

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pepsin A (Porcine) | BDBM50022520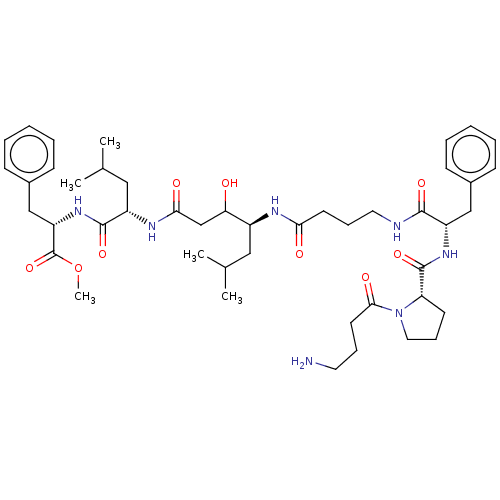 (CHEMBL3349395 | H2N-Abu-Pro-Phe-Abu-Sta-Leu-PheOMe) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human renin at pH 6.0 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50227343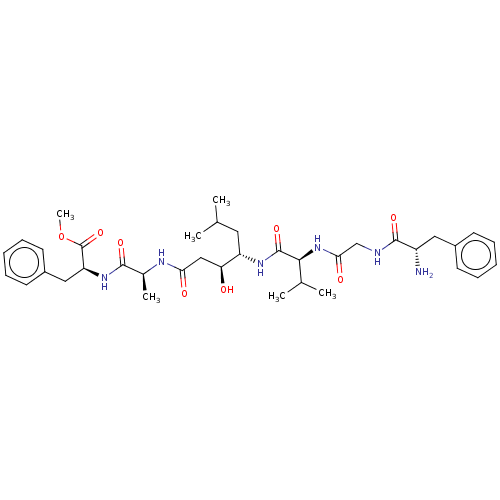 (CHEMBL3349391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of porcine pepsin at pH 4.0 (time-dependent inhibition, T1/2>30 s) | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50022512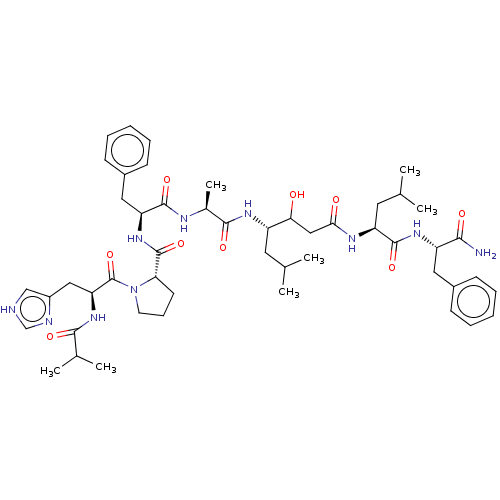 (CHEMBL3349394 | Ibu-His-Pro-Phe-Ala-Sta-Leu-PheNH2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of porcine pepsin at pH 4.0 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022507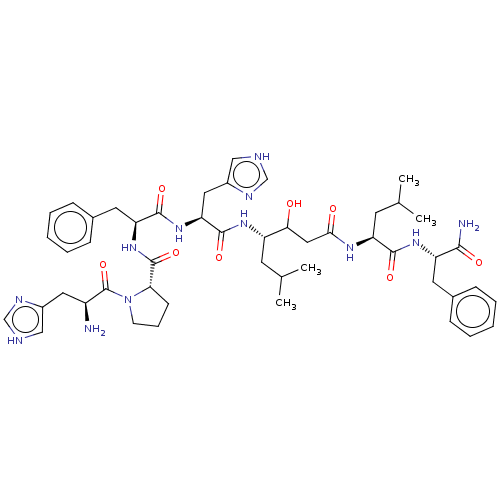 (CHEMBL3349407 | H2N-His-Pro-Phe-His-Sta-Leu-PheOMe) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human renin at pH 7.4 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022520 (CHEMBL3349395 | H2N-Abu-Pro-Phe-Abu-Sta-Leu-PheOMe) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human renin at pH 7.4 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50022517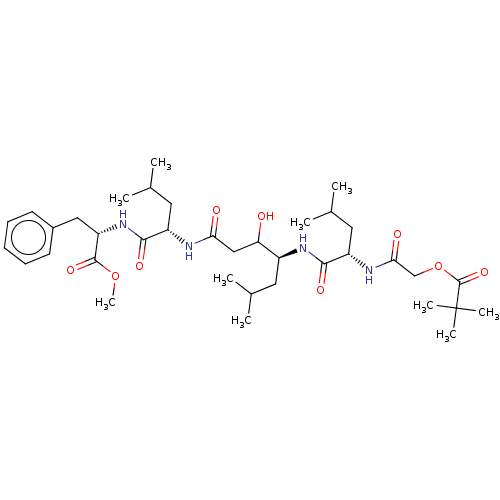 (CHEMBL3349397 | POA-Leu-Sta-Leu-PheOMe) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of porcine pepsin at pH 4.0 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022519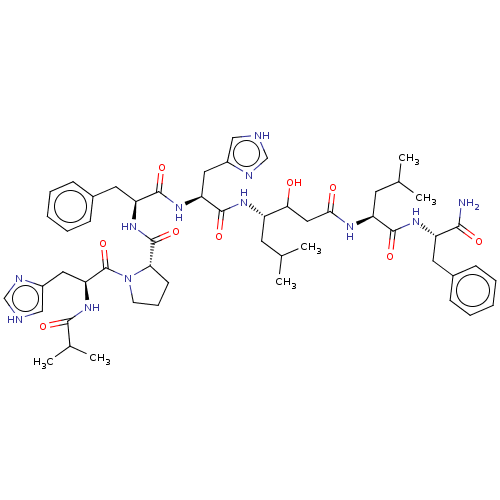 (CHEMBL3349393 | Ibu-His-Pro-Phe-His-Sta-Leu-PheNH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human renin at pH 7.4 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50022519 (CHEMBL3349393 | Ibu-His-Pro-Phe-His-Sta-Leu-PheNH2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of porcine pepsin at pH 4.0 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50022507 (CHEMBL3349407 | H2N-His-Pro-Phe-His-Sta-Leu-PheOMe) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of porcine pepsin at pH 4.0 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50022517 (CHEMBL3349397 | POA-Leu-Sta-Leu-PheOMe) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of rabbit liver cathepsin D at pH 4.0 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50022515 (CHEMBL3349396 | POA-His-Sta-Leu-PheOMe) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of porcine pepsin at pH 4.0 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022512 (CHEMBL3349394 | Ibu-His-Pro-Phe-Ala-Sta-Leu-PheNH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human renin at pH 7.4 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50022513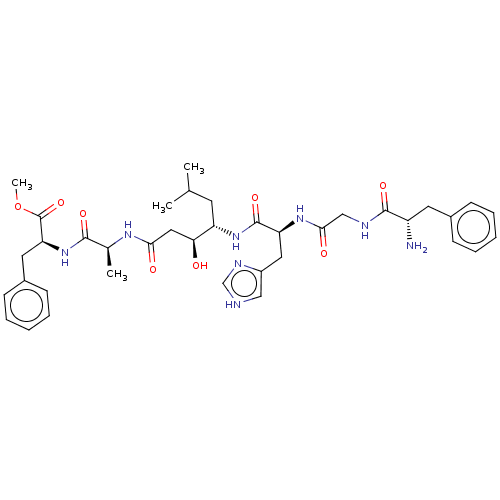 (CHEMBL3349389 | H2N-Phe-Gly-His-(S,S)-Sta-Ala-Phe-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of porcine pepsin at pH 4 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022515 (CHEMBL3349396 | POA-His-Sta-Leu-PheOMe) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human renin at pH 7.4 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022517 (CHEMBL3349397 | POA-Leu-Sta-Leu-PheOMe) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human renin at pH 7.4 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50022515 (CHEMBL3349396 | POA-His-Sta-Leu-PheOMe) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of rabbit liver cathepsin D at pH 4.0 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50022523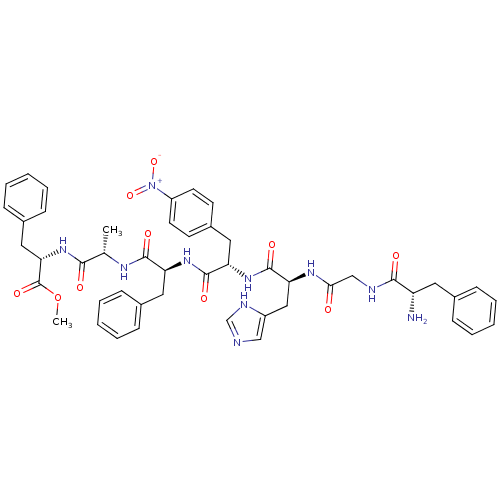 (CHEMBL347185 | H2N-Phe-Gly-His-Nph-Phe-Ala-Phe-OMe) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of porcine pepsin at pH 4 | J Med Chem 31: 625-9 (1988) BindingDB Entry DOI: 10.7270/Q2B85742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase LATS1 (Homo sapiens (Human)) | BDBM574325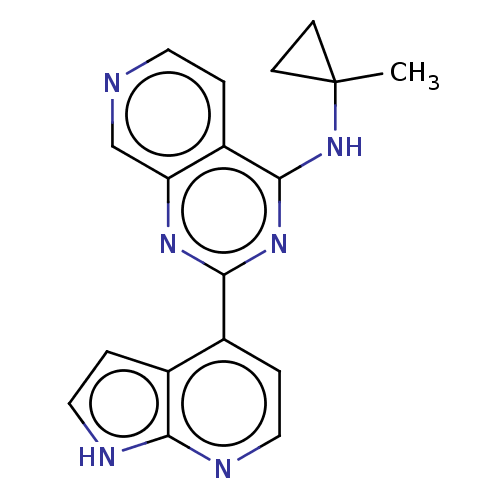 (US11458138, Example 154) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LATS1 biochemical HTRF assay was performed using the HTRF KinEASE-STK S1 kit (CisBio, catalogue number 62ST1PEC) according to manufacturer's ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21Z47NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330345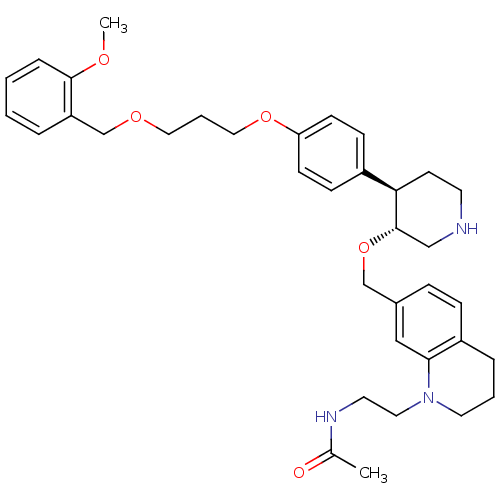 (CHEMBL1276275 | N-(2-(7-(((3R,4R)-4-(4-(3-(2-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330350 ((R)-3-((3S,4R,5R)-4-(4-(3-(2-methoxybenzyloxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012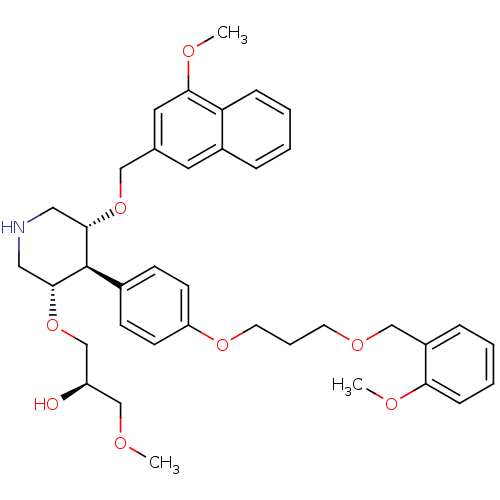 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17945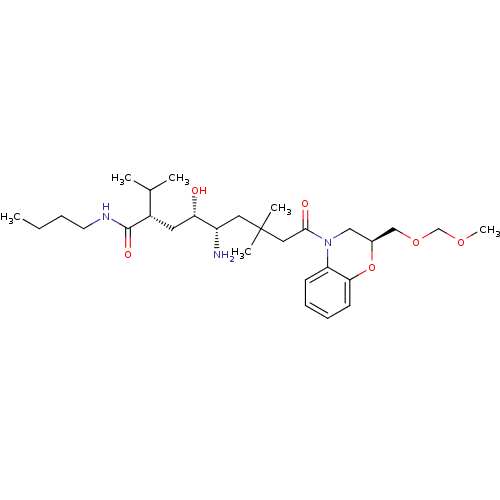 ((2S,4S,5S)-5-amino-N-butyl-4-hydroxy-9-[(2S)-2-[(m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330354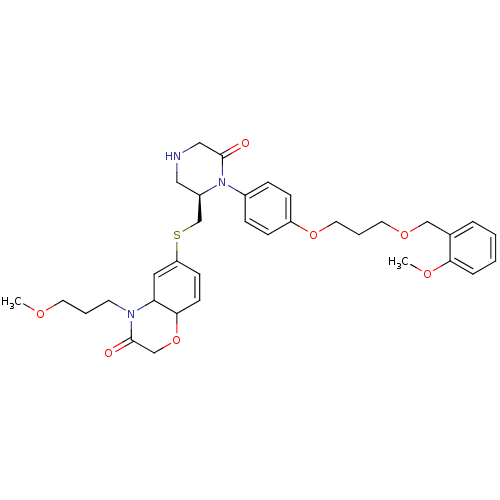 (6-(((R)-1-(4-(3-(2-methoxybenzyloxy)propoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684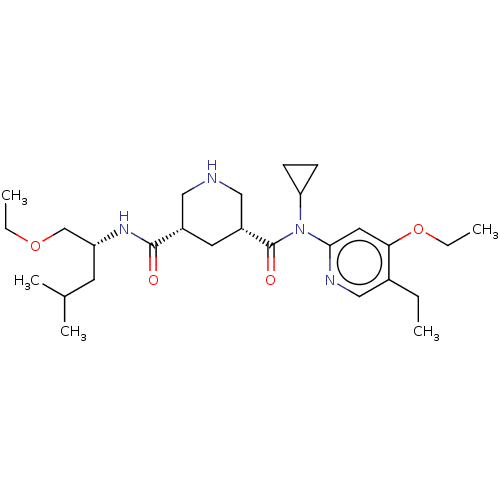 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98678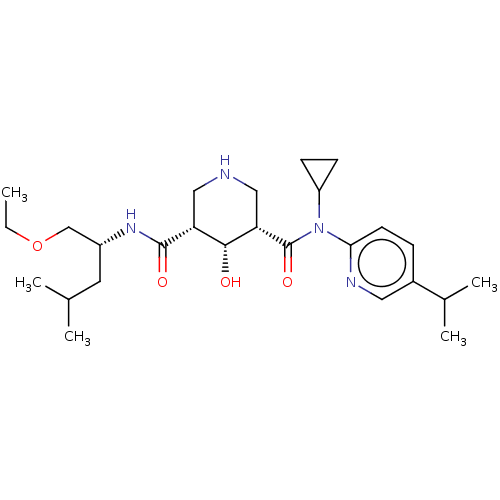 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330355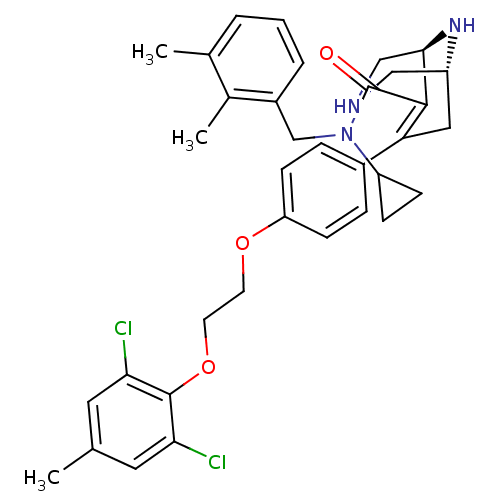 ((1R,5S)-N-cyclopropyl-7-(4-(2-(2,6-dichloro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077699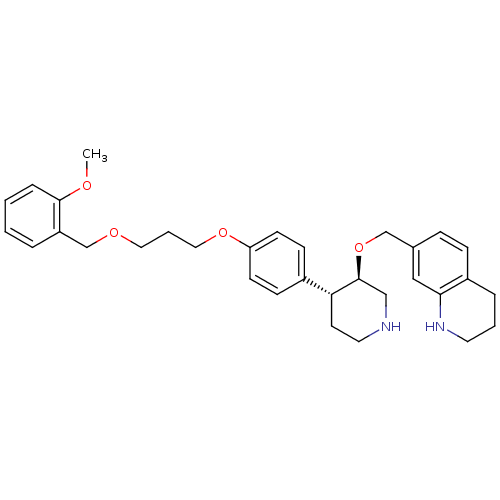 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330352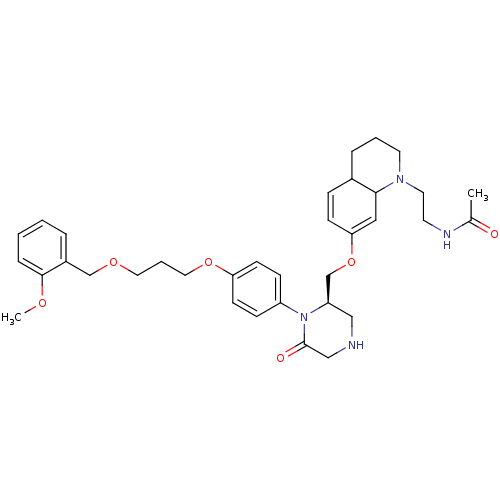 (CHEMBL1276278 | N-(2-(7-(((R)-1-(4-(3-(2-methoxybe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330360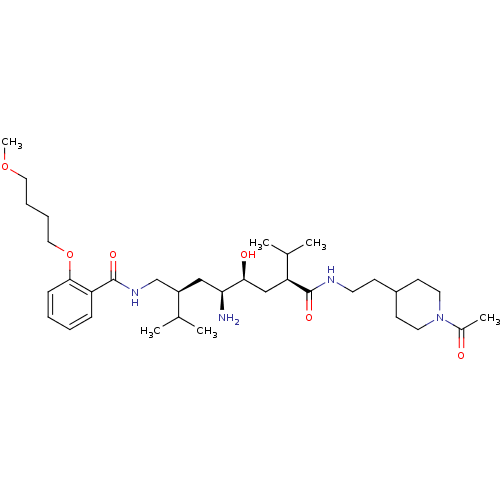 (CHEMBL1276272 | N-((2S,4S,5S,7S)-7-(2-(1-acetylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of renin in plasma | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054540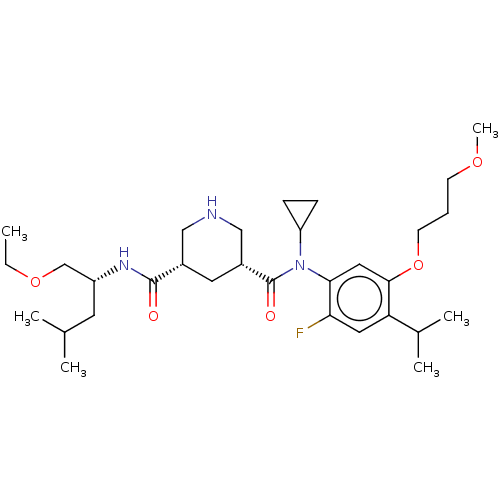 (CHEMBL3318939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054632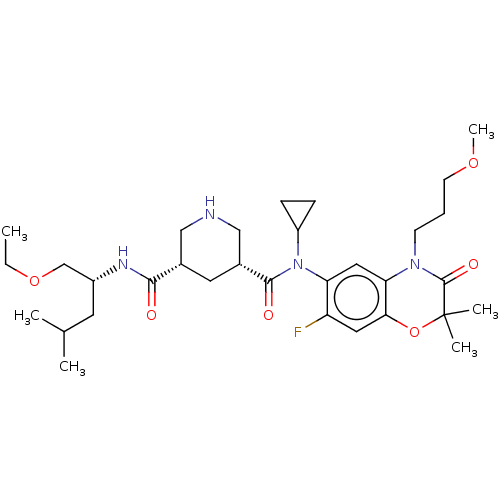 (CHEMBL3318940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18313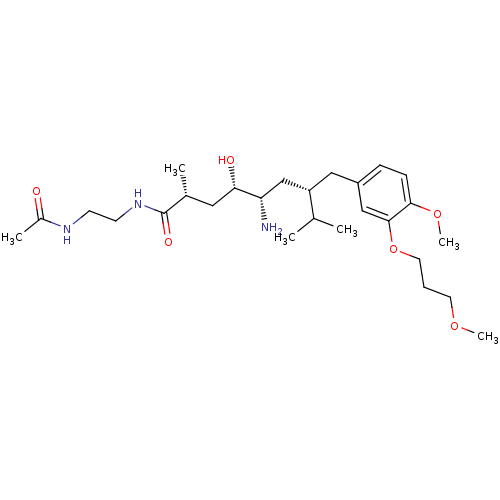 ((2R,4S,5S,7S)-5-amino-N-(2-acetamidoethyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259465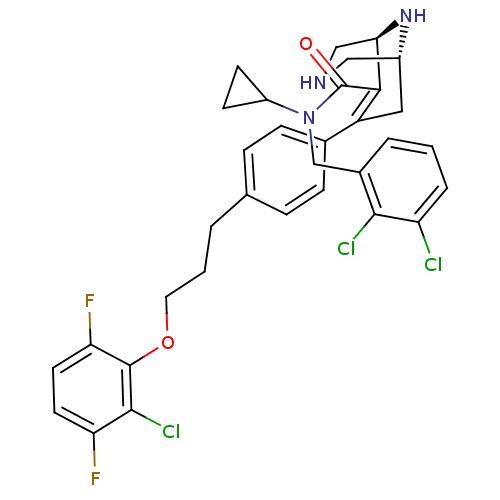 ((1R,5S)-7-{4-[3-(2-Chloro-3,6-difluoro-phenoxy)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542738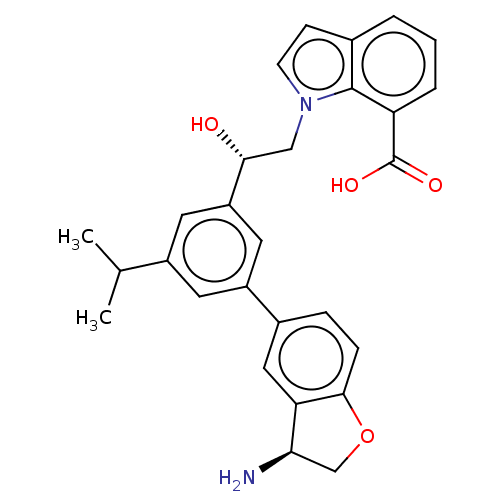 (CHEMBL4637027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18288 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17949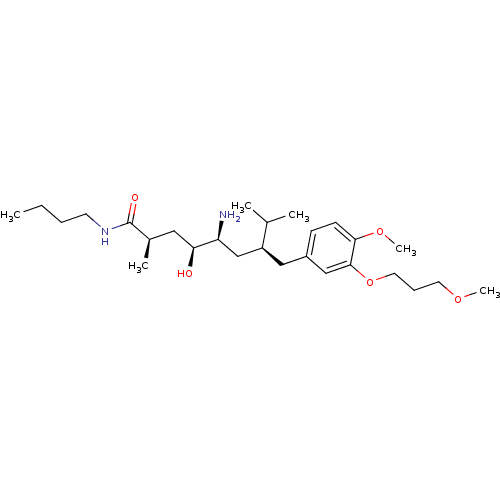 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18342 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18337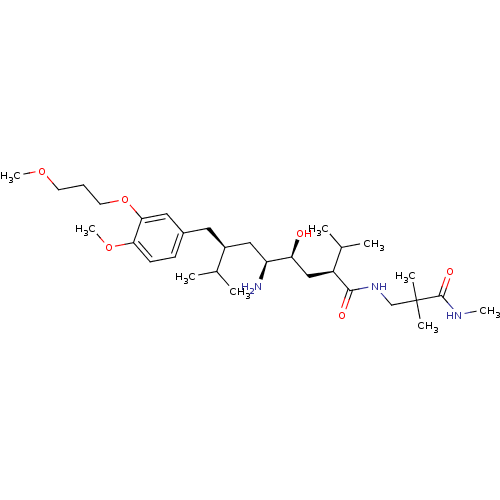 ((2S,4S,5S,7S)-5-amino-N-[2,2-dimethyl-2-(methylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054633 (CHEMBL3318941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054637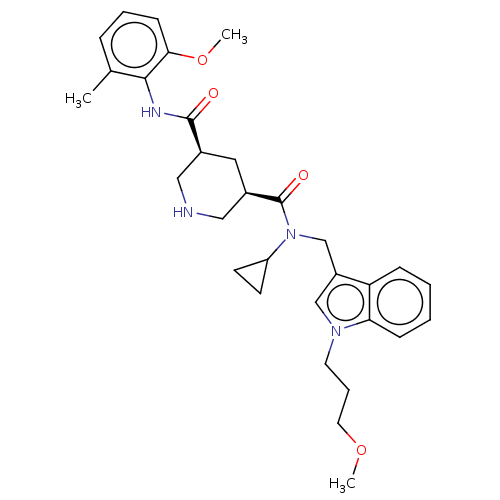 (CHEMBL3318938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054636 (CHEMBL3318937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330351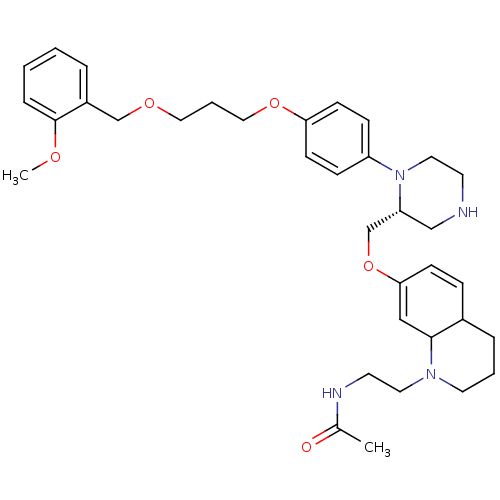 (CHEMBL1276277 | N-(2-(7-(((R)-1-(4-(3-(2-methoxybe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17944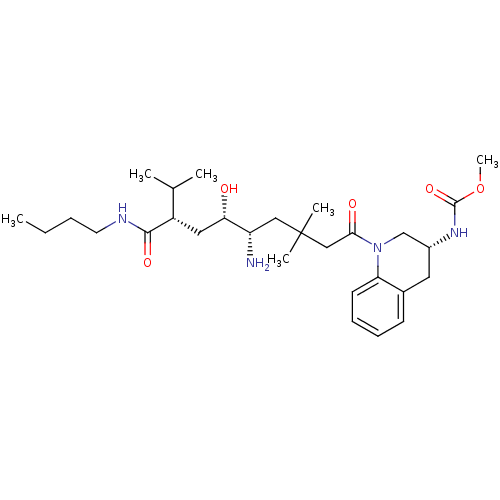 (Renin nonpeptide inhibitor, 4 | methyl N-[(3R)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17944 (Renin nonpeptide inhibitor, 4 | methyl N-[(3R)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of renin in plasma | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki International University Curated by ChEMBL | Assay Description Inhibition of purified recombinant human renin | Bioorg Med Chem Lett 19: 4863-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.128 BindingDB Entry DOI: 10.7270/Q2F18ZRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki International University Curated by ChEMBL | Assay Description Inhibition of human plasma renin | Bioorg Med Chem Lett 19: 4863-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.128 BindingDB Entry DOI: 10.7270/Q2F18ZRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of renin in plasma | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2400 total ) | Next | Last >> |