-2.62,)| Show InChI InChI=1S/C22H32NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2,4-5,8-9,17,19-20,25H,3,6-7,10-16H2,1H3/q+1/t17?,20-,22-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M3 was determined in guinea pig ileum |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50369230
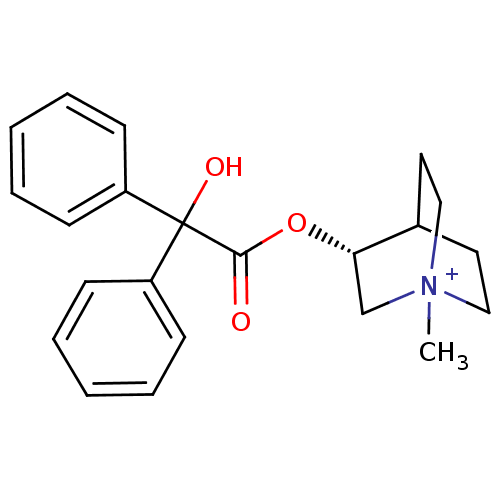
(CHEMBL1398637 | CLIDINIUM)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)C(O)(c1ccccc1)c1ccccc1 |wU:7.10,THB:9:7:2.3:6.5,(-1.62,-6.55,;-1.6,-5.01,;-3.17,-5.68,;-3.37,-4.27,;-1.87,-3.61,;-1.8,-1.94,;-1.34,-3.07,;-.49,-4.23,;-.2,-5.66,;.72,-3.27,;.49,-1.75,;-.95,-1.19,;1.69,-.79,;.73,.42,;2.9,.17,;2.67,1.69,;3.87,2.65,;5.3,2.09,;5.53,.57,;4.33,-.39,;2.65,-1.99,;4.17,-1.76,;5.13,-2.97,;4.57,-4.4,;3.05,-4.63,;2.09,-3.43,)| Show InChI InChI=1S/C22H26NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2-11,17,20,25H,12-16H2,1H3/q+1/t17?,20-,23?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50055978

(4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...)Show SMILES C[N+]1(C)CCC(CC1)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 Show InChI InChI=1S/C21H32NO3/c1-22(2)15-13-19(14-16-22)25-20(23)21(24,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3,5-6,9-10,18-19,24H,4,7-8,11-16H2,1-2H3/q+1/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073587
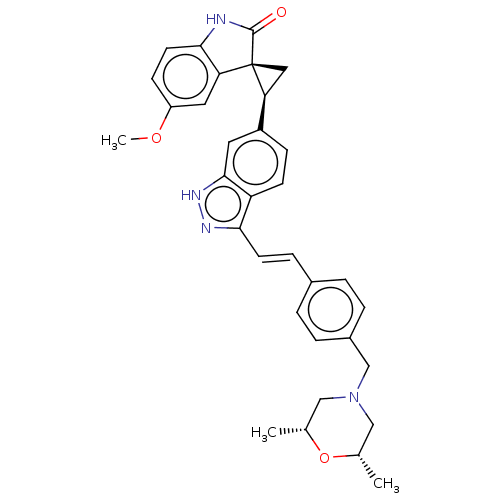
(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Muscarinic receptor M1
(Bos taurus) | BDBM50452855

(Isoptpo Hyoscine | Scopolamine)Show SMILES [H][C@@]12O[C@@]1([H])[C@]1([H])C[C@@H](C[C@@]2([H])N1C)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:14:8:12:1.3,2:1:12:8.7.9,2:3:12:8.7.9| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11-,12-,13-,14+,15+,16+/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M1 was determined in calf brain membrane |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50369230

(CHEMBL1398637 | CLIDINIUM)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)C(O)(c1ccccc1)c1ccccc1 |wU:7.10,THB:9:7:2.3:6.5,(-1.62,-6.55,;-1.6,-5.01,;-3.17,-5.68,;-3.37,-4.27,;-1.87,-3.61,;-1.8,-1.94,;-1.34,-3.07,;-.49,-4.23,;-.2,-5.66,;.72,-3.27,;.49,-1.75,;-.95,-1.19,;1.69,-.79,;.73,.42,;2.9,.17,;2.67,1.69,;3.87,2.65,;5.3,2.09,;5.53,.57,;4.33,-.39,;2.65,-1.99,;4.17,-1.76,;5.13,-2.97,;4.57,-4.4,;3.05,-4.63,;2.09,-3.43,)| Show InChI InChI=1S/C22H26NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2-11,17,20,25H,12-16H2,1H3/q+1/t17?,20-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50452855

(Isoptpo Hyoscine | Scopolamine)Show SMILES [H][C@@]12O[C@@]1([H])[C@]1([H])C[C@@H](C[C@@]2([H])N1C)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:14:8:12:1.3,2:1:12:8.7.9,2:3:12:8.7.9| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11-,12-,13-,14+,15+,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor
(Cavia porcellus) | BDBM50369230

(CHEMBL1398637 | CLIDINIUM)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)C(O)(c1ccccc1)c1ccccc1 |wU:7.10,THB:9:7:2.3:6.5,(-1.62,-6.55,;-1.6,-5.01,;-3.17,-5.68,;-3.37,-4.27,;-1.87,-3.61,;-1.8,-1.94,;-1.34,-3.07,;-.49,-4.23,;-.2,-5.66,;.72,-3.27,;.49,-1.75,;-.95,-1.19,;1.69,-.79,;.73,.42,;2.9,.17,;2.67,1.69,;3.87,2.65,;5.3,2.09,;5.53,.57,;4.33,-.39,;2.65,-1.99,;4.17,-1.76,;5.13,-2.97,;4.57,-4.4,;3.05,-4.63,;2.09,-3.43,)| Show InChI InChI=1S/C22H26NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2-11,17,20,25H,12-16H2,1H3/q+1/t17?,20-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M3 was determined in guinea pig ileum |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50081537
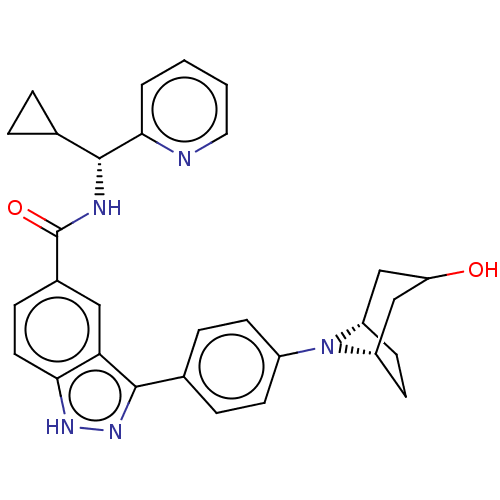
(CHEMBL3422092)Show SMILES [H][C@]12CC[C@@]([H])(CC(O)C1)N2c1ccc(cc1)-c1n[nH]c2ccc(cc12)C(=O)N[C@H](C1CC1)c1ccccn1 |r,@:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate by Lineweaver-Burk plot analysis in prese... |
J Med Chem 58: 3366-92 (2015)
Article DOI: 10.1021/jm501740a
BindingDB Entry DOI: 10.7270/Q2Q52RCN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073586
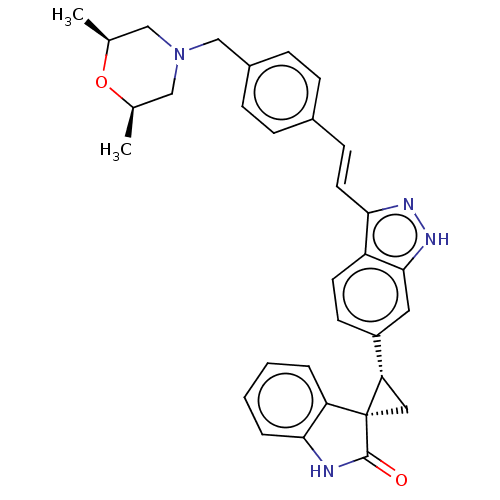
(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50183019
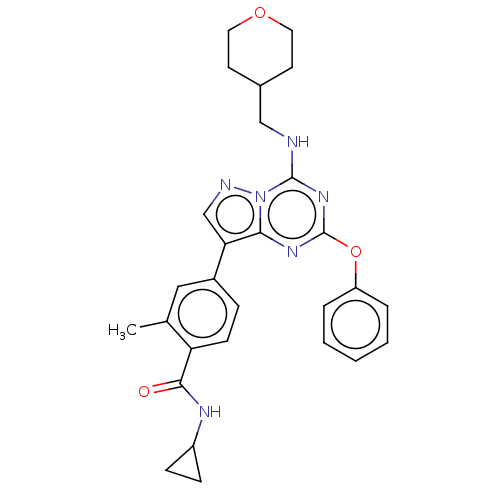
(CHEMBL3819210)Show SMILES Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NCC3CCOCC3)nc(Oc3ccccc3)nc12 Show InChI InChI=1S/C28H30N6O3/c1-18-15-20(7-10-23(18)26(35)31-21-8-9-21)24-17-30-34-25(24)32-28(37-22-5-3-2-4-6-22)33-27(34)29-16-19-11-13-36-14-12-19/h2-7,10,15,17,19,21H,8-9,11-14,16H2,1H3,(H,31,35)(H,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of TTK (unknown origin) by double reciprocal plot analysis in presence of ATP |
Bioorg Med Chem Lett 26: 3562-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.021
BindingDB Entry DOI: 10.7270/Q28G8NNB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM84745
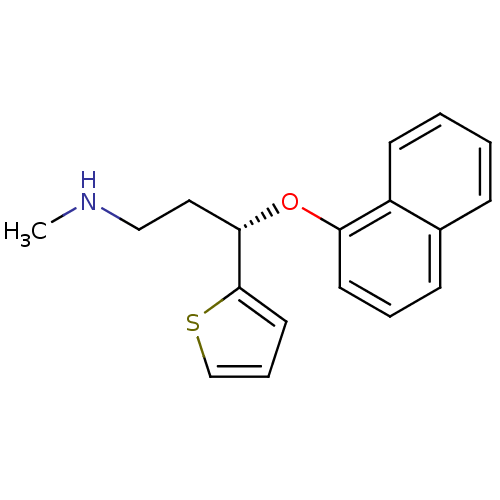
(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-paroxetine binding to cloned human serotonin (5-HT) transporter |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50148370
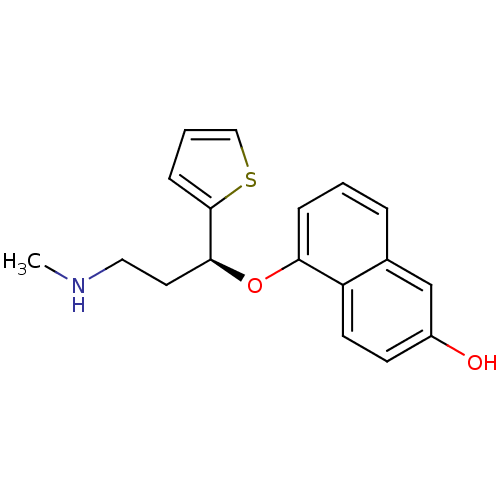
(5-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO2S/c1-19-10-9-17(18-6-3-11-22-18)21-16-5-2-4-13-12-14(20)7-8-15(13)16/h2-8,11-12,17,19-20H,9-10H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-paroxetine binding to cloned human serototnin (5-HT) transporter; Average of two experiments |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM3033

(3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0...)Show SMILES Cn1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4n(CCC#N)c3c12 Show InChI InChI=1S/C24H18N4O/c1-27-17-9-4-2-7-14(17)20-21-16(13-26-24(21)29)19-15-8-3-5-10-18(15)28(12-6-11-25)23(19)22(20)27/h2-5,7-10H,6,12-13H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50148369
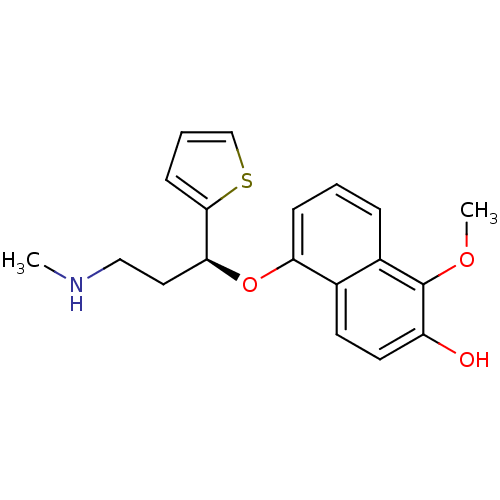
(1-Methoxy-5-((S)-3-methylamino-1-thiophen-2-yl-pro...)Show InChI InChI=1S/C19H21NO3S/c1-20-11-10-17(18-7-4-12-24-18)23-16-6-3-5-14-13(16)8-9-15(21)19(14)22-2/h3-9,12,17,20-21H,10-11H2,1-2H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-paroxetine binding to cloned human serototnin (5-HT) transporter; Average of two experiments |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM25117

(AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(\C=C\c3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM25117

(AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(\C=C\c3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) |
J Med Chem 58: 130-46 (2015)
Article DOI: 10.1021/jm500537u
BindingDB Entry DOI: 10.7270/Q2125V9W |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50452855

(Isoptpo Hyoscine | Scopolamine)Show SMILES [H][C@@]12O[C@@]1([H])[C@]1([H])C[C@@H](C[C@@]2([H])N1C)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:14:8:12:1.3,2:1:12:8.7.9,2:3:12:8.7.9| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11-,12-,13-,14+,15+,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50148370

(5-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO2S/c1-19-10-9-17(18-6-3-11-22-18)21-16-5-2-4-13-12-14(20)7-8-15(13)16/h2-8,11-12,17,19-20H,9-10H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-nisoxatine binding to cloned human norepinephrine (NE) transporter; Average of two experiments |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 7.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-nisoxatine binding to cloned human norepinephrine (NE) transporter |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50148377
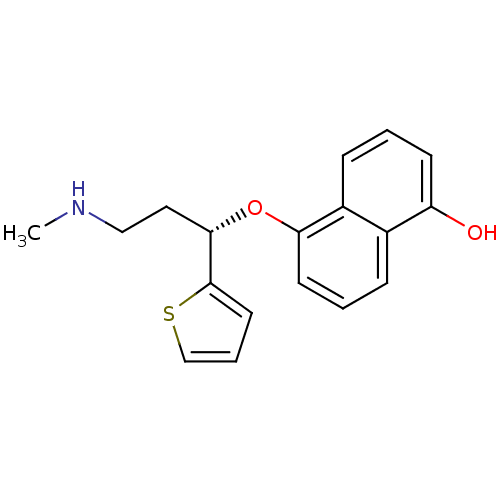
(5-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO2S/c1-19-11-10-17(18-9-4-12-22-18)21-16-8-3-5-13-14(16)6-2-7-15(13)20/h2-9,12,17,19-20H,10-11H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-paroxetine binding to cloned human serototnin (5-HT) transporter; Average of two experiments |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50426474
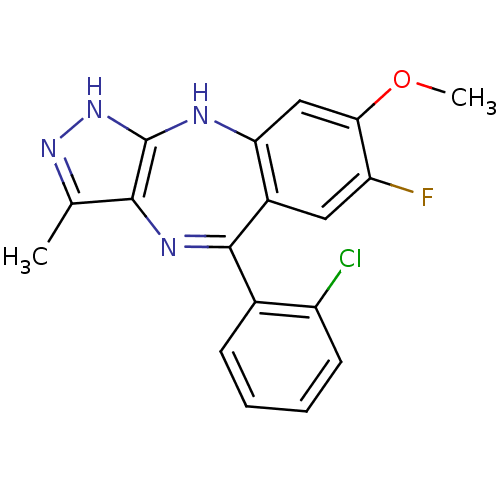
(CHEMBL1980391)Show SMILES COc1cc2Nc3[nH]nc(C)c3N=C(c3ccccc3Cl)c2cc1F |t:13| Show InChI InChI=1S/C18H14ClFN4O/c1-9-16-18(24-23-9)21-14-8-15(25-2)13(20)7-11(14)17(22-16)10-5-3-4-6-12(10)19/h3-8H,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50004205
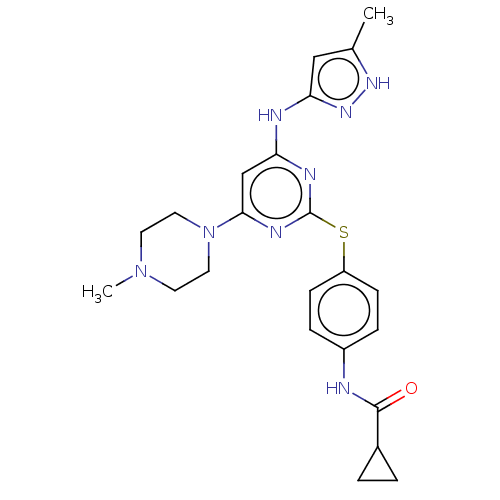
(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) |
J Med Chem 58: 130-46 (2015)
Article DOI: 10.1021/jm500537u
BindingDB Entry DOI: 10.7270/Q2125V9W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50148377

(5-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO2S/c1-19-11-10-17(18-9-4-12-22-18)21-16-8-3-5-13-14(16)6-2-7-15(13)20/h2-9,12,17,19-20H,10-11H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-nisoxatine binding to cloned human norepinephrine (NE) transporter; Average of two experiments |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50437839
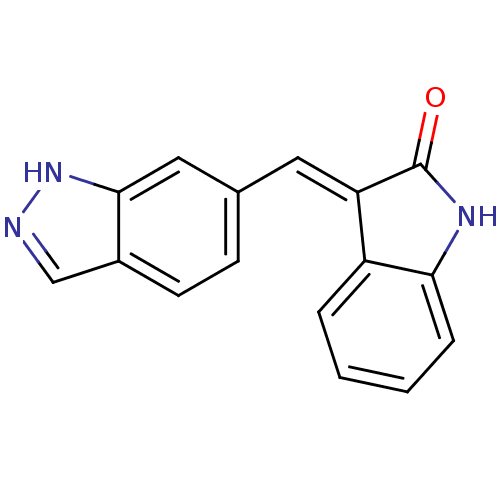
(CHEMBL2407911)Show InChI InChI=1S/C16H11N3O/c20-16-13(12-3-1-2-4-14(12)18-16)7-10-5-6-11-9-17-19-15(11)8-10/h1-9H,(H,17,19)(H,18,20)/b13-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal GST-tagged human PLK4 (1 to 391 amino acids) expressed in Escherichia coli in the presence of ATP |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50437870

(CHEMBL2407901)Show SMILES COCCOc1ccc2Nc3ncnc4[nH]cc(CN(C)CCCN(C)C(=O)COc1c2)c34 Show InChI InChI=1S/C23H30N6O4/c1-28-7-4-8-29(2)20(30)14-33-19-11-17(5-6-18(19)32-10-9-31-3)27-23-21-16(13-28)12-24-22(21)25-15-26-23/h5-6,11-12,15H,4,7-10,13-14H2,1-3H3,(H2,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50148379

(4-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO2S/c1-19-11-10-17(18-7-4-12-22-18)21-16-9-8-15(20)13-5-2-3-6-14(13)16/h2-9,12,17,19-20H,10-11H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-paroxetine binding to cloned human serototnin (5-HT) transporter; Average of two experiments |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor
(Cavia porcellus) | BDBM50176065
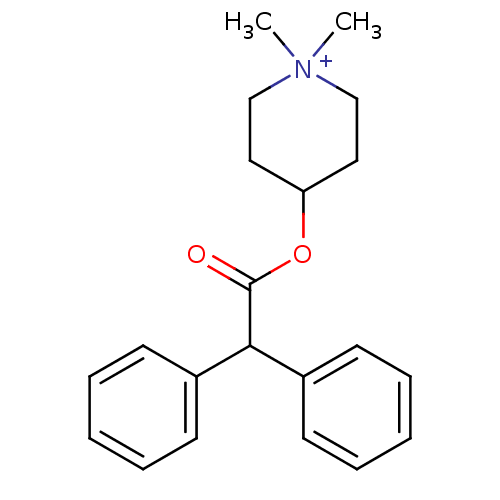
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M3 was determined in guinea pig ileum |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50148379

(4-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO2S/c1-19-11-10-17(18-7-4-12-22-18)21-16-9-8-15(20)13-5-2-3-6-14(13)16/h2-9,12,17,19-20H,10-11H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-nisoxatine binding to cloned human norepinephrine (NE) transporter; Average of two experiments |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444934
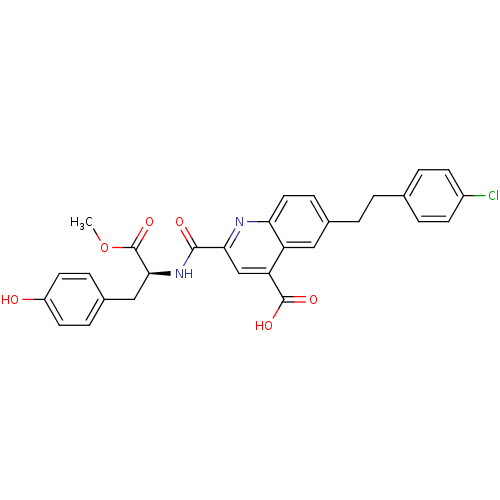
(CHEMBL3099762)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(CCc3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H25ClN2O6/c1-38-29(37)26(15-19-6-11-21(33)12-7-19)32-27(34)25-16-23(28(35)36)22-14-18(8-13-24(22)31-25)3-2-17-4-9-20(30)10-5-17/h4-14,16,26,33H,2-3,15H2,1H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50437869
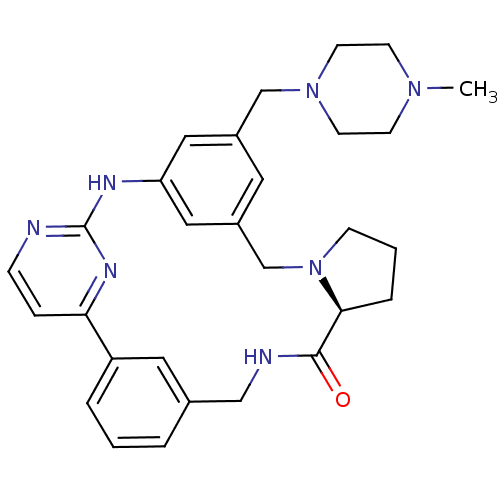
(CHEMBL2407902)Show SMILES CN1CCN(Cc2cc3CN4CCC[C@H]4C(=O)NCc4cccc(c4)-c4ccnc(Nc(c2)c3)n4)CC1 |r| Show InChI InChI=1S/C29H35N7O/c1-34-10-12-35(13-11-34)19-22-14-23-17-25(16-22)32-29-30-8-7-26(33-29)24-5-2-4-21(15-24)18-31-28(37)27-6-3-9-36(27)20-23/h2,4-5,7-8,14-17,27H,3,6,9-13,18-20H2,1H3,(H,31,37)(H,30,32,33)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444921

(CHEMBL3099750)Show SMILES CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H24ClN3O5/c1-31-27(35)25(15-19-6-11-21(34)12-7-19)33-28(36)26-16-23(29(37)38)22-14-18(8-13-24(22)32-26)3-2-17-4-9-20(30)10-5-17/h2-14,16,25,34H,15H2,1H3,(H,31,35)(H,33,36)(H,37,38)/b3-2+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444933
(CHEMBL3099763)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H23ClN2O6/c1-38-29(37)26(15-19-6-11-21(33)12-7-19)32-27(34)25-16-23(28(35)36)22-14-18(8-13-24(22)31-25)3-2-17-4-9-20(30)10-5-17/h2-14,16,26,33H,15H2,1H3,(H,32,34)(H,35,36)/b3-2+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444932
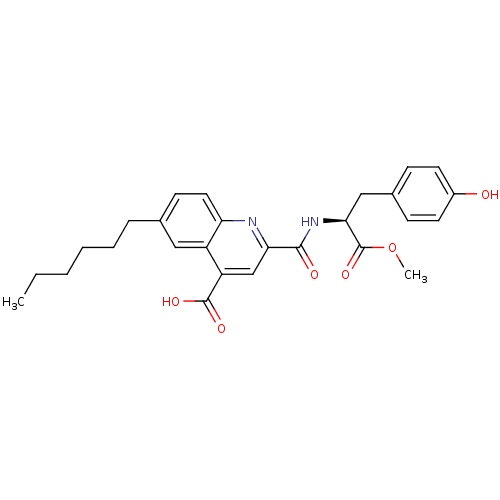
(CHEMBL3099764)Show SMILES CCCCCCc1ccc2nc(cc(C(O)=O)c2c1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)OC |r| Show InChI InChI=1S/C27H30N2O6/c1-3-4-5-6-7-17-10-13-22-20(14-17)21(26(32)33)16-23(28-22)25(31)29-24(27(34)35-2)15-18-8-11-19(30)12-9-18/h8-14,16,24,30H,3-7,15H2,1-2H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
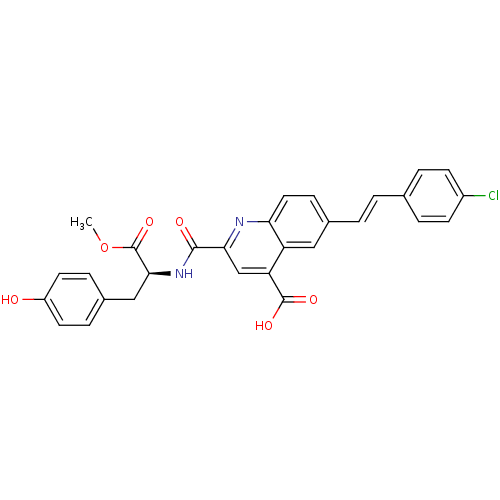
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444918
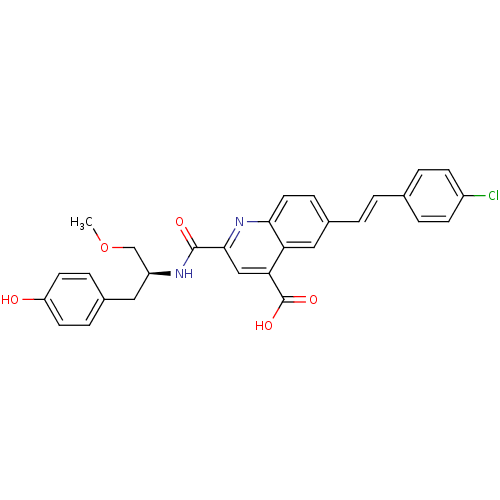
(CHEMBL3099753)Show SMILES COC[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H25ClN2O5/c1-37-17-22(14-19-6-11-23(33)12-7-19)31-28(34)27-16-25(29(35)36)24-15-20(8-13-26(24)32-27)3-2-18-4-9-21(30)10-5-18/h2-13,15-16,22,33H,14,17H2,1H3,(H,31,34)(H,35,36)/b3-2+/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444935
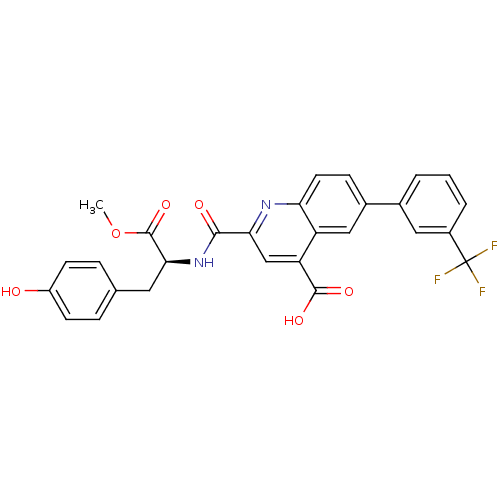
(CHEMBL3099761)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H21F3N2O6/c1-39-27(38)24(11-15-5-8-19(34)9-6-15)33-25(35)23-14-21(26(36)37)20-13-17(7-10-22(20)32-23)16-3-2-4-18(12-16)28(29,30)31/h2-10,12-14,24,34H,11H2,1H3,(H,33,35)(H,36,37)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444919
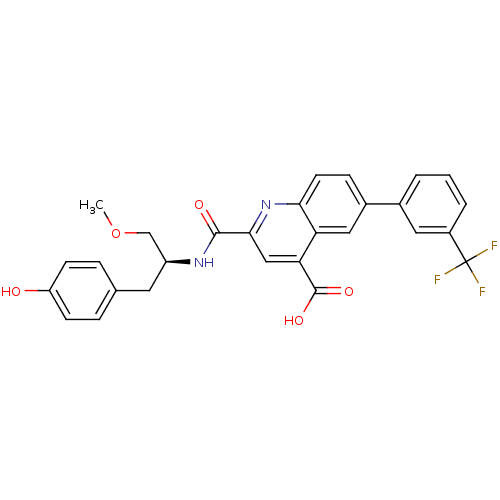
(CHEMBL3099752)Show SMILES COC[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H23F3N2O5/c1-38-15-20(11-16-5-8-21(34)9-6-16)32-26(35)25-14-23(27(36)37)22-13-18(7-10-24(22)33-25)17-3-2-4-19(12-17)28(29,30)31/h2-10,12-14,20,34H,11,15H2,1H3,(H,32,35)(H,36,37)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50148373
(5-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO3S/c1-19-10-9-16(17-6-3-11-23-17)22-15-5-2-4-13-12(15)7-8-14(20)18(13)21/h2-8,11,16,19-21H,9-10H2,1H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-mazindol binding to cloned human dopamine (DA) transporter |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50148373

(5-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO3S/c1-19-10-9-16(17-6-3-11-23-17)22-15-5-2-4-13-12(15)7-8-14(20)18(13)21/h2-8,11,16,19-21H,9-10H2,1H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-paroxetine binding to cloned human serotonin (5-HT) transporter |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50148379

(4-((S)-3-Methylamino-1-thiophen-2-yl-propoxy)-naph...)Show InChI InChI=1S/C18H19NO2S/c1-19-11-10-17(18-7-4-12-22-18)21-16-9-8-15(20)13-5-2-3-6-14(13)16/h2-9,12,17,19-20H,10-11H2,1H3/t17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-mazindol binding to cloned human dopamine (DA) transporter; Average of two experiments |
Bioorg Med Chem Lett 14: 3481-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.066
BindingDB Entry DOI: 10.7270/Q24Q7TFH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data