

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114409 (CHEMBL3609809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as disappearance of NADH fluorescence | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114409 (CHEMBL3609809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using NADH as substrate assessed as disappearance of NADH fluorescence | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114404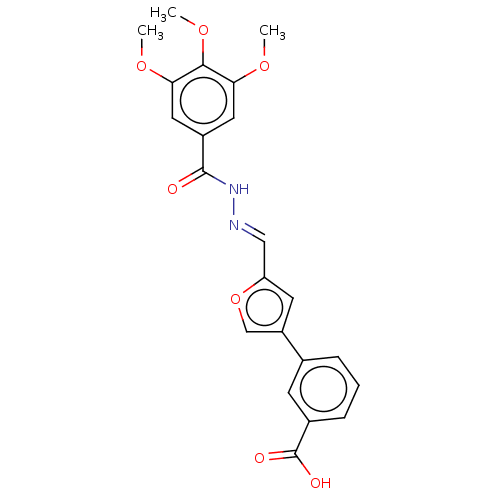 (CHEMBL3608325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114408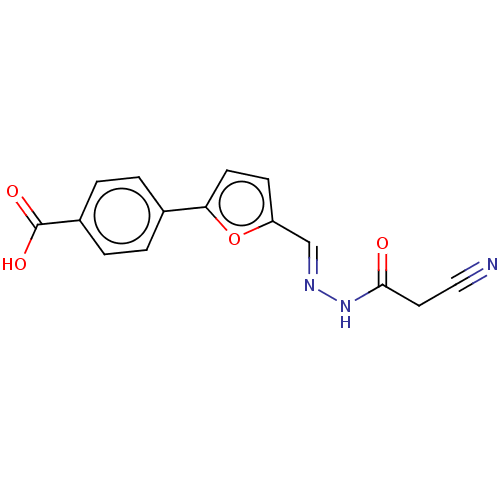 (CHEMBL3609810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114409 (CHEMBL3609809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114400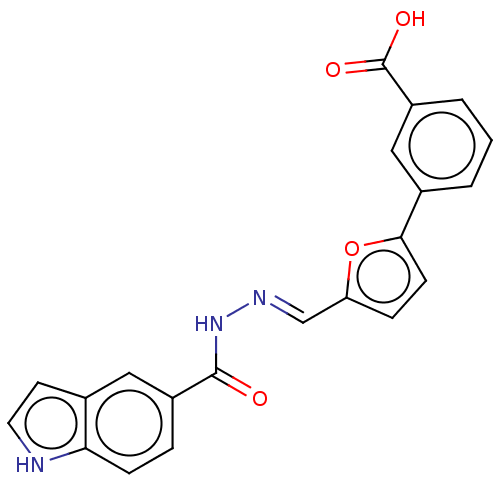 (CHEMBL3608329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114403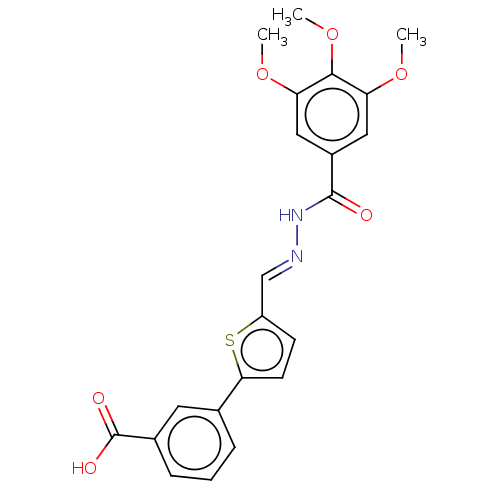 (CHEMBL3608326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114401 (CHEMBL3608328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114409 (CHEMBL3609809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114407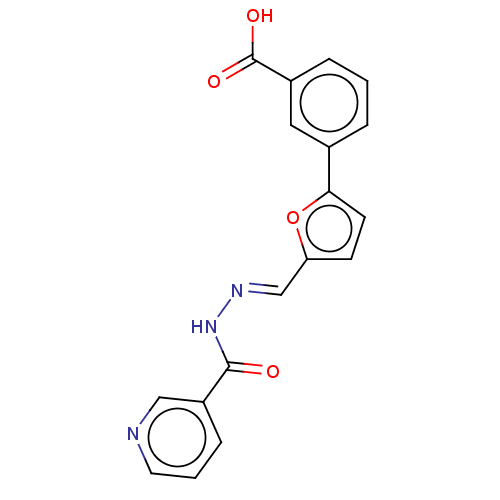 (CHEMBL3197264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114402 (CHEMBL3608327) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114399 (CHEMBL3608330) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114404 (CHEMBL3608325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114400 (CHEMBL3608329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114403 (CHEMBL3608326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114399 (CHEMBL3608330) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114405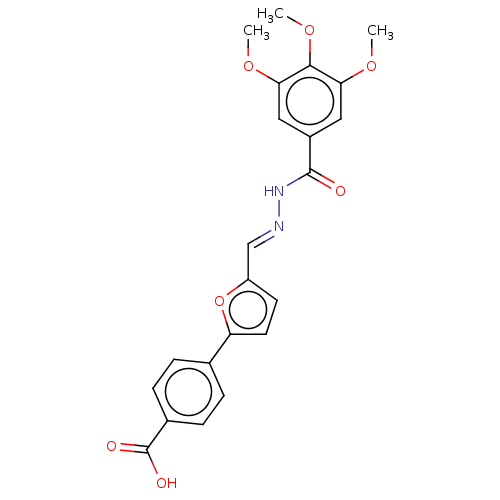 (CHEMBL3609813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114405 (CHEMBL3609813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114411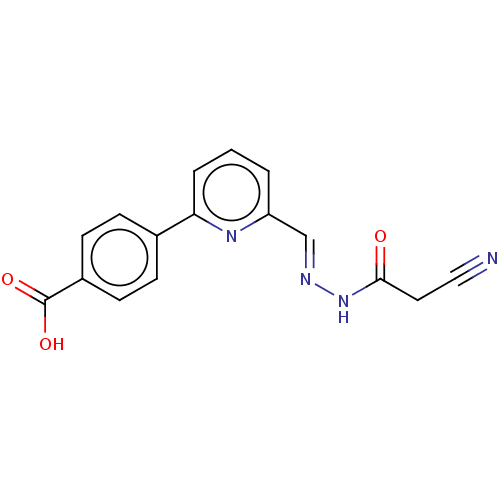 (CHEMBL3608331) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114412 (CHEMBL3609812) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114407 (CHEMBL3197264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114402 (CHEMBL3608327) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114401 (CHEMBL3608328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114410 (CHEMBL3608332) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human liver purified lactate dehydrogenase-A using pyruvate as substrate assessed as NADH oxidation for 3 mins by fluorimetric method | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114406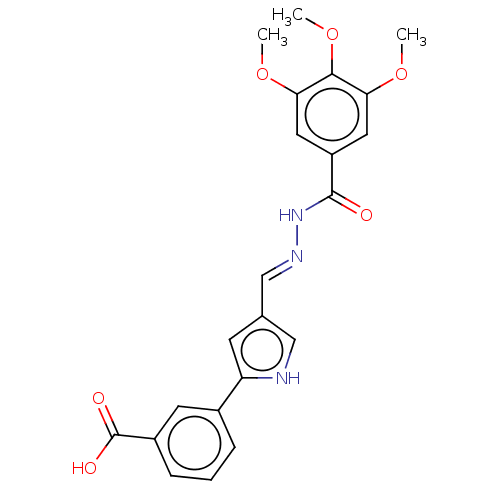 (CHEMBL3609811) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50114408 (CHEMBL3609810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of lactate dehydrogenase-A in human Raji cells assessed as reduction of intracellular lactate level after 3 hrs | Eur J Med Chem 101: 63-70 (2015) Article DOI: 10.1016/j.ejmech.2015.06.028 BindingDB Entry DOI: 10.7270/Q2NS0WQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Breast cancer type 2 susceptibility protein/DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM65519 (F0665-0303 | N-cyclopentyl-2-((5-((2-oxobenzo[d]th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia | Assay Description This assay is an efficient tool for directly measuring inhibition of the BRC4−Rad51 interaction, at a molecular level, which is described by Ra... | ACS Chem Biol 12: 2491-2497 (2017) Article DOI: 10.1021/acschembio.7b00707 BindingDB Entry DOI: 10.7270/Q2WS8RD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Breast cancer type 2 susceptibility protein/DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM65520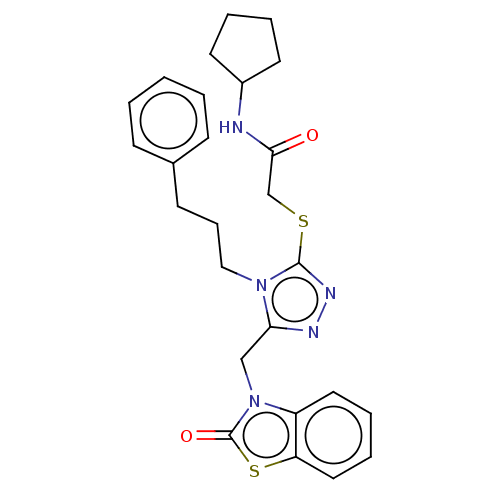 (N-cyclopentyl-2-((5-((2-oxobenzo[d]thiazol-3(2H)-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia | Assay Description This assay is an efficient tool for directly measuring inhibition of the BRC4−Rad51 interaction, at a molecular level, which is described by Ra... | ACS Chem Biol 12: 2491-2497 (2017) Article DOI: 10.1021/acschembio.7b00707 BindingDB Entry DOI: 10.7270/Q2WS8RD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50536924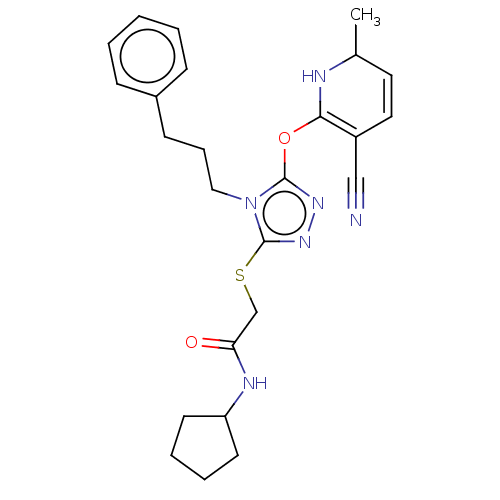 (CHEMBL4538360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50536925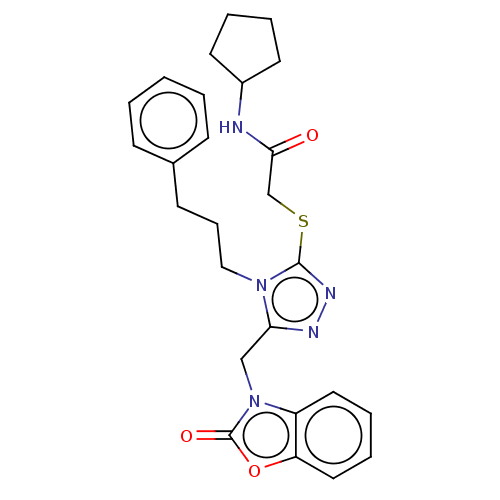 (CHEMBL4552593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50536926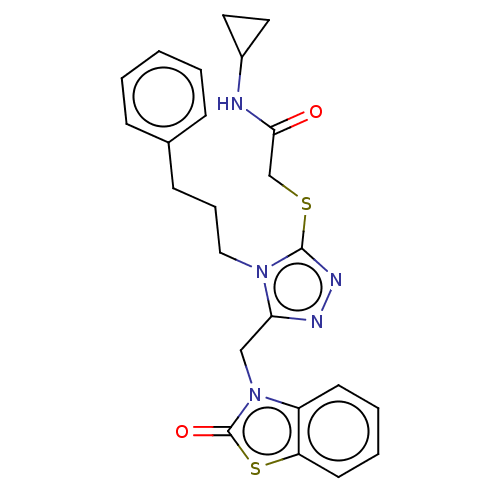 (CHEMBL4559055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50536927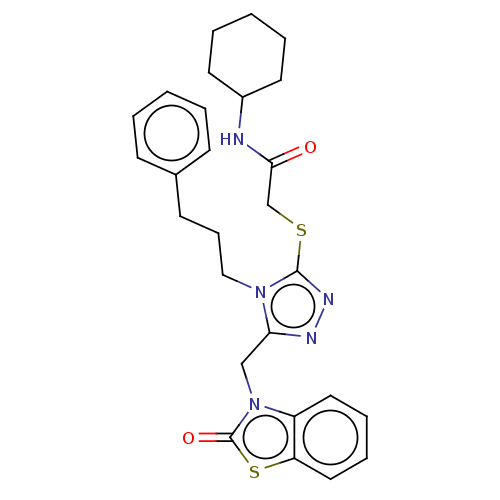 (CHEMBL4530772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM65520 (N-cyclopentyl-2-((5-((2-oxobenzo[d]thiazol-3(2H)-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM65519 (F0665-0303 | N-cyclopentyl-2-((5-((2-oxobenzo[d]th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50536928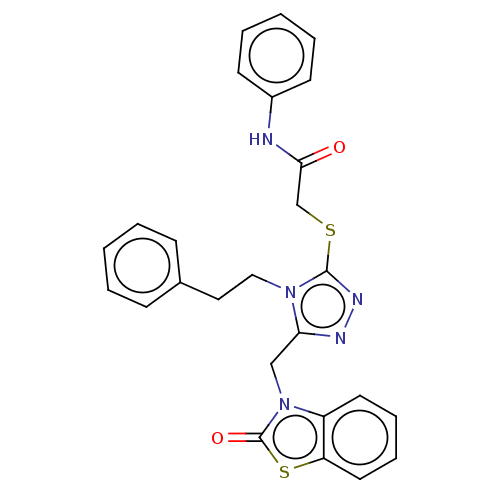 (CHEMBL4553930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50536929 (CHEMBL4551898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50536930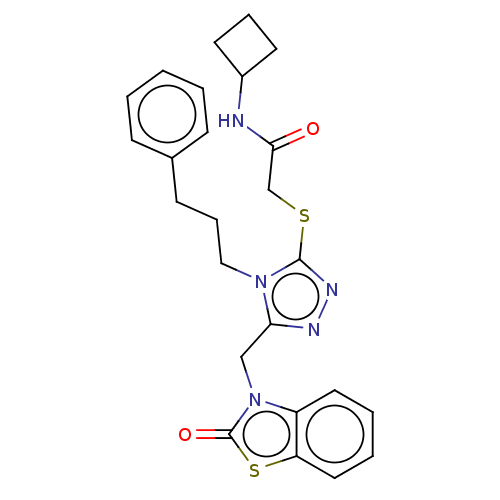 (CHEMBL4540385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of N-terminal biotinylated-BRC4 from recombinant human Rad51 expressed in Escherichia coli by ELISA | Eur J Med Chem 165: 80-92 (2019) Article DOI: 10.1016/j.ejmech.2019.01.008 BindingDB Entry DOI: 10.7270/Q26H4MZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527105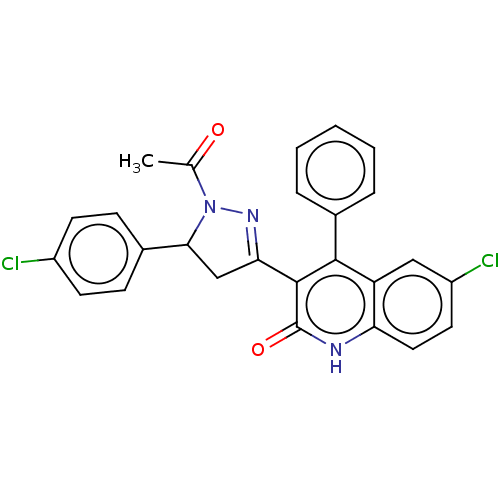 (CHEMBL4574296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527106 (CHEMBL4559865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527107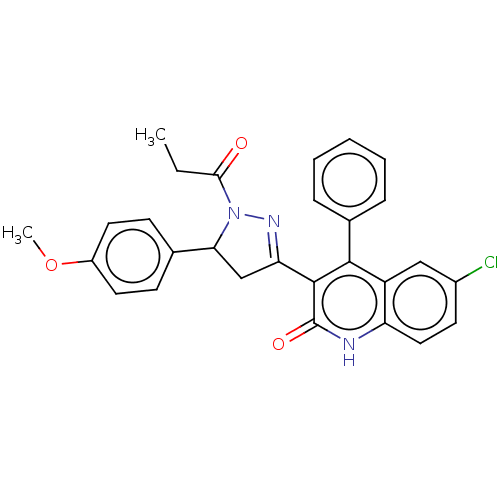 (CHEMBL4450955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Binding affinity to recombinant His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells by microscale thermophoresis analysis | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527108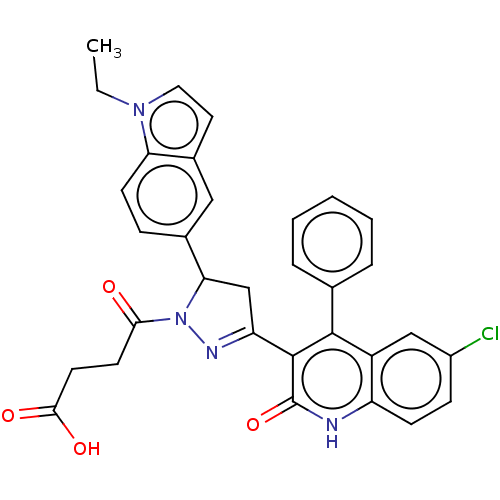 (CHEMBL4470365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527109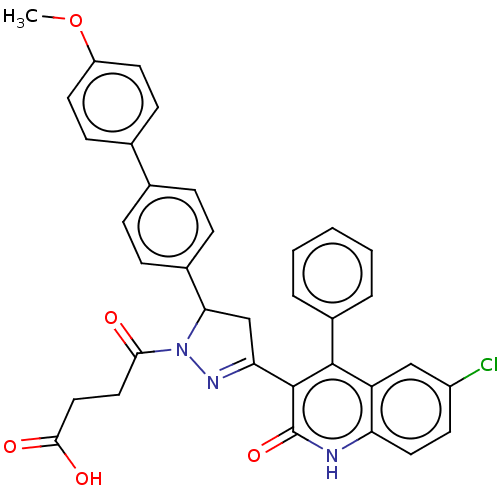 (CHEMBL4461983) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527110 (CHEMBL4549187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50440089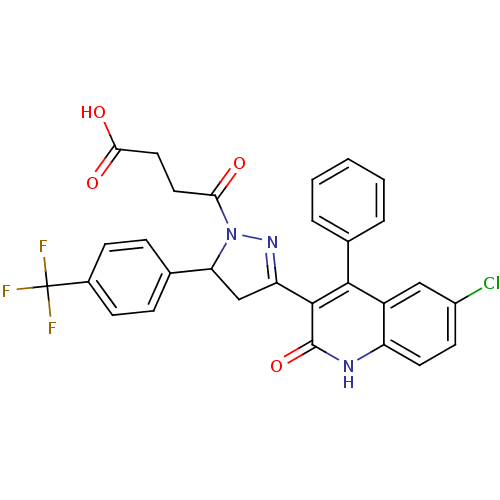 (CHEMBL2426089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50440092 (CHEMBL2426085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527111 (CHEMBL4555660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527112 (CHEMBL4589515) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527113 (CHEMBL4593903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527107 (CHEMBL4450955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50527114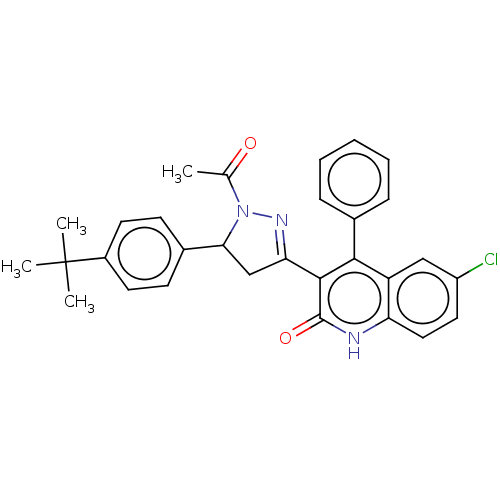 (CHEMBL4572566) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of biotinylated BRC4 peptide binding to His-tagged human RAD51 expressed in Escherichia coli Rosetta(DE3) pLysS cells assessed as BRC4-RAD... | J Med Chem 63: 2588-2619 (2020) Article DOI: 10.1021/acs.jmedchem.9b01526 BindingDB Entry DOI: 10.7270/Q2SF30MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |