 Found 602 hits with Last Name = 'maj' and Initial = 'r'
Found 602 hits with Last Name = 'maj' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92649
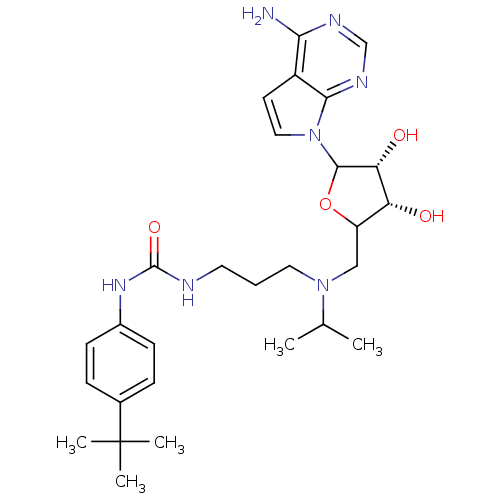
(EPZ004777)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1OC([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21?,22-,23-,26?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92648
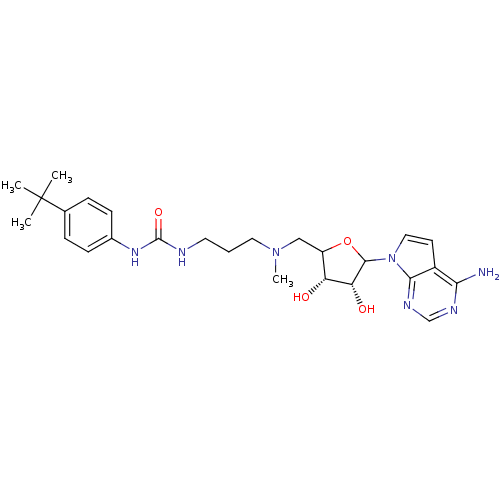
(EPZ004450)Show SMILES CN(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1OC([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C26H37N7O4/c1-26(2,3)16-6-8-17(9-7-16)31-25(36)28-11-5-12-32(4)14-19-20(34)21(35)24(37-19)33-13-10-18-22(27)29-15-30-23(18)33/h6-10,13,15,19-21,24,34-35H,5,11-12,14H2,1-4H3,(H2,27,29,30)(H2,28,31,36)/t19?,20-,21-,24?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92647
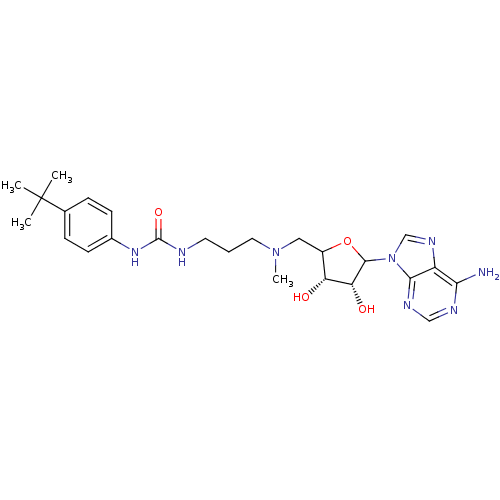
(EPZ003696)Show SMILES CN(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C25H36N8O4/c1-25(2,3)15-6-8-16(9-7-15)31-24(36)27-10-5-11-32(4)12-17-19(34)20(35)23(37-17)33-14-30-18-21(26)28-13-29-22(18)33/h6-9,13-14,17,19-20,23,34-35H,5,10-12H2,1-4H3,(H2,26,28,29)(H2,27,31,36)/t17?,19-,20-,23?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition measured after 10 mins by... |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM81348
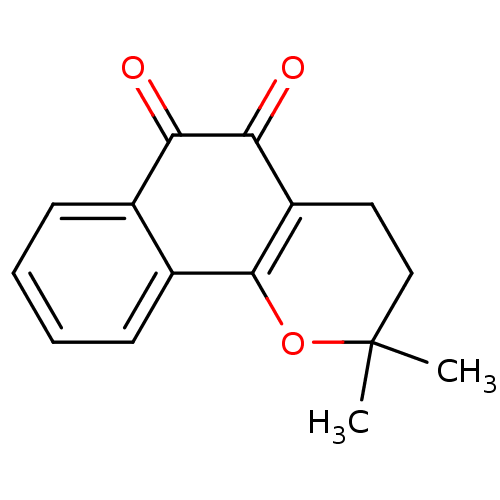
(β-Lapachone (A3) | Beta lapachone | R115 (Rea...)Show InChI InChI=1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant IDO1 expressed in Escherichia coli by Michaelis-Menton nonlinear regression plot analysis in presence ... |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92642
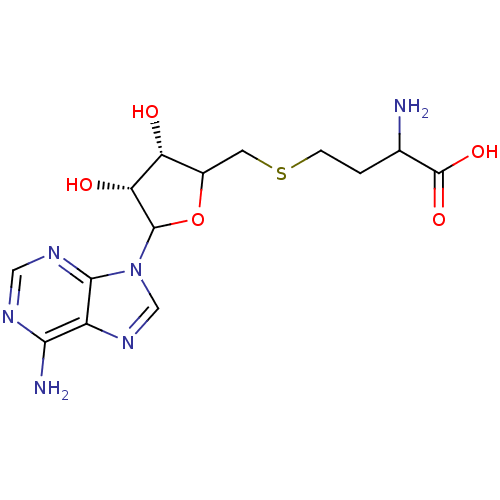
(SAH)Show SMILES NC(CCSCC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6?,7?,9-,10-,13?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92646
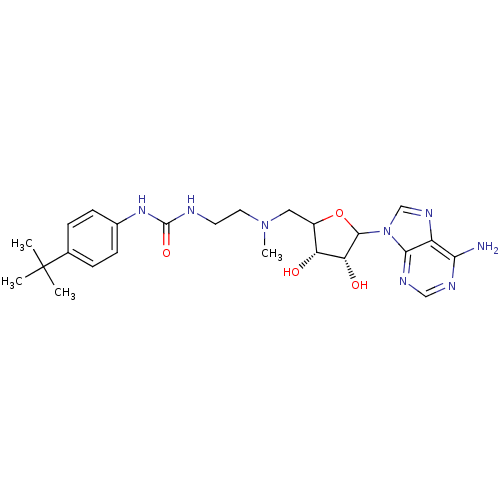
(EPZ003647)Show SMILES CN(CCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C24H34N8O4/c1-24(2,3)14-5-7-15(8-6-14)30-23(35)26-9-10-31(4)11-16-18(33)19(34)22(36-16)32-13-29-17-20(25)27-12-28-21(17)32/h5-8,12-13,16,18-19,22,33-34H,9-11H2,1-4H3,(H2,25,27,28)(H2,26,30,35)/t16?,18-,19-,22?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 845 | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50183456
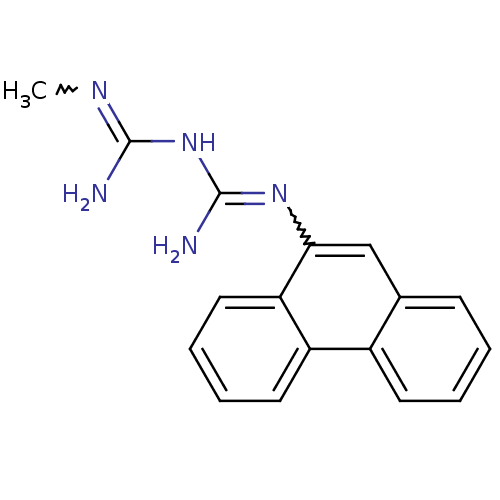
(CHEMBL425403 | N-methyl-N'-9-phenanthrylimidodicar...)Show SMILES CN=C(N)NC(N)=Nc1cc2ccccc2c2ccccc12 |w:7.7,1.0| Show InChI InChI=1S/C17H17N5/c1-20-16(18)22-17(19)21-15-10-11-6-2-3-7-12(11)13-8-4-5-9-14(13)15/h2-10H,1H3,(H5,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO1 expressed in yeast IS20-2B using tryptophan as substrate by methylene blue/ascorbate assay |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50279937
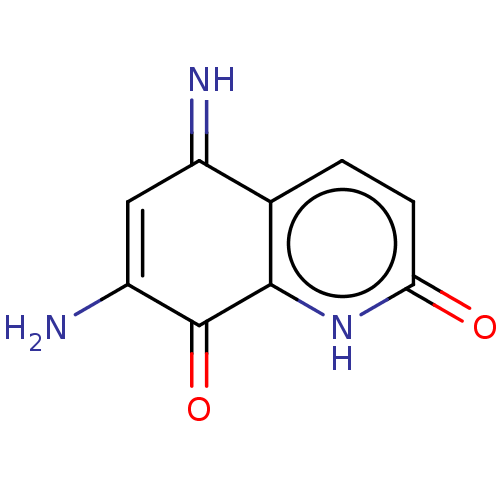
(CHEMBL1985550)Show InChI InChI=1S/C9H7N3O2/c10-5-3-6(11)9(14)8-4(5)1-2-7(13)12-8/h1-3,10H,11H2,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO1 using L-tryptophan as substrate after 15 mins by Dixon plot analysis |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM36371
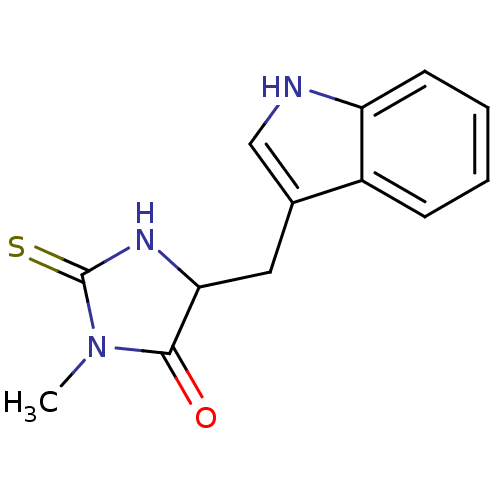
(5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4-Imidaz...)Show InChI InChI=1S/C13H13N3OS/c1-16-12(17)11(15-13(16)18)6-8-7-14-10-5-3-2-4-9(8)10/h2-5,7,11,14H,6H2,1H3,(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Competitive inhibition of 6His-tagged human recombinant IDO expressed in Escherichia coli BL21DE3pLys |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92644
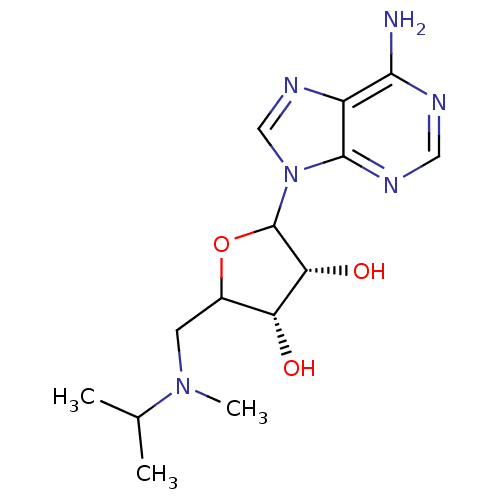
(EPZ002446)Show SMILES CC(C)N(C)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H22N6O3/c1-7(2)19(3)4-8-10(21)11(22)14(23-8)20-6-18-9-12(15)16-5-17-13(9)20/h5-8,10-11,14,21-22H,4H2,1-3H3,(H2,15,16,17)/t8?,10-,11-,14?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92645
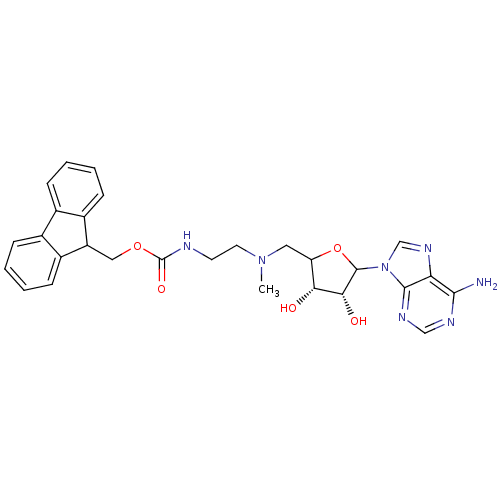
(EPZ003144)Show SMILES CN(CCNC(=O)OCC1c2ccccc2-c2ccccc12)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H31N7O5/c1-34(12-21-23(36)24(37)27(40-21)35-15-33-22-25(29)31-14-32-26(22)35)11-10-30-28(38)39-13-20-18-8-4-2-6-16(18)17-7-3-5-9-19(17)20/h2-9,14-15,20-21,23-24,27,36-37H,10-13H2,1H3,(H,30,38)(H2,29,31,32)/t21?,23-,24-,27?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92643
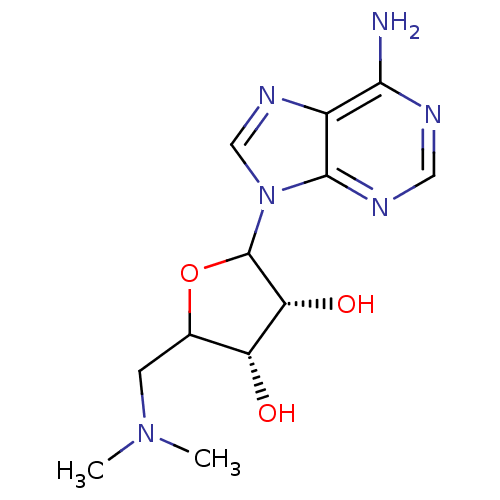
(EPZ000004)Show SMILES CN(C)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H18N6O3/c1-17(2)3-6-8(19)9(20)12(21-6)18-5-16-7-10(13)14-4-15-11(7)18/h4-6,8-9,12,19-20H,3H2,1-2H3,(H2,13,14,15)/t6?,8-,9-,12?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50024210
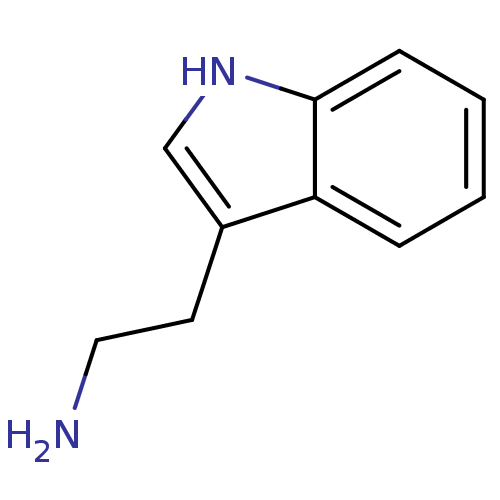
(1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...)Show InChI InChI=1S/C10H12N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant IDO1 expressed in Escherichia coli BL21 by Lineweaver-Burk double-reciprocal plot analysis |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM168435
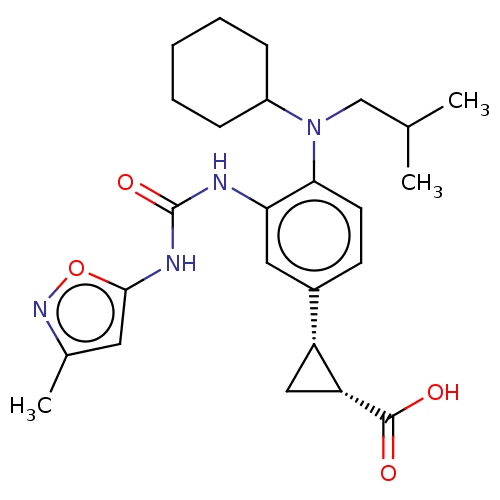
(US9675571, 129)Show SMILES CC(C)CN(C1CCCCC1)c1ccc(cc1NC(=O)Nc1cc(C)no1)[C@H]1C[C@H]1C(O)=O |r| Show InChI InChI=1S/C25H34N4O4/c1-15(2)14-29(18-7-5-4-6-8-18)22-10-9-17(19-13-20(19)24(30)31)12-21(22)26-25(32)27-23-11-16(3)28-33-23/h9-12,15,18-20H,4-8,13-14H2,1-3H3,(H,30,31)(H2,26,27,32)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50391363
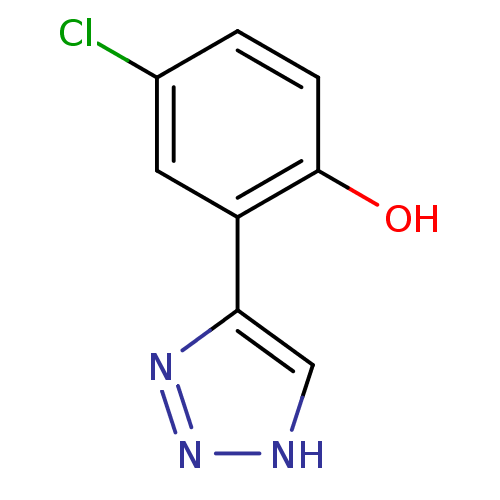
(CHEMBL2148074)Show InChI InChI=1S/C8H6ClN3O/c9-5-1-2-8(13)6(3-5)7-4-10-12-11-7/h1-4,13H,(H,10,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis |
J Med Chem 55: 5270-90 (2012)
Article DOI: 10.1021/jm300260v
BindingDB Entry DOI: 10.7270/Q27H1KNW |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478032
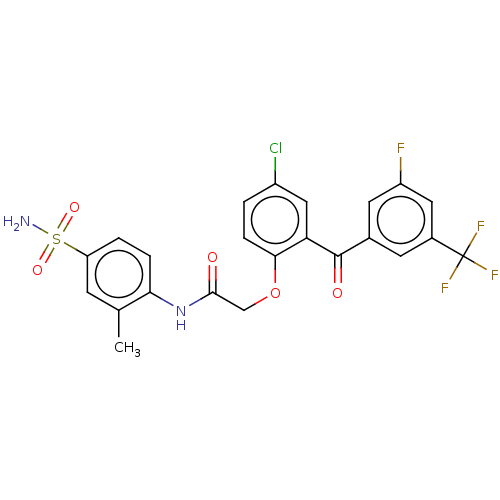
(CHEMBL275658 | GW4511 | GW564511 | GW69564)Show SMILES Cc1cc(ccc1NC(=O)COc1ccc(Cl)cc1C(=O)c1cc(F)cc(c1)C(F)(F)F)S(N)(=O)=O Show InChI InChI=1S/C23H17ClF4N2O5S/c1-12-6-17(36(29,33)34)3-4-19(12)30-21(31)11-35-20-5-2-15(24)10-18(20)22(32)13-7-14(23(26,27)28)9-16(25)8-13/h2-10H,11H2,1H3,(H,30,31)(H2,29,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of wild-type HIV1 reverse transcriptase |
Bioorg Med Chem 17: 5744-62 (2009)
Article DOI: 10.1016/j.bmc.2009.06.060
BindingDB Entry DOI: 10.7270/Q20G3NXJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049642
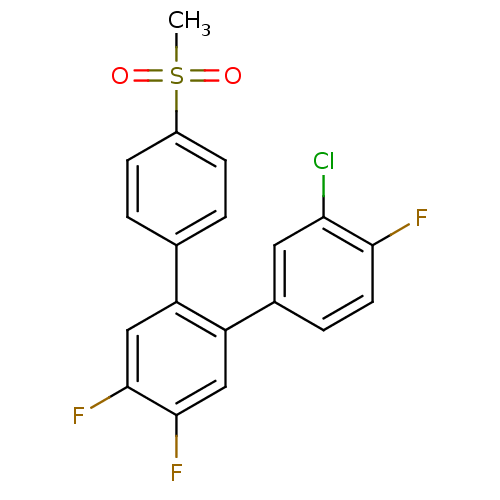
(3-Chloro-4,4',5'-trifluoro-4''-methanesulfonyl-[1,...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C19H12ClF3O2S/c1-26(24,25)13-5-2-11(3-6-13)14-9-18(22)19(23)10-15(14)12-4-7-17(21)16(20)8-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288291
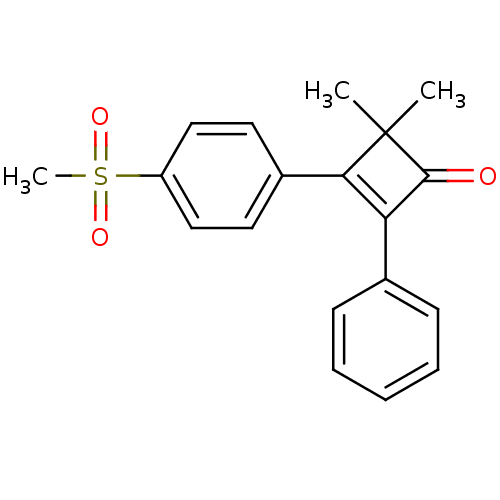
(3-(4-Methanesulfonyl-phenyl)-4,4-dimethyl-2-phenyl...)Show SMILES CC1(C)C(=O)C(=C1c1ccc(cc1)S(C)(=O)=O)c1ccccc1 |c:5| Show InChI InChI=1S/C19H18O3S/c1-19(2)17(14-9-11-15(12-10-14)23(3,21)22)16(18(19)20)13-7-5-4-6-8-13/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in human whole blood |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM340834
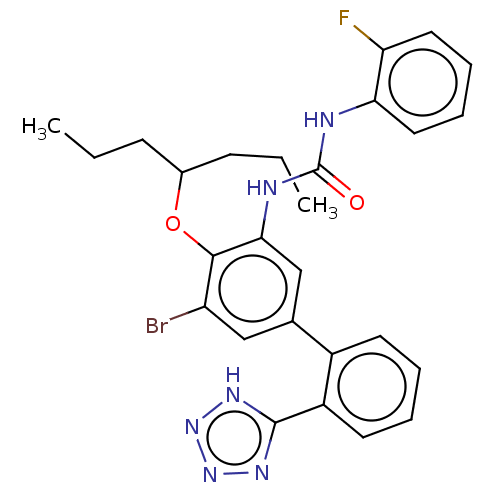
(US9765018, Example 287)Show SMILES CCCC(CCC)Oc1c(Br)cc(cc1NC(=O)Nc1ccccc1F)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H28BrFN6O2/c1-3-9-18(10-4-2)37-25-21(28)15-17(19-11-5-6-12-20(19)26-32-34-35-33-26)16-24(25)31-27(36)30-23-14-8-7-13-22(23)29/h5-8,11-16,18H,3-4,9-10H2,1-2H3,(H2,30,31,36)(H,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM317549
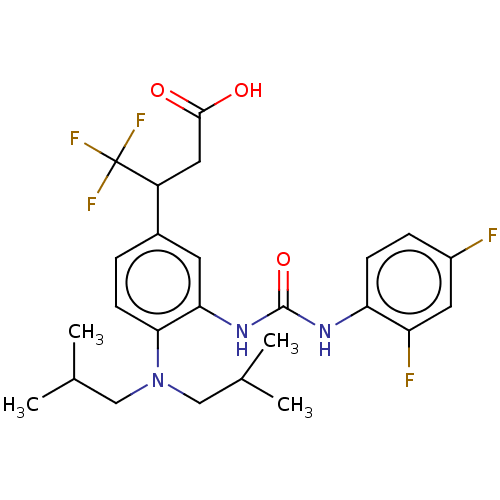
(3-(3-(3-(2,4- difluorophenyl)ureido)-4- (diisobuty...)Show SMILES CC(C)CN(CC(C)C)c1ccc(cc1NC(=O)Nc1ccc(F)cc1F)C(CC(O)=O)C(F)(F)F Show InChI InChI=1S/C25H30F5N3O3/c1-14(2)12-33(13-15(3)4)22-8-5-16(18(11-23(34)35)25(28,29)30)9-21(22)32-24(36)31-20-7-6-17(26)10-19(20)27/h5-10,14-15,18H,11-13H2,1-4H3,(H,34,35)(H2,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031688
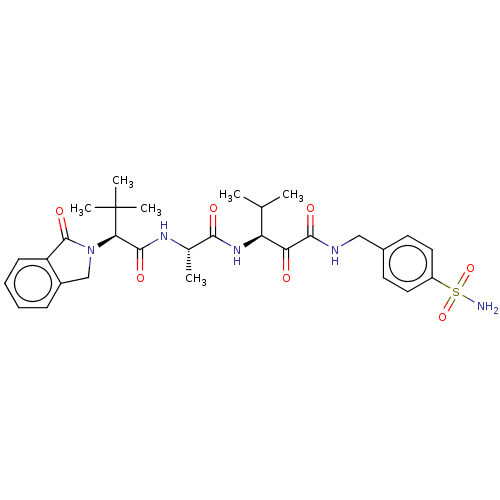
(CHEMBL3360299)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N1Cc2ccccc2C1=O)C(C)(C)C)C(=O)C(=O)NCc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C30H39N5O7S/c1-17(2)23(24(36)27(38)32-15-19-11-13-21(14-12-19)43(31,41)42)34-26(37)18(3)33-28(39)25(30(4,5)6)35-16-20-9-7-8-10-22(20)29(35)40/h7-14,17-18,23,25H,15-16H2,1-6H3,(H,32,38)(H,33,39)(H,34,37)(H2,31,41,42)/t18-,23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM178085

(US10307413, Compound 188 | US10391089, Compound 18...)Show SMILES O[C@@H](CNC(=O)c1ccnc(NC2CCC2)c1)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C22H28N4O2/c27-20(15-26-11-9-16-4-1-2-5-18(16)14-26)13-24-22(28)17-8-10-23-21(12-17)25-19-6-3-7-19/h1-2,4-5,8,10,12,19-20,27H,3,6-7,9,11,13-15H2,(H,23,25)(H,24,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.5b00380
BindingDB Entry DOI: 10.7270/Q2SQ941H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50474760
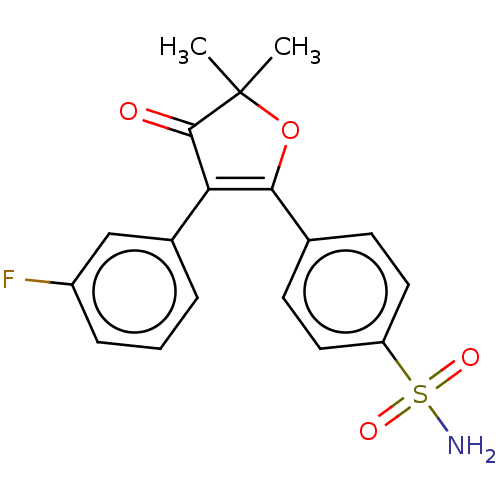
(CG-100649 | CG100649 | Polmacoxib)Show SMILES CC1(C)OC(=C(C1=O)c1cccc(F)c1)c1ccc(cc1)S(N)(=O)=O |c:4| Show InChI InChI=1S/C18H16FNO4S/c1-18(2)17(21)15(12-4-3-5-13(19)10-12)16(24-18)11-6-8-14(9-7-11)25(20,22)23/h3-10H,1-2H3,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 in LPS-induced mouse peritoneal macrophages |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50153982
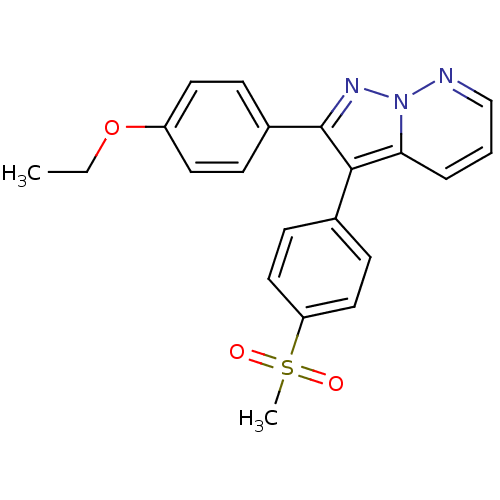
(2-(4-Ethoxy-phenyl)-3-(4-methanesulfonyl-phenyl)-p...)Show SMILES CCOc1ccc(cc1)-c1nn2ncccc2c1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C21H19N3O3S/c1-3-27-17-10-6-16(7-11-17)21-20(19-5-4-14-22-24(19)23-21)15-8-12-18(13-9-15)28(2,25)26/h4-14H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human COX-2 expressed in human COS cells using arachidonic acid as substrate |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50289328

(5-(4-Methanesulfonyl-phenyl)-6-phenyl-2-trifluorom...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1sc2nc(nn2c1-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C18H12F3N3O2S2/c1-28(25,26)13-9-7-12(8-10-13)15-14(11-5-3-2-4-6-11)24-17(27-15)22-16(23-24)18(19,20)21/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in CHO-K1 cells using arachidonic acid as substrate preincubated for 15 mins followed by substrate ad... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031689

(CHEMBL3360298)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccnc2ccccc12)C(C)(C)C)C(=O)C(=O)NCC(=O)N1CCN(CC1)C(C)C |r| Show InChI InChI=1S/C34H49N7O6/c1-20(2)27(28(43)32(46)36-19-26(42)41-17-15-40(16-18-41)21(3)4)38-30(44)22(5)37-33(47)29(34(6,7)8)39-31(45)24-13-14-35-25-12-10-9-11-23(24)25/h9-14,20-22,27,29H,15-19H2,1-8H3,(H,36,46)(H,37,47)(H,38,44)(H,39,45)/t22-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031684

(CHEMBL3360302)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1cccc2ccccc12)C(C)(C)C)C(=O)C(=O)NCc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C33H41N5O7S/c1-19(2)26(27(39)31(42)35-18-21-14-16-23(17-15-21)46(34,44)45)37-29(40)20(3)36-32(43)28(33(4,5)6)38-30(41)25-13-9-11-22-10-7-8-12-24(22)25/h7-17,19-20,26,28H,18H2,1-6H3,(H,35,42)(H,36,43)(H,37,40)(H,38,41)(H2,34,44,45)/t20-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031685

(CHEMBL3360301)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N1Cc2ccccc2C1=O)C(C)(C)C)C(=O)C(=O)NCc1ccc(CN(C)C)cc1 |r| Show InChI InChI=1S/C33H45N5O5/c1-20(2)26(27(39)30(41)34-17-22-13-15-23(16-14-22)18-37(7)8)36-29(40)21(3)35-31(42)28(33(4,5)6)38-19-24-11-9-10-12-25(24)32(38)43/h9-16,20-21,26,28H,17-19H2,1-8H3,(H,34,41)(H,35,42)(H,36,40)/t21-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM178085

(US10307413, Compound 188 | US10391089, Compound 18...)Show SMILES O[C@@H](CNC(=O)c1ccnc(NC2CCC2)c1)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C22H28N4O2/c27-20(15-26-11-9-16-4-1-2-5-18(16)14-26)13-24-22(28)17-8-10-23-21(12-17)25-19-6-3-7-19/h1-2,4-5,8,10,12,19-20,27H,3,6-7,9,11,13-15H2,(H,23,25)(H,24,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.5b00380
BindingDB Entry DOI: 10.7270/Q2SQ941H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031695
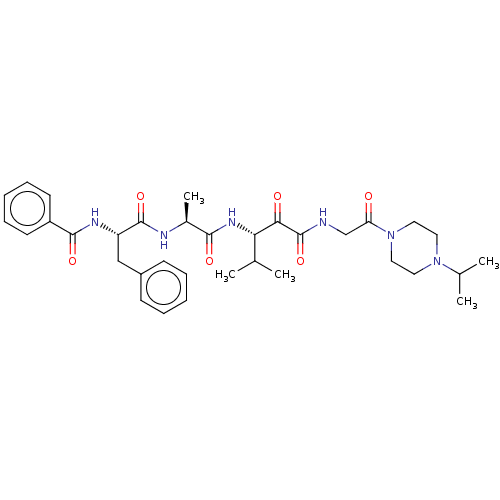
(CHEMBL3360292)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1)C(=O)C(=O)NCC(=O)N1CCN(CC1)C(C)C |r| Show InChI InChI=1S/C34H46N6O6/c1-22(2)29(30(42)34(46)35-21-28(41)40-18-16-39(17-19-40)23(3)4)38-31(43)24(5)36-33(45)27(20-25-12-8-6-9-13-25)37-32(44)26-14-10-7-11-15-26/h6-15,22-24,27,29H,16-21H2,1-5H3,(H,35,46)(H,36,45)(H,37,44)(H,38,43)/t24-,27-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031687
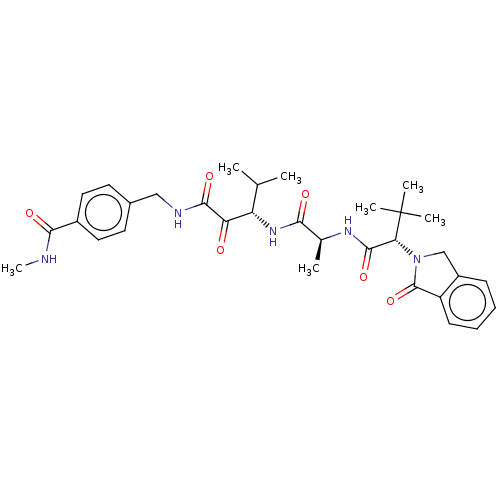
(CHEMBL3360300)Show SMILES CNC(=O)c1ccc(CNC(=O)C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](N2Cc3ccccc3C2=O)C(C)(C)C)C(C)C)cc1 |r| Show InChI InChI=1S/C32H41N5O6/c1-18(2)24(25(38)29(41)34-16-20-12-14-21(15-13-20)28(40)33-7)36-27(39)19(3)35-30(42)26(32(4,5)6)37-17-22-10-8-9-11-23(22)31(37)43/h8-15,18-19,24,26H,16-17H2,1-7H3,(H,33,40)(H,34,41)(H,35,42)(H,36,39)/t19-,24-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50500137

(CHEMBL3747340)Show SMILES Cc1ccc(CC(=O)Nc2cc(ccc2N2CC3CC2CN3Cc2ccccc2)-c2ccccc2-c2nnn[nH]2)cc1 |THB:22:21:15.16:18,14:15:21.20:18| Show InChI InChI=1S/C34H33N7O/c1-23-11-13-24(14-12-23)17-33(42)35-31-18-26(29-9-5-6-10-30(29)34-36-38-39-37-34)15-16-32(31)41-22-27-19-28(41)21-40(27)20-25-7-3-2-4-8-25/h2-16,18,27-28H,17,19-22H2,1H3,(H,35,42)(H,36,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50131593
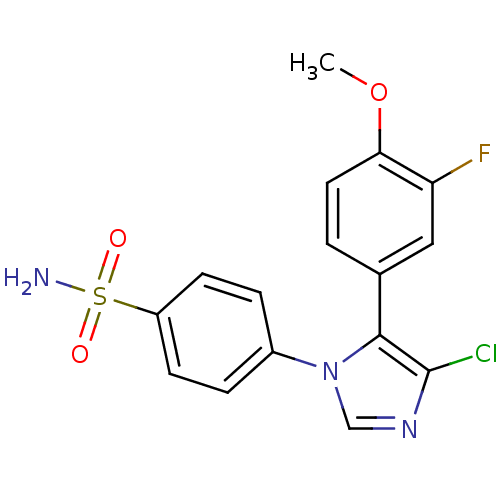
(4-[4-Chloro-5-(3-fluoro-4-methoxy-phenyl)-imidazol...)Show SMILES COc1ccc(cc1F)-c1c(Cl)ncn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13ClFN3O3S/c1-24-14-7-2-10(8-13(14)18)15-16(17)20-9-21(15)11-3-5-12(6-4-11)25(19,22)23/h2-9H,1H3,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in human 143982 cells |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031690

(CHEMBL3360297)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C12CC3CC(CC(C3)C1)C2)C(=O)C(=O)NCC(=O)N1CCN(C)CC1 |r,TLB:12:22:25:29.27.28,THB:27:26:23:29.28.30,27:28:25.26.31:23,30:28:25:31.22.23,30:22:25:29.27.28| Show InChI InChI=1S/C35H50N6O6/c1-21(2)28(29(43)33(46)36-20-27(42)41-12-10-40(4)11-13-41)38-31(44)22(3)37-34(47)30(39-32(45)26-8-6-5-7-9-26)35-17-23-14-24(18-35)16-25(15-23)19-35/h5-9,21-25,28,30H,10-20H2,1-4H3,(H,36,46)(H,37,47)(H,38,44)(H,39,45)/t22-,23?,24?,25?,28-,30+,35?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031713

(CHEMBL3360282)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NCc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C29H39N5O7S/c1-17(2)22(23(35)27(38)31-16-19-12-14-21(15-13-19)42(30,40)41)33-25(36)18(3)32-28(39)24(29(4,5)6)34-26(37)20-10-8-7-9-11-20/h7-15,17-18,22,24H,16H2,1-6H3,(H,31,38)(H,32,39)(H,33,36)(H,34,37)(H2,30,40,41)/t18-,22-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031693
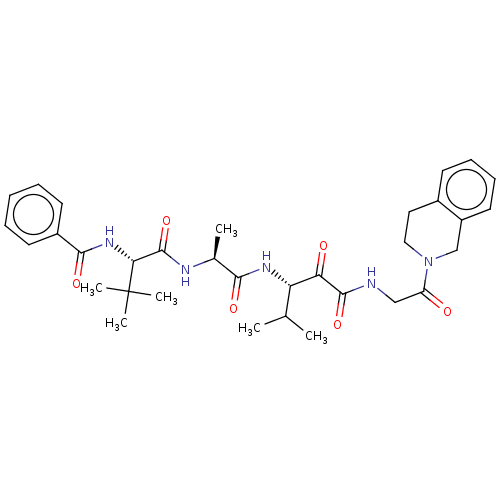
(CHEMBL3360294)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NCC(=O)N1CCc2ccccc2C1 |r| Show InChI InChI=1S/C33H43N5O6/c1-20(2)26(27(40)31(43)34-18-25(39)38-17-16-22-12-10-11-15-24(22)19-38)36-29(41)21(3)35-32(44)28(33(4,5)6)37-30(42)23-13-8-7-9-14-23/h7-15,20-21,26,28H,16-19H2,1-6H3,(H,34,43)(H,35,44)(H,36,41)(H,37,42)/t21-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031635

(CHEMBL3359786)Show SMILES [O-]C=O.CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C1CC[N+](C)(C)CC1)C(C)(C)C)C(=O)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C30H47N5O5/c1-19(2)23(24(36)28(39)31-18-21-12-10-9-11-13-21)33-26(37)20(3)32-29(40)25(30(4,5)6)34-27(38)22-14-16-35(7,8)17-15-22/h9-13,19-20,22-23,25H,14-18H2,1-8H3,(H3-,31,32,33,34,37,38,39,40)/p+1/t20-,23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031632
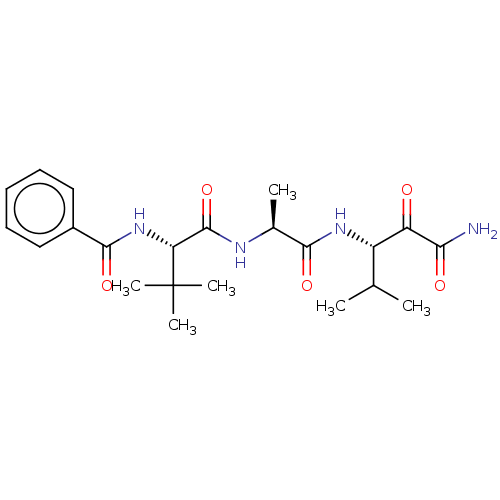
(CHEMBL3359790)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(N)=O |r| Show InChI InChI=1S/C22H32N4O5/c1-12(2)15(16(27)18(23)28)25-19(29)13(3)24-21(31)17(22(4,5)6)26-20(30)14-10-8-7-9-11-14/h7-13,15,17H,1-6H3,(H2,23,28)(H,24,31)(H,25,29)(H,26,30)/t13-,15-,17+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM103023
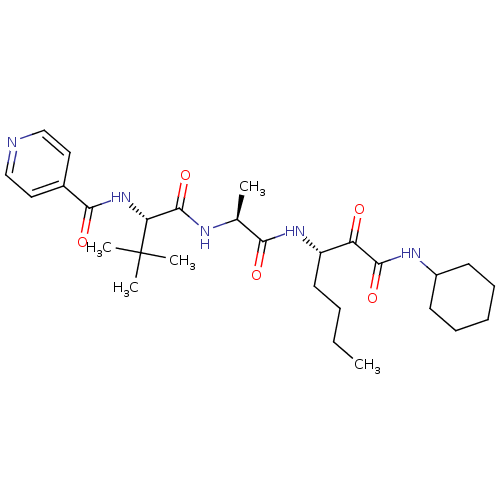
(US8541363, PVA-039)Show SMILES CCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccncc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C28H43N5O5/c1-6-7-13-21(22(34)26(37)31-20-11-9-8-10-12-20)32-24(35)18(2)30-27(38)23(28(3,4)5)33-25(36)19-14-16-29-17-15-19/h14-18,20-21,23H,6-13H2,1-5H3,(H,30,38)(H,31,37)(H,32,35)(H,33,36)/t18-,21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester
US Patent
| Assay Description
The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... |
US Patent US8541363 (2013)
BindingDB Entry DOI: 10.7270/Q2PC3105 |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031668

(CHEMBL3359782)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N1Cc2ccccc2C1=O)C(C)(C)C)C(=O)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C30H38N4O5/c1-18(2)23(24(35)27(37)31-16-20-12-8-7-9-13-20)33-26(36)19(3)32-28(38)25(30(4,5)6)34-17-21-14-10-11-15-22(21)29(34)39/h7-15,18-19,23,25H,16-17H2,1-6H3,(H,31,37)(H,32,38)(H,33,36)/t19-,23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IFN-gamma-stimulated IDO1 activity in human HeLa cells using L-tryptophan as substrate after 48 hrs by microplate reader analysis |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049041
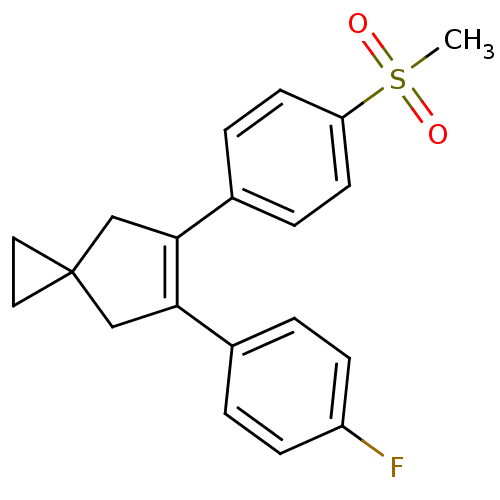
(5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C20H19FO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM103022
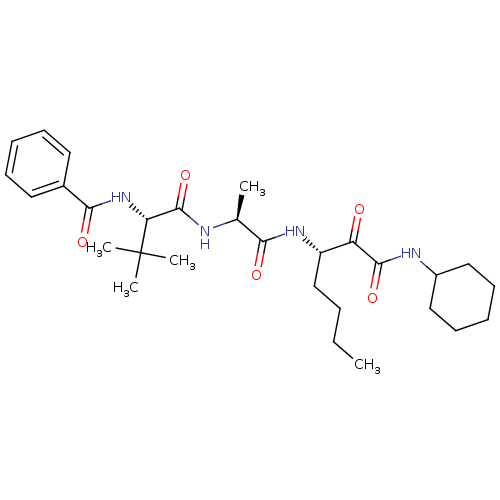
(US8541363, PVA-037)Show SMILES CCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C29H44N4O5/c1-6-7-18-22(23(34)27(37)31-21-16-12-9-13-17-21)32-25(35)19(2)30-28(38)24(29(3,4)5)33-26(36)20-14-10-8-11-15-20/h8,10-11,14-15,19,21-22,24H,6-7,9,12-13,16-18H2,1-5H3,(H,30,38)(H,31,37)(H,32,35)(H,33,36)/t19-,22-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.85 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester
US Patent
| Assay Description
The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... |
US Patent US8541363 (2013)
BindingDB Entry DOI: 10.7270/Q2PC3105 |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031719
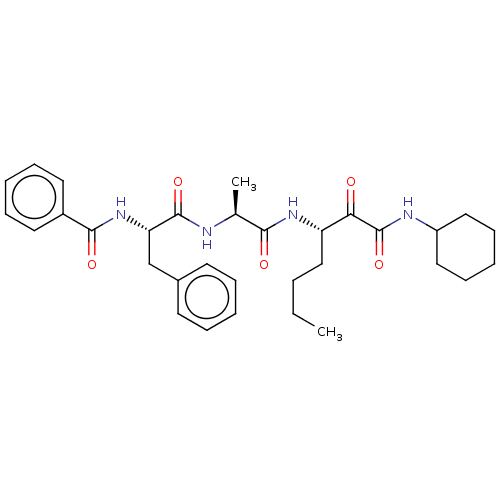
(CHEMBL3359772)Show SMILES CCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C32H42N4O5/c1-3-4-20-26(28(37)32(41)34-25-18-12-7-13-19-25)35-29(38)22(2)33-31(40)27(21-23-14-8-5-9-15-23)36-30(39)24-16-10-6-11-17-24/h5-6,8-11,14-17,22,25-27H,3-4,7,12-13,18-21H2,1-2H3,(H,33,40)(H,34,41)(H,35,38)(H,36,39)/t22-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031711
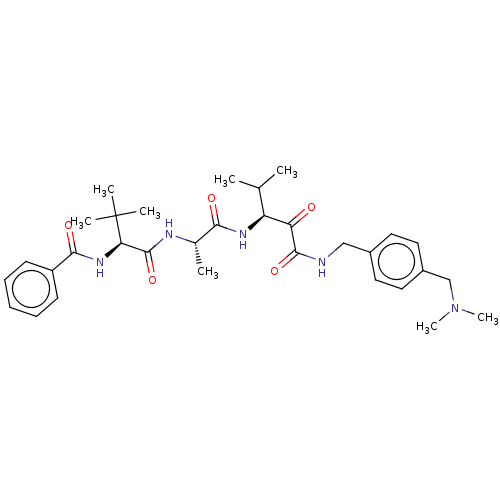
(CHEMBL3360284)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NCc1ccc(CN(C)C)cc1 |r| Show InChI InChI=1S/C32H45N5O5/c1-20(2)25(26(38)30(41)33-18-22-14-16-23(17-15-22)19-37(7)8)35-28(39)21(3)34-31(42)27(32(4,5)6)36-29(40)24-12-10-9-11-13-24/h9-17,20-21,25,27H,18-19H2,1-8H3,(H,33,41)(H,34,42)(H,35,39)(H,36,40)/t21-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM190405
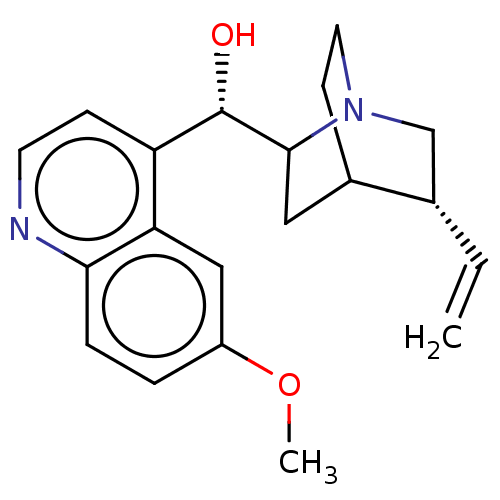
(US9180183, Quinidine)Show SMILES COc1ccc2nccc([C@H](O)C3CC4CCN3C[C@@H]4C=C)c2c1 |r,TLB:10:12:16.15:19.18,THB:20:19:12.13:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14?,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA
US Patent
| Assay Description
The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... |
US Patent US9173935 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P8S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM190405

(US9180183, Quinidine)Show SMILES COc1ccc2nccc([C@H](O)C3CC4CCN3C[C@@H]4C=C)c2c1 |r,TLB:10:12:16.15:19.18,THB:20:19:12.13:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14?,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA
US Patent
| Assay Description
The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... |
US Patent US9180183 (2015)
BindingDB Entry DOI: 10.7270/Q2B27T2W |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM177922

(US10307413, Compound 337 | US10391089, Compound 16...)Show SMILES O[C@@H](CNC(=O)c1cc(NC2COC2)ncn1)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C20H25N5O3/c26-17(10-25-6-5-14-3-1-2-4-15(14)9-25)8-21-20(27)18-7-19(23-13-22-18)24-16-11-28-12-16/h1-4,7,13,16-17,26H,5-6,8-12H2,(H,21,27)(H,22,23,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.5b00380
BindingDB Entry DOI: 10.7270/Q2SQ941H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data