

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014480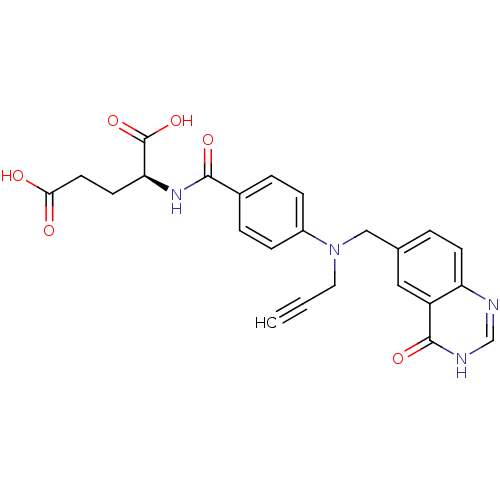 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of thymidylate synthase | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50014480 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081273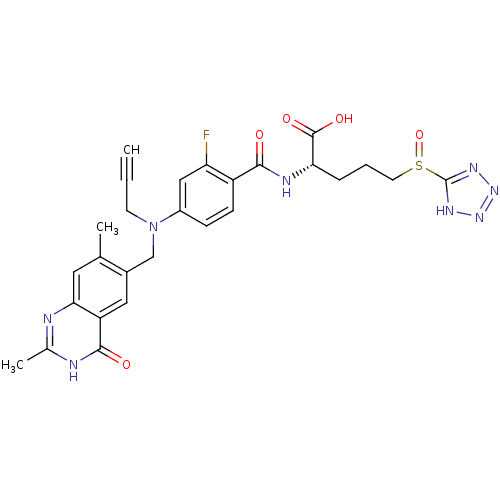 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081256 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081259 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081265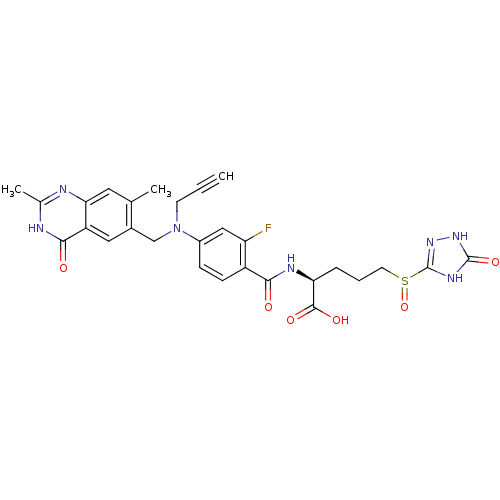 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116610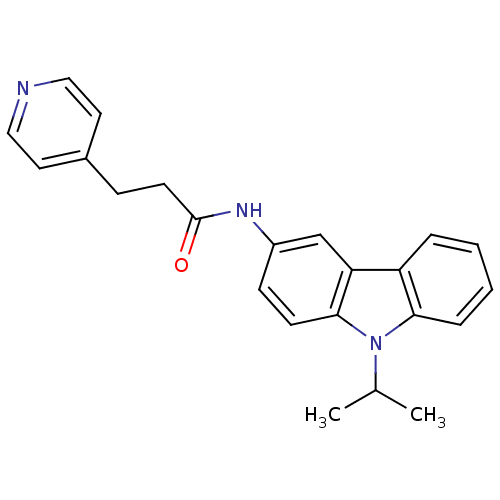 (CHEMBL119743 | N-(9-Isopropyl-9H-carbazol-3-yl)-3-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116590 (CHEMBL325486 | N-(9-Methanesulfonyl-9H-carbazol-3-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081240 (2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081252 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081246 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081243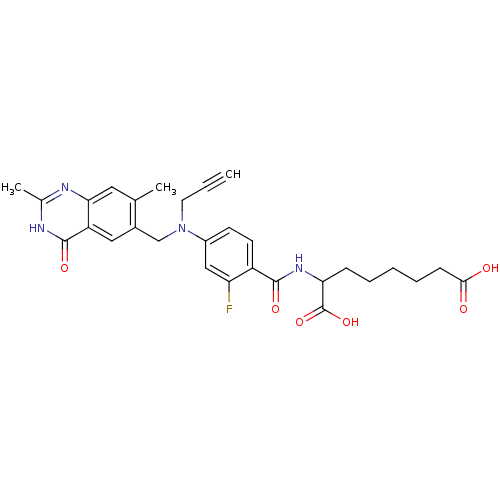 (2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081260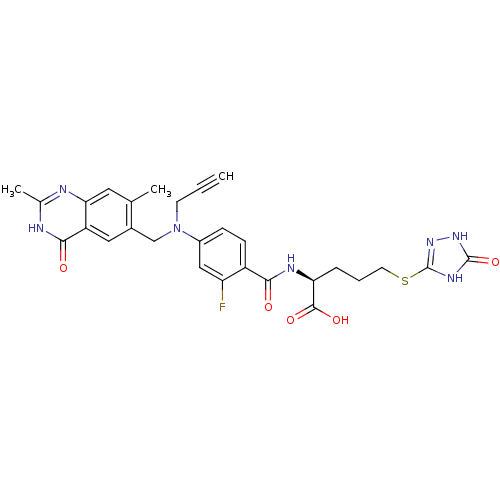 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081261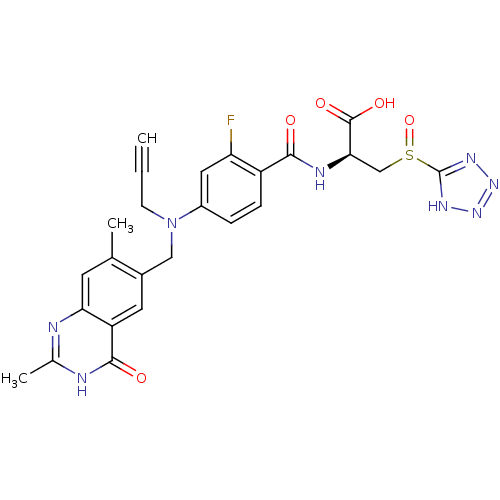 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081247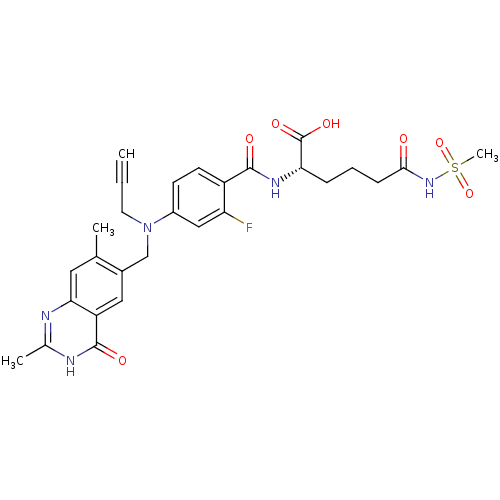 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116619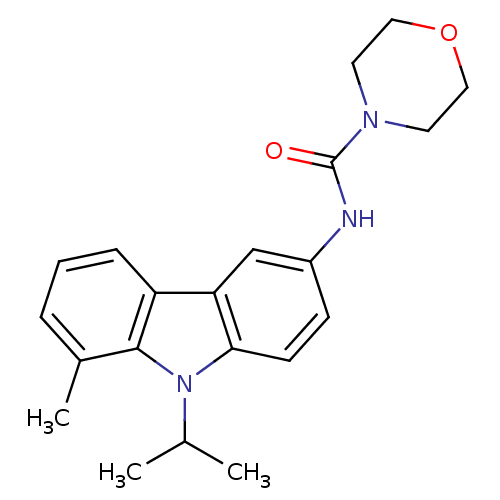 (CHEMBL117563 | Morpholine-4-carboxylic acid (9-iso...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116602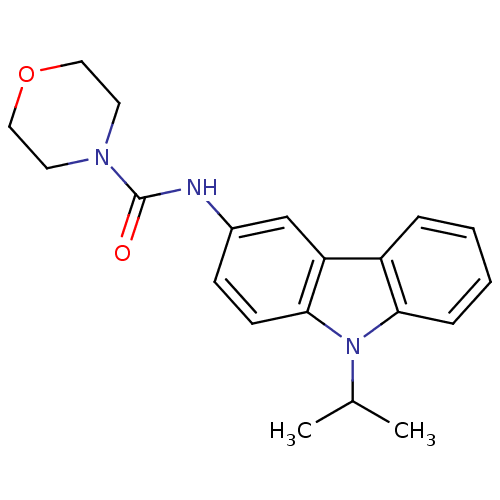 (CHEMBL419951 | Morpholine-4-carboxylic acid (9-iso...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116600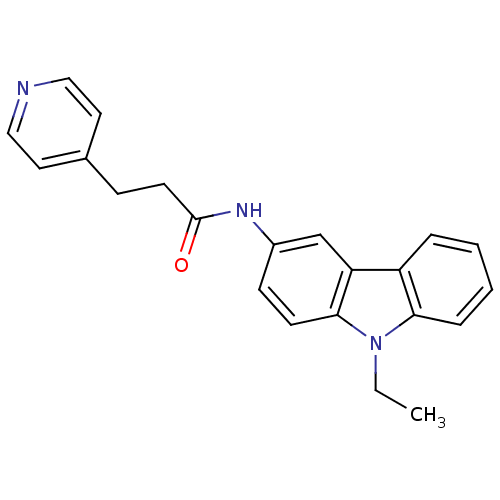 (CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081239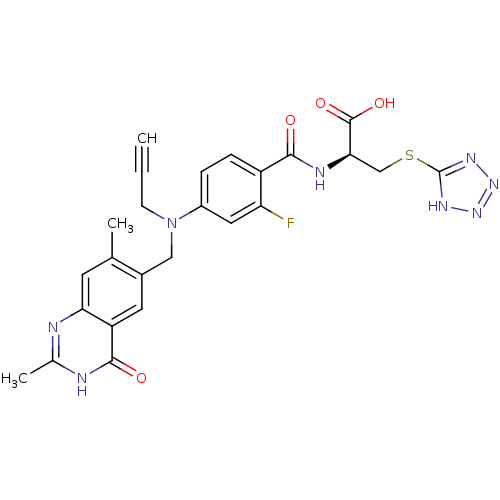 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081253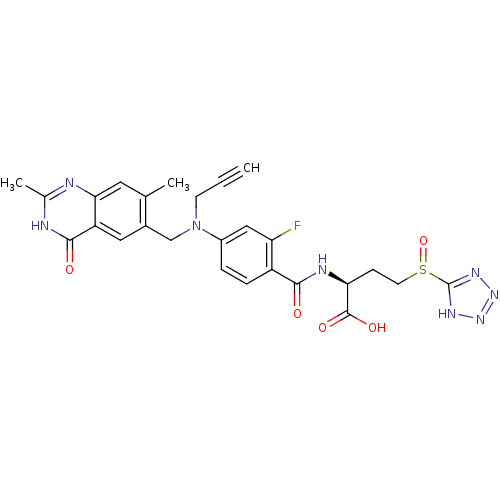 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081272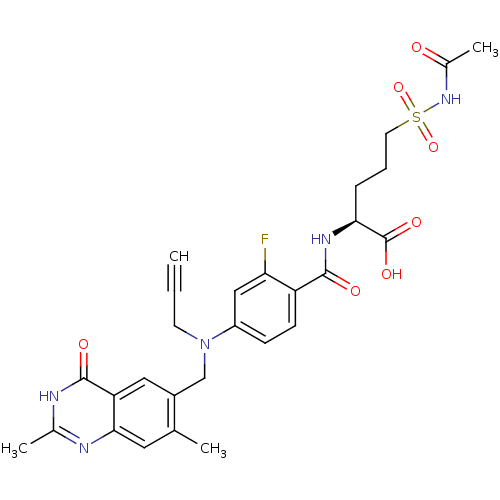 ((S)-5-Acetylsulfamoyl-2-{4-[(2,7-dimethyl-4-oxo-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081264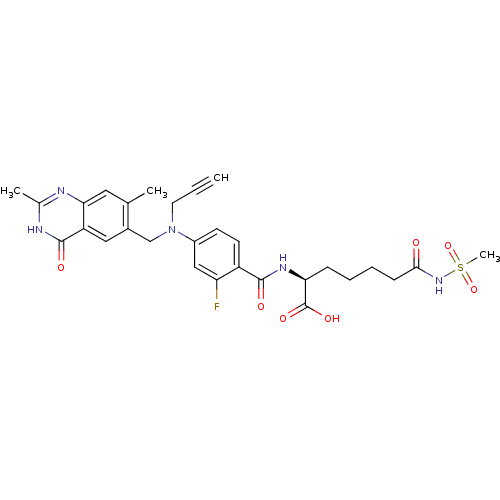 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081249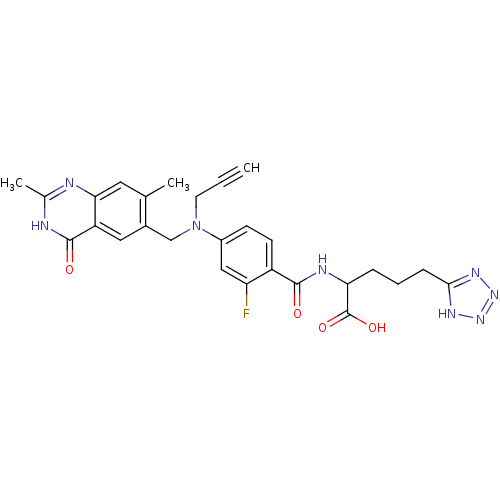 (2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116592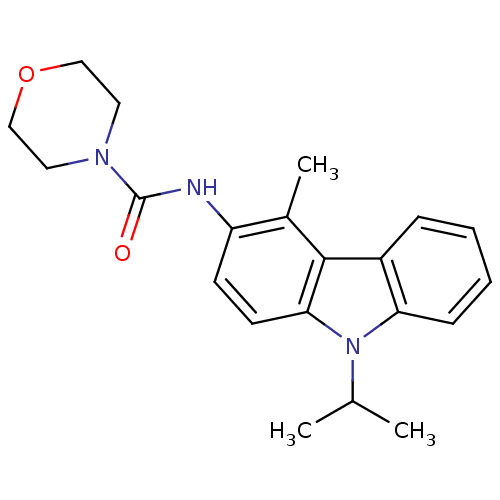 (CHEMBL325226 | Morpholine-4-carboxylic acid (9-iso...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116605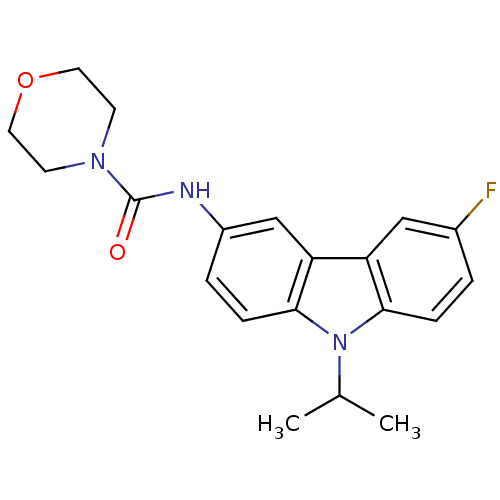 (CHEMBL432628 | Morpholine-4-carboxylic acid (6-flu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116617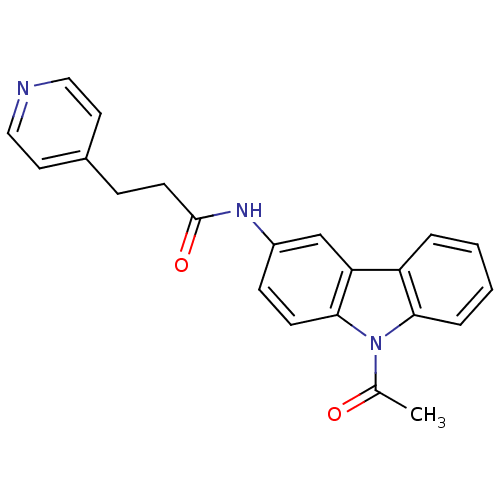 (CHEMBL116210 | N-(9-Acetyl-9H-carbazol-3-yl)-3-pyr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081258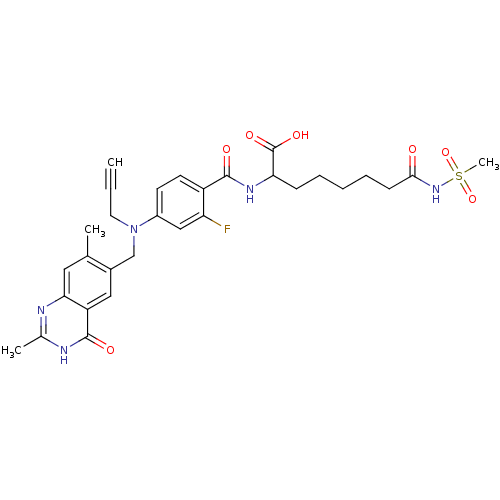 (2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081251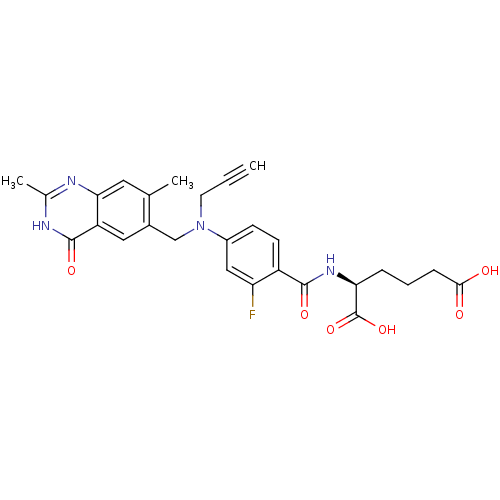 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081262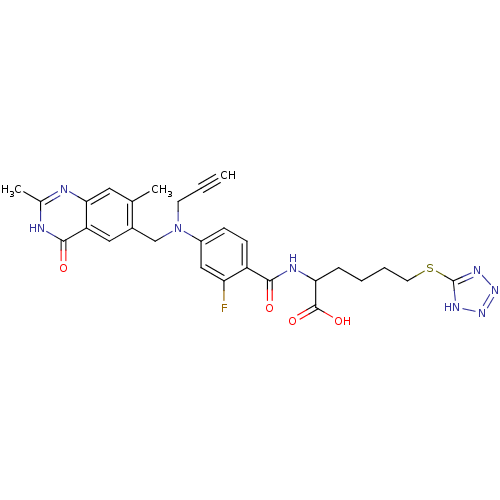 (2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081248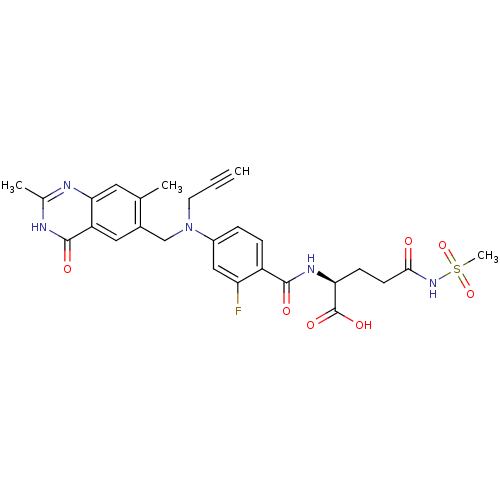 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033933 ((R)-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081266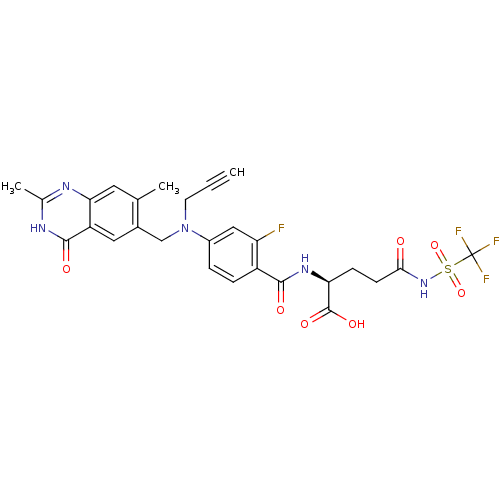 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081241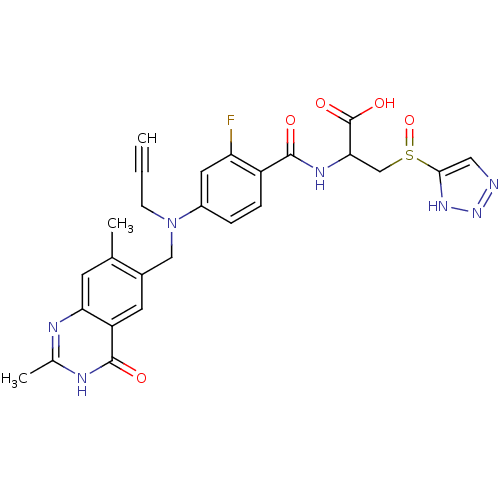 (2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081267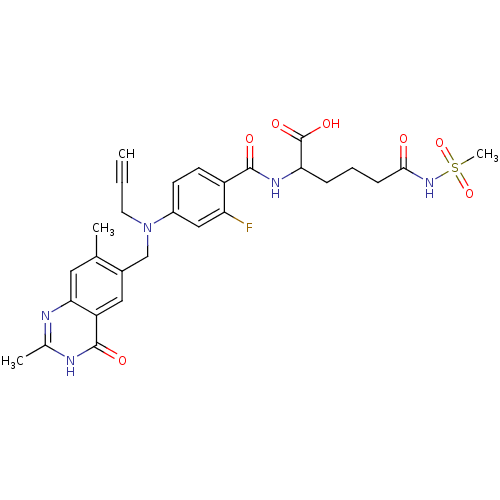 (2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081271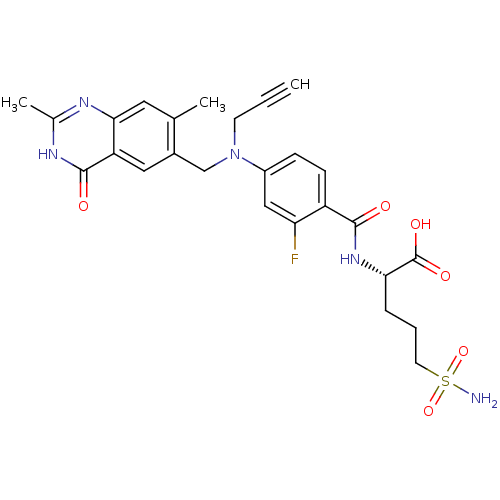 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081270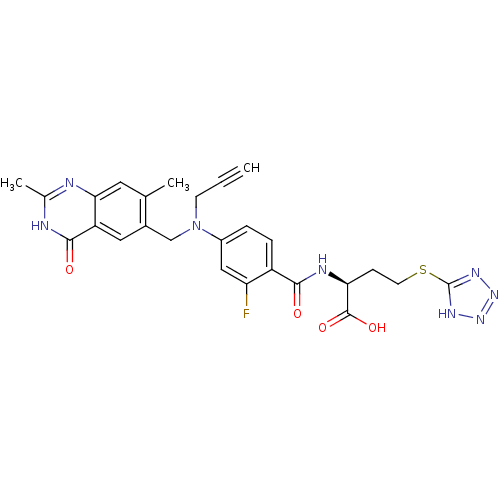 ((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033920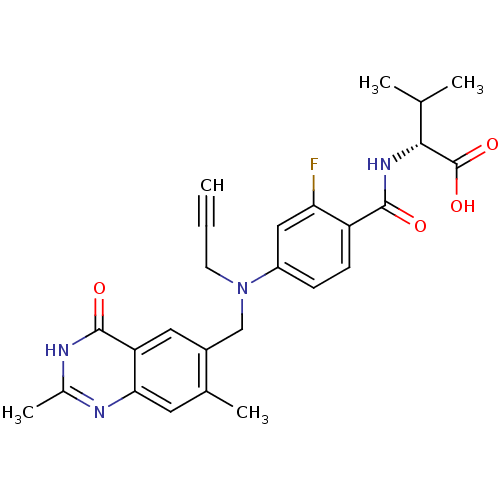 ((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit Thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116592 (CHEMBL325226 | Morpholine-4-carboxylic acid (9-iso...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Compound was evaluated for functional antagonism of Neuropeptide Y receptor Y5 activity in cellular Ca flux | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081255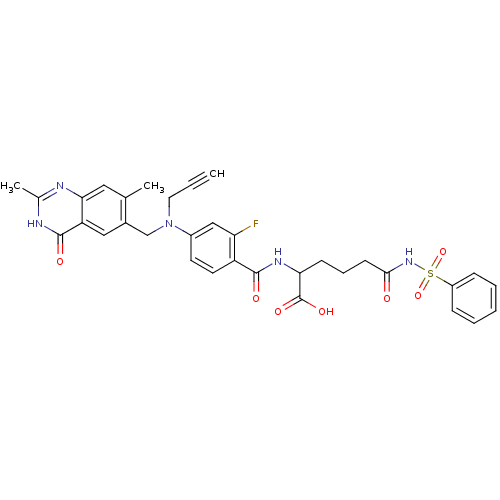 (6-Benzenesulfonylamino-2-{4-[(2,7-dimethyl-4-oxo-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50116600 (CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity against rat Neuropeptide Y receptor Y5 | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116597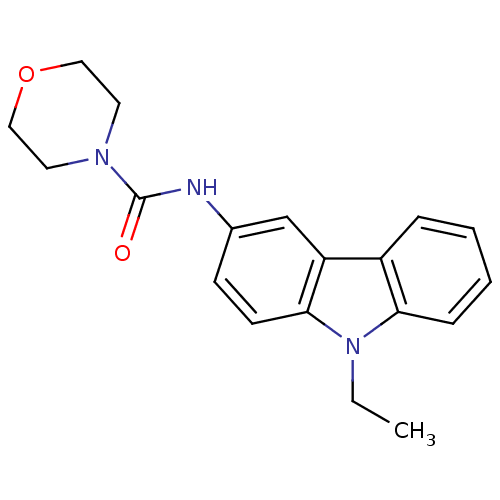 (CHEMBL119247 | Morpholine-4-carboxylic acid (9-eth...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033901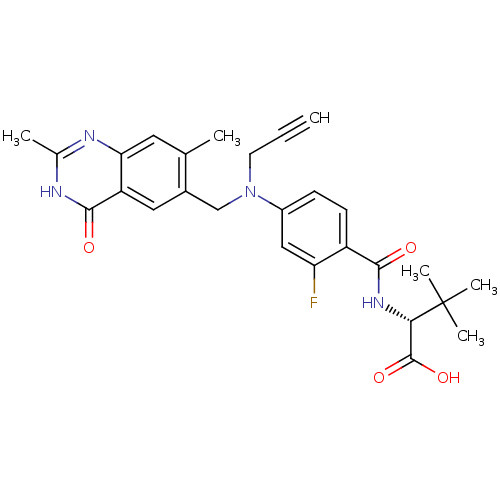 ((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit Thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50081257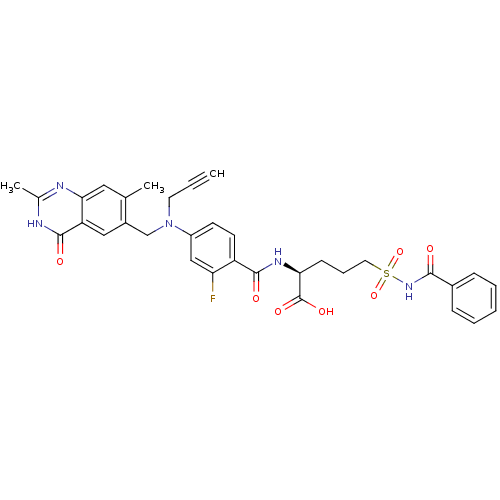 ((S)-5-Benzoylsulfamoyl-2-{4-[(2,7-dimethyl-4-oxo-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50116608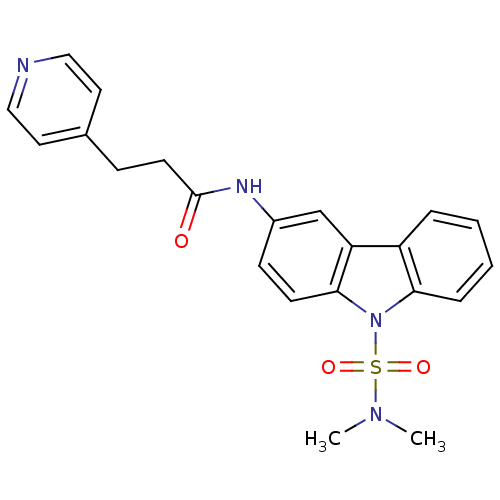 (CHEMBL117922 | N-(9-Dimethylsulfamoyl-9H-carbazol-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) | J Med Chem 45: 3509-23 (2002) BindingDB Entry DOI: 10.7270/Q27S7N3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014498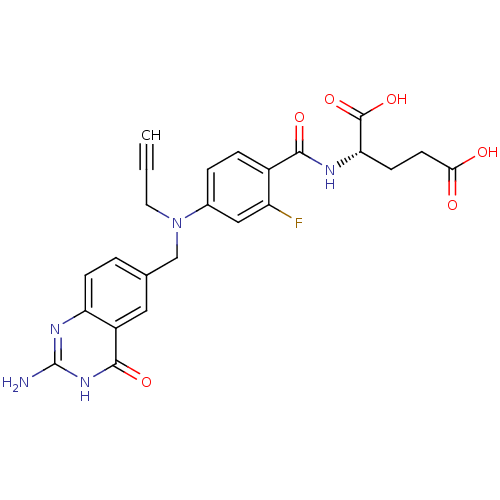 ((S)-2-(4-(((2-amino-4-oxo-3,4-dihydroquinazolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033913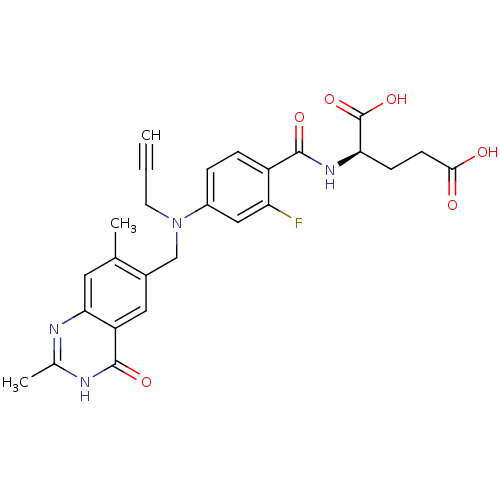 ((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 311 total ) | Next | Last >> |