 Found 1430 hits with Last Name = 'martin' and Initial = 'f'
Found 1430 hits with Last Name = 'martin' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50027230
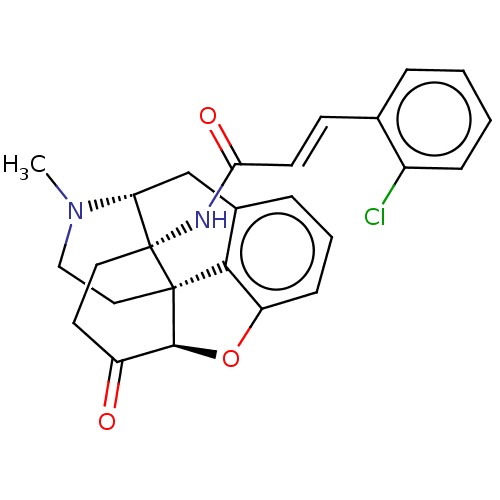
(CHEMBL2113666)Show SMILES [H][C@@]12Oc3cccc4C[C@@]5([H])N(C)CC[C@@]1(c34)[C@]5(CCC2=O)NC(=O)\C=C\c1ccccc1Cl |r,THB:12:11:17:8.7.16| Show InChI InChI=1S/C26H25ClN2O3/c1-29-14-13-25-23-17-6-4-8-20(23)32-24(25)19(30)11-12-26(25,21(29)15-17)28-22(31)10-9-16-5-2-3-7-18(16)27/h2-10,21,24H,11-15H2,1H3,(H,28,31)/b10-9+/t21-,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human cloned delta opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM197
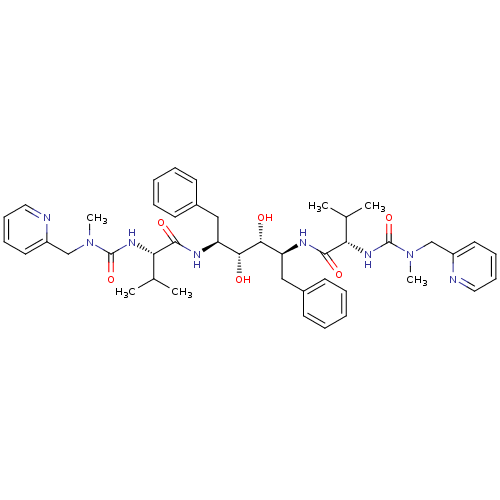
((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36-,37-,38-,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50451631

(CHEMBL4212454)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(nn1C)C(C)(C)C |r| Show InChI InChI=1S/C21H28N6O2/c1-21(2,3)18-13-17(26(5)24-18)19(28)23-16(12-15-10-8-7-9-11-15)20(29)27(6)25(4)14-22/h7-11,13,16H,12H2,1-6H3,(H,23,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027235
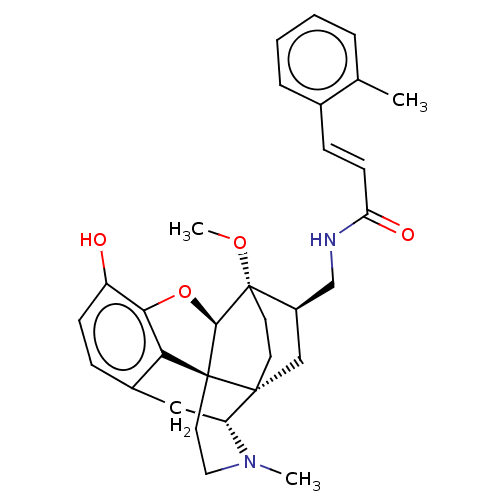
(CHEMBL2113304)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccccc2C)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C31H36N2O4/c1-19-6-4-5-7-20(19)9-11-25(35)32-18-22-17-29-12-13-31(22,36-3)28-30(29)14-15-33(2)24(29)16-21-8-10-23(34)27(37-28)26(21)30/h4-11,22,24,28,34H,12-18H2,1-3H3,(H,32,35)/b11-9+/t22-,24-,28-,29-,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130607
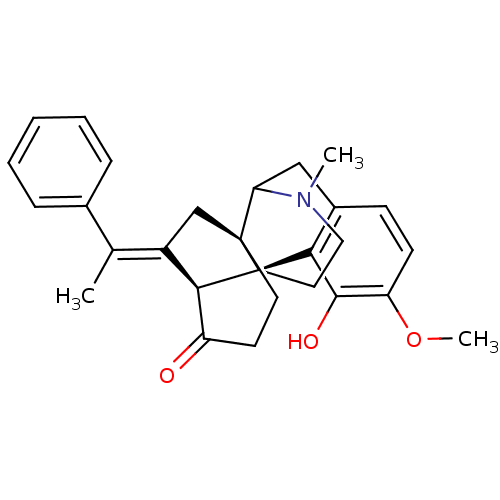
(3-hydroxy-4-methoxy-17-methyl-18-[1-phenyl-(E)-eth...)Show SMILES COc1ccc2CC3N(C)CC[C@@]4([C@@H]5\C(C[C@]34CCC5=O)=C(/C)c3ccccc3)c2c1O |TLB:4:5:16:8.11.10,30:29:16:8.11.10,THB:21:14:12:19.17.18| Show InChI InChI=1S/C28H31NO3/c1-17(18-7-5-4-6-8-18)20-16-27-12-11-21(30)25(20)28(27)13-14-29(2)23(27)15-19-9-10-22(32-3)26(31)24(19)28/h4-10,23,25,31H,11-16H2,1-3H3/b20-17+/t23?,25-,27-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity in recombinant human Opioid receptor kappa 1 transfected into chinese hamster ovary cells by displacing [3H]U-69593 radioligand |
J Med Chem 46: 3174-7 (2003)
Article DOI: 10.1021/jm030801n
BindingDB Entry DOI: 10.7270/Q2TH8NGD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027230

(CHEMBL2113666)Show SMILES [H][C@@]12Oc3cccc4C[C@@]5([H])N(C)CC[C@@]1(c34)[C@]5(CCC2=O)NC(=O)\C=C\c1ccccc1Cl |r,THB:12:11:17:8.7.16| Show InChI InChI=1S/C26H25ClN2O3/c1-29-14-13-25-23-17-6-4-8-20(23)32-24(25)19(30)11-12-26(25,21(29)15-17)28-22(31)10-9-16-5-2-3-7-18(16)27/h2-10,21,24H,11-15H2,1H3,(H,28,31)/b10-9+/t21-,24+,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064201
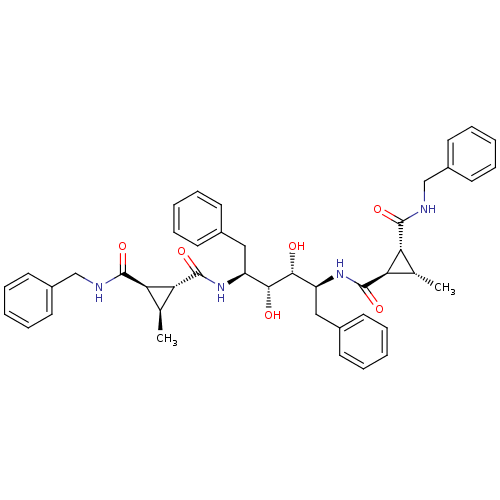
(1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoyl-3-meth...)Show SMILES C[C@H]1[C@H]([C@@H]1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1[C@@H](C)[C@H]1C(=O)NCc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C44H50N4O6/c1-27-35(41(51)45-25-31-19-11-5-12-20-31)37(27)43(53)47-33(23-29-15-7-3-8-16-29)39(49)40(50)34(24-30-17-9-4-10-18-30)48-44(54)38-28(2)36(38)42(52)46-26-32-21-13-6-14-22-32/h3-22,27-28,33-40,49-50H,23-26H2,1-2H3,(H,45,51)(H,46,52)(H,47,53)(H,48,54)/t27-,28-,33-,34-,35+,36+,37+,38+,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064202
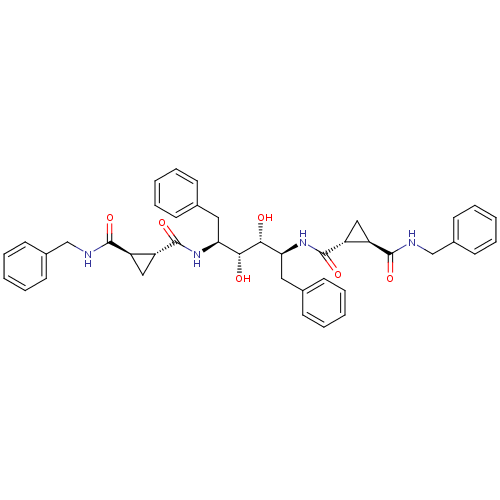
(1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoylcyclopr...)Show SMILES O[C@@H]([C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1C[C@H]1C(=O)NCc1ccccc1)[C@H](Cc1ccccc1)NC(=O)[C@@H]1C[C@H]1C(=O)NCc1ccccc1 Show InChI InChI=1S/C42H46N4O6/c47-37(35(21-27-13-5-1-6-14-27)45-41(51)33-23-31(33)39(49)43-25-29-17-9-3-10-18-29)38(48)36(22-28-15-7-2-8-16-28)46-42(52)34-24-32(34)40(50)44-26-30-19-11-4-12-20-30/h1-20,31-38,47-48H,21-26H2,(H,43,49)(H,44,50)(H,45,51)(H,46,52)/t31-,32-,33-,34-,35+,36+,37-,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027244

(CHEMBL2113307)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccc(Cl)cc2)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C30H33ClN2O4/c1-33-14-13-29-25-19-6-9-22(34)26(25)37-27(29)30(36-2)12-11-28(29,23(33)15-19)16-20(30)17-32-24(35)10-5-18-3-7-21(31)8-4-18/h3-10,20,23,27,34H,11-17H2,1-2H3,(H,32,35)/b10-5+/t20-,23-,27-,28-,29+,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027242

(CHEMBL2113308)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccc(cc2)[N+]([O-])=O)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C30H33N3O6/c1-32-14-13-29-25-19-6-9-22(34)26(25)39-27(29)30(38-2)12-11-28(29,23(32)15-19)16-20(30)17-31-24(35)10-5-18-3-7-21(8-4-18)33(36)37/h3-10,20,23,27,34H,11-17H2,1-2H3,(H,31,35)/b10-5+/t20-,23-,27-,28-,29+,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130602
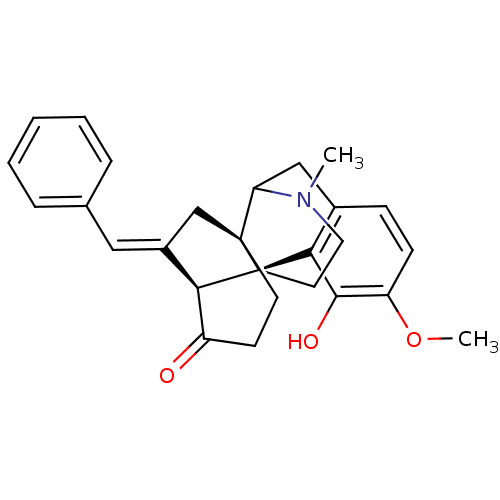
(3-hydroxy-4-methoxy-17-methyl-18-[1-phenyl-(E)-met...)Show SMILES COc1ccc2CC3N(C)CC[C@@]4([C@@H]5\C(C[C@]34CCC5=O)=C\c3ccccc3)c2c1O |TLB:4:5:16:8.11.10,29:28:16:8.11.10,THB:21:14:12:17.19.18| Show InChI InChI=1S/C27H29NO3/c1-28-13-12-27-23-19(14-17-6-4-3-5-7-17)16-26(27,11-10-20(23)29)22(28)15-18-8-9-21(31-2)25(30)24(18)27/h3-9,14,22-23,30H,10-13,15-16H2,1-2H3/b19-14+/t22?,23-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity in recombinant human Opioid receptor kappa 1 transfected into chinese hamster ovary cells by displacing [3H]U-69593 radioligand |
J Med Chem 46: 3174-7 (2003)
Article DOI: 10.1021/jm030801n
BindingDB Entry DOI: 10.7270/Q2TH8NGD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity for human Opioid receptor delta 1 transfected into chinese hamster ovary cells by displacing [3H]CI-DPDPE radioligand |
J Med Chem 46: 3174-7 (2003)
Article DOI: 10.1021/jm030801n
BindingDB Entry DOI: 10.7270/Q2TH8NGD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity for human Opioid receptor mu 1 transfected into chinese hamster ovary cells by displacing [3H]DAMGO radioligand |
J Med Chem 46: 3174-7 (2003)
Article DOI: 10.1021/jm030801n
BindingDB Entry DOI: 10.7270/Q2TH8NGD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027246
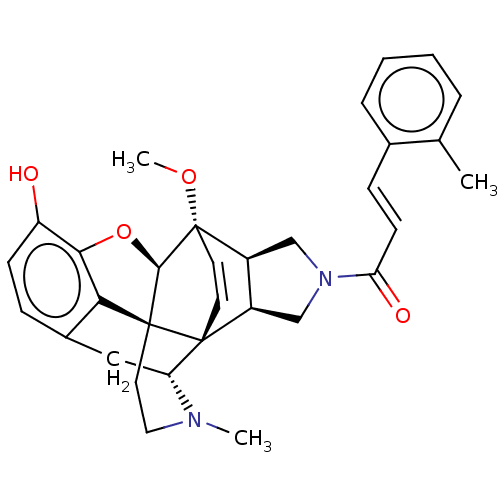
(CHEMBL2113313)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]13C=C[C@@]2(OC)[C@]2([H])Oc4c5c(C[C@@]1([H])N(C)CC[C@@]325)ccc4O)C(=O)\C=C\c1ccccc1C |c:9,TLB:23:22:18.17.19:7| Show InChI InChI=1S/C32H34N2O4/c1-19-6-4-5-7-20(19)9-11-26(36)34-17-22-23(18-34)32(37-3)13-12-30(22)25-16-21-8-10-24(35)28-27(21)31(30,29(32)38-28)14-15-33(25)2/h4-13,22-23,25,29,35H,14-18H2,1-3H3/b11-9+/t22-,23+,25-,29-,30-,31+,32+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027243
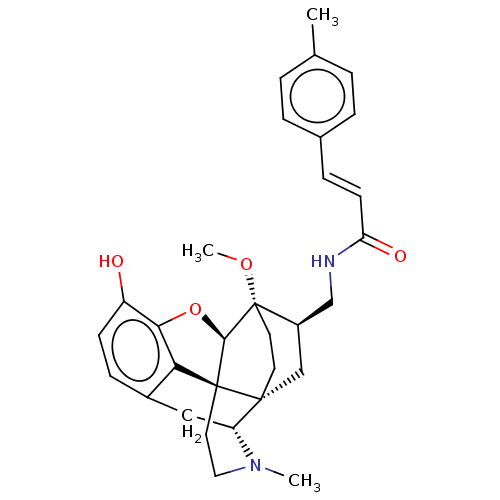
(CHEMBL2113306)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccc(C)cc2)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C31H36N2O4/c1-19-4-6-20(7-5-19)8-11-25(35)32-18-22-17-29-12-13-31(22,36-3)28-30(29)14-15-33(2)24(29)16-21-9-10-23(34)27(37-28)26(21)30/h4-11,22,24,28,34H,12-18H2,1-3H3,(H,32,35)/b11-8+/t22-,24-,28-,29-,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027236
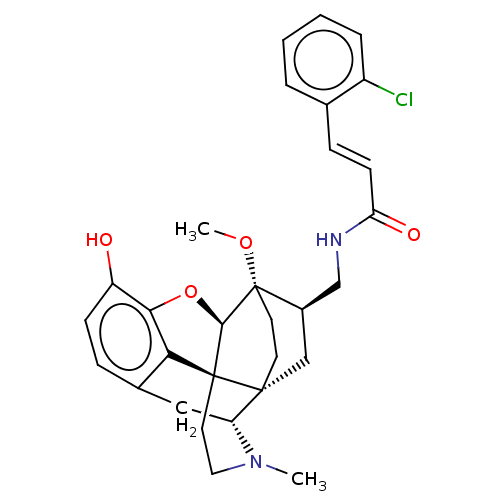
(CHEMBL2113305)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccccc2Cl)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C30H33ClN2O4/c1-33-14-13-29-25-19-7-9-22(34)26(25)37-27(29)30(36-2)12-11-28(29,23(33)15-19)16-20(30)17-32-24(35)10-8-18-5-3-4-6-21(18)31/h3-10,20,23,27,34H,11-17H2,1-2H3,(H,32,35)/b10-8+/t20-,23-,27-,28-,29+,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027238
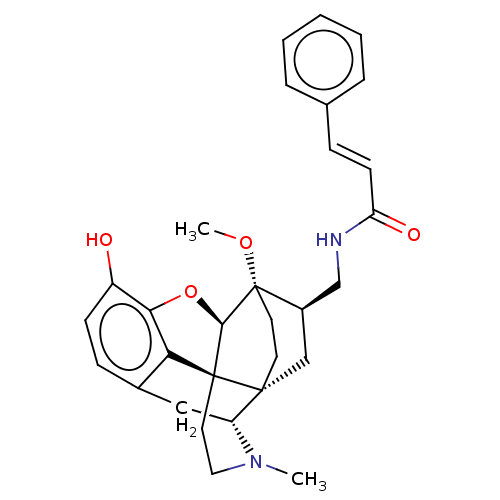
(CHEMBL2113303)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccccc2)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C30H34N2O4/c1-32-15-14-29-25-20-9-10-22(33)26(25)36-27(29)30(35-2)13-12-28(29,23(32)16-20)17-21(30)18-31-24(34)11-8-19-6-4-3-5-7-19/h3-11,21,23,27,33H,12-18H2,1-2H3,(H,31,34)/b11-8+/t21-,23-,27-,28-,29+,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549366

(CHEMBL4755248)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NS(=O)(=O)c1cccc(Br)c1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064199
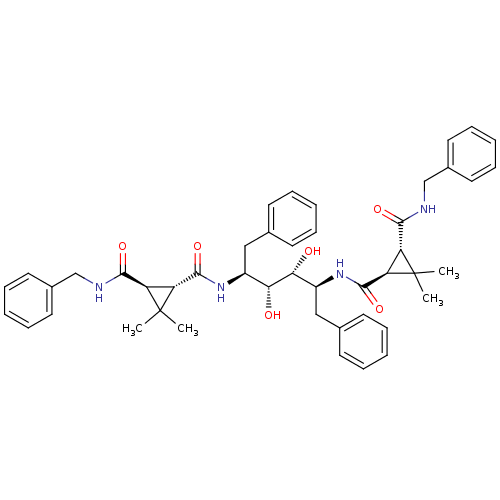
(1N-benzyl-2N-[1-benzyl-4-(3-benzylcarbamoyl-2,2-di...)Show SMILES CC1(C)[C@H]([C@@H]1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H]1[C@H](C(=O)NCc2ccccc2)C1(C)C)C(=O)NCc1ccccc1 Show InChI InChI=1S/C46H54N4O6/c1-45(2)35(41(53)47-27-31-21-13-7-14-22-31)37(45)43(55)49-33(25-29-17-9-5-10-18-29)39(51)40(52)34(26-30-19-11-6-12-20-30)50-44(56)38-36(46(38,3)4)42(54)48-28-32-23-15-8-16-24-32/h5-24,33-40,51-52H,25-28H2,1-4H3,(H,47,53)(H,48,54)(H,49,55)(H,50,56)/t33-,34-,35+,36+,37+,38+,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027248
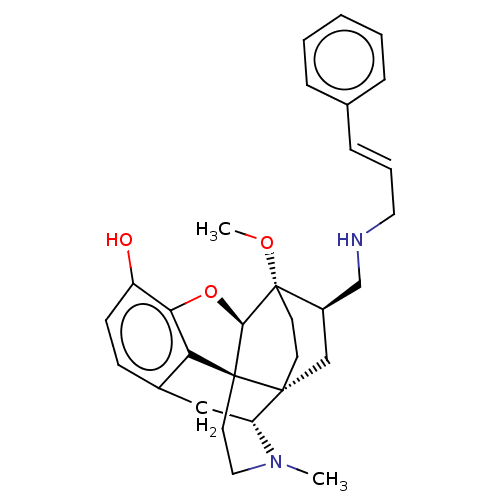
(CHEMBL2113311)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC\C=C\c2ccccc2)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C30H36N2O3/c1-32-16-14-29-25-21-10-11-23(33)26(25)35-27(29)30(34-2)13-12-28(29,24(32)17-21)18-22(30)19-31-15-6-9-20-7-4-3-5-8-20/h3-11,22,24,27,31,33H,12-19H2,1-2H3/b9-6+/t22-,24-,27-,28-,29+,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity in recombinant human Opioid receptor kappa 1 transfected into chinese hamster ovary cells by displacing [3H]U-69593 radioligand |
J Med Chem 46: 3174-7 (2003)
Article DOI: 10.1021/jm030801n
BindingDB Entry DOI: 10.7270/Q2TH8NGD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human cloned kappa opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50130602

(3-hydroxy-4-methoxy-17-methyl-18-[1-phenyl-(E)-met...)Show SMILES COc1ccc2CC3N(C)CC[C@@]4([C@@H]5\C(C[C@]34CCC5=O)=C\c3ccccc3)c2c1O |TLB:4:5:16:8.11.10,29:28:16:8.11.10,THB:21:14:12:17.19.18| Show InChI InChI=1S/C27H29NO3/c1-28-13-12-27-23-19(14-17-6-4-3-5-7-17)16-26(27,11-10-20(23)29)22(28)15-18-8-9-21(31-2)25(30)24(18)27/h3-9,14,22-23,30H,10-13,15-16H2,1-2H3/b19-14+/t22?,23-,26-,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity for human Opioid receptor mu 1 transfected into chinese hamster ovary cells by displacing [3H]DAMGO radioligand |
J Med Chem 46: 3174-7 (2003)
Article DOI: 10.1021/jm030801n
BindingDB Entry DOI: 10.7270/Q2TH8NGD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064203
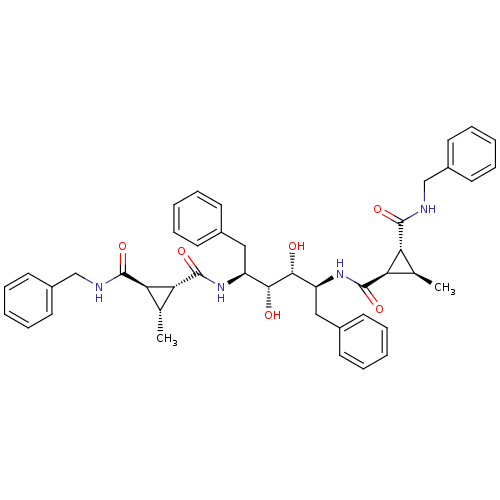
(1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoyl-3-meth...)Show SMILES C[C@@H]1[C@H]([C@@H]1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1[C@H](C)[C@H]1C(=O)NCc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C44H50N4O6/c1-27-35(41(51)45-25-31-19-11-5-12-20-31)37(27)43(53)47-33(23-29-15-7-3-8-16-29)39(49)40(50)34(24-30-17-9-4-10-18-30)48-44(54)38-28(2)36(38)42(52)46-26-32-21-13-6-14-22-32/h3-22,27-28,33-40,49-50H,23-26H2,1-2H3,(H,45,51)(H,46,52)(H,47,53)(H,48,54)/t27-,28-,33+,34+,35-,36-,37-,38-,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027247

(CHEMBL2113314)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]13C=C[C@@]2(OC)[C@]2([H])Oc4c5c(C[C@@]1([H])N(C)CC[C@@]325)ccc4O)C(=O)\C=C\c1ccc(C)cc1 |c:9,TLB:23:22:18.17.19:7| Show InChI InChI=1S/C32H34N2O4/c1-19-4-6-20(7-5-19)8-11-26(36)34-17-22-23(18-34)32(37-3)13-12-30(22)25-16-21-9-10-24(35)28-27(21)31(30,29(32)38-28)14-15-33(25)2/h4-13,22-23,25,29,35H,14-18H2,1-3H3/b11-8+/t22-,23+,25-,29-,30-,31+,32+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50451625

(CHEMBL4217414)Show SMILES CC(C)C[C@H](NC(=O)c1cc(nn1C)C(C)(C)C)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C18H30N6O2/c1-12(2)9-13(17(26)24(8)22(6)11-19)20-16(25)14-10-15(18(3,4)5)21-23(14)7/h10,12-13H,9H2,1-8H3,(H,20,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549370

(CHEMBL4746022)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Br)c1)NC(=O)c1cc(nn1C)C(C)(C)C)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027246

(CHEMBL2113313)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]13C=C[C@@]2(OC)[C@]2([H])Oc4c5c(C[C@@]1([H])N(C)CC[C@@]325)ccc4O)C(=O)\C=C\c1ccccc1C |c:9,TLB:23:22:18.17.19:7| Show InChI InChI=1S/C32H34N2O4/c1-19-6-4-5-7-20(19)9-11-26(36)34-17-22-23(18-34)32(37-3)13-12-30(22)25-16-21-8-10-24(35)28-27(21)31(30,29(32)38-28)14-15-33(25)2/h4-13,22-23,25,29,35H,14-18H2,1-3H3/b11-9+/t22-,23+,25-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human cloned kappa opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027230

(CHEMBL2113666)Show SMILES [H][C@@]12Oc3cccc4C[C@@]5([H])N(C)CC[C@@]1(c34)[C@]5(CCC2=O)NC(=O)\C=C\c1ccccc1Cl |r,THB:12:11:17:8.7.16| Show InChI InChI=1S/C26H25ClN2O3/c1-29-14-13-25-23-17-6-4-8-20(23)32-24(25)19(30)11-12-26(25,21(29)15-17)28-22(31)10-9-16-5-2-3-7-18(16)27/h2-10,21,24H,11-15H2,1H3,(H,28,31)/b10-9+/t21-,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human cloned kappa opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130600
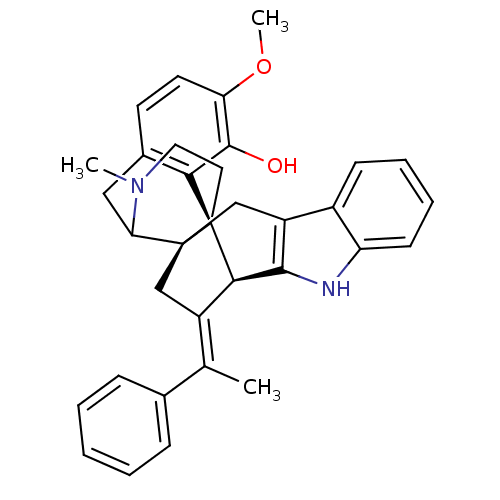
(19-methoxy-24-methyl-26-[1-phenyl-(E)-ethylidene]-...)Show SMILES COc1ccc2CC3N(C)CC[C@@]4([C@@H]5\C(C[C@]34Cc3c5[nH]c4ccccc34)=C(/C)c3ccccc3)c2c1O |TLB:36:35:16:8.11.10,4:5:16:8.11.10| Show InChI InChI=1S/C34H34N2O2/c1-20(21-9-5-4-6-10-21)24-18-33-19-25-23-11-7-8-12-26(23)35-31(25)30(24)34(33)15-16-36(2)28(33)17-22-13-14-27(38-3)32(37)29(22)34/h4-14,28,30,35,37H,15-19H2,1-3H3/b24-20+/t28?,30-,33+,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity in recombinant human Opioid receptor kappa 1 transfected into chinese hamster ovary cells by displacing [3H]U-69593 radioligand |
J Med Chem 46: 3174-7 (2003)
Article DOI: 10.1021/jm030801n
BindingDB Entry DOI: 10.7270/Q2TH8NGD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027255
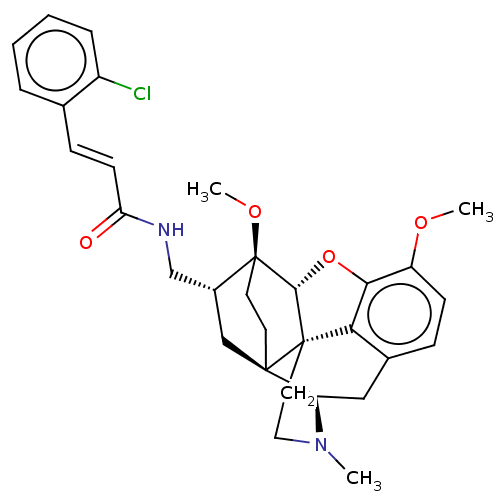
(CHEMBL2113296)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccccc2Cl)C1)ccc3OC |THB:10:9:5.4.6:14| Show InChI InChI=1S/C31H35ClN2O4/c1-34-15-14-30-26-20-8-10-23(36-2)27(26)38-28(30)31(37-3)13-12-29(30,24(34)16-20)17-21(31)18-33-25(35)11-9-19-6-4-5-7-22(19)32/h4-11,21,24,28H,12-18H2,1-3H3,(H,33,35)/b11-9+/t21-,24-,28-,29-,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549372
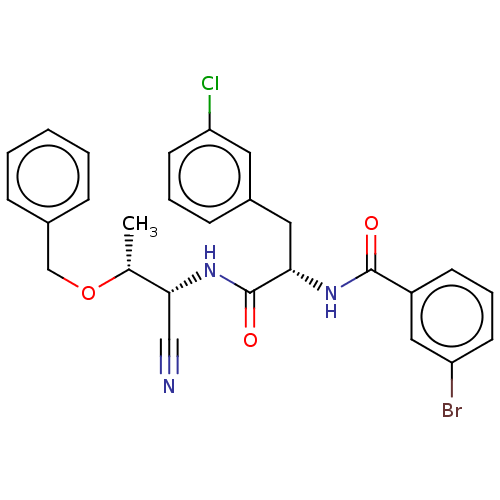
(CHEMBL4744431)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(Br)c1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027249

(CHEMBL2113312)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC\C=C\c2ccc(Cl)cc2)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C30H35ClN2O3/c1-33-15-13-29-25-20-7-10-23(34)26(25)36-27(29)30(35-2)12-11-28(29,24(33)16-20)17-21(30)18-32-14-3-4-19-5-8-22(31)9-6-19/h3-10,21,24,27,32,34H,11-18H2,1-2H3/b4-3+/t21-,24-,27-,28-,29+,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027254
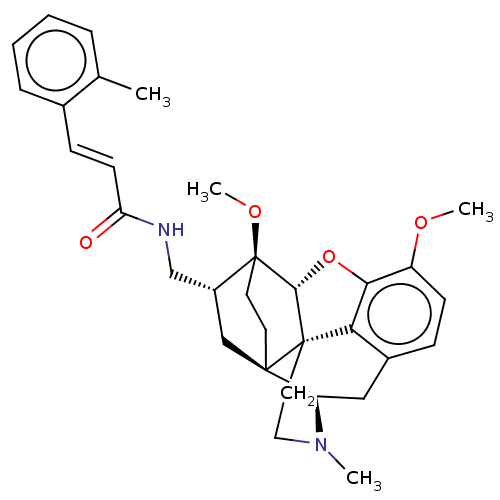
(CHEMBL2113295)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccccc2C)C1)ccc3OC |THB:10:9:5.4.6:14| Show InChI InChI=1S/C32H38N2O4/c1-20-7-5-6-8-21(20)10-12-26(35)33-19-23-18-30-13-14-32(23,37-4)29-31(30)15-16-34(2)25(30)17-22-9-11-24(36-3)28(38-29)27(22)31/h5-12,23,25,29H,13-19H2,1-4H3,(H,33,35)/b12-10+/t23-,25-,29-,30-,31+,32+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human cloned kappa opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50027241
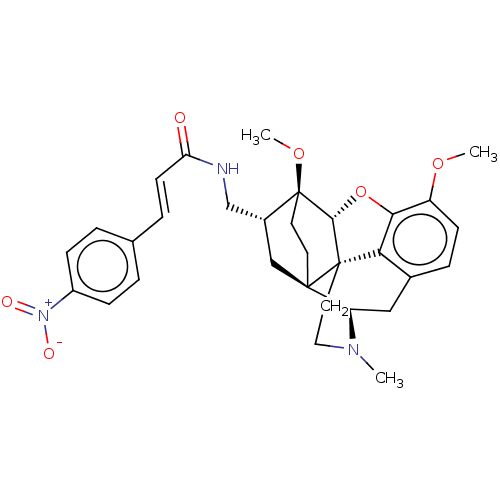
(CHEMBL2113299)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccc(cc2)[N+]([O-])=O)C1)ccc3OC |THB:10:9:5.4.6:14| Show InChI InChI=1S/C31H35N3O6/c1-33-15-14-30-26-20-7-10-23(38-2)27(26)40-28(30)31(39-3)13-12-29(30,24(33)16-20)17-21(31)18-32-25(35)11-6-19-4-8-22(9-5-19)34(36)37/h4-11,21,24,28H,12-18H2,1-3H3,(H,32,35)/b11-6+/t21-,24-,28-,29-,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549374

(CHEMBL4746374)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(c1)-c1cccnc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM31992
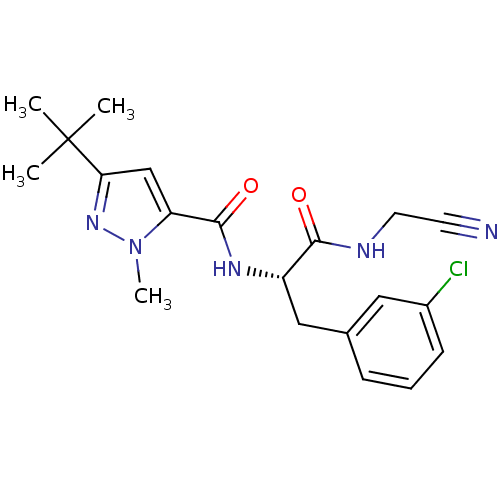
(Dipeptidyl nitrile inhibitor, 25)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N)C(C)(C)C |r| Show InChI InChI=1S/C20H24ClN5O2/c1-20(2,3)17-12-16(26(4)25-17)19(28)24-15(18(27)23-9-8-22)11-13-6-5-7-14(21)10-13/h5-7,10,12,15H,9,11H2,1-4H3,(H,23,27)(H,24,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027247

(CHEMBL2113314)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]13C=C[C@@]2(OC)[C@]2([H])Oc4c5c(C[C@@]1([H])N(C)CC[C@@]325)ccc4O)C(=O)\C=C\c1ccc(C)cc1 |c:9,TLB:23:22:18.17.19:7| Show InChI InChI=1S/C32H34N2O4/c1-19-4-6-20(7-5-19)8-11-26(36)34-17-22-23(18-34)32(37-3)13-12-30(22)25-16-21-9-10-24(35)28-27(21)31(30,29(32)38-28)14-15-33(25)2/h4-13,22-23,25,29,35H,14-18H2,1-3H3/b11-8+/t22-,23+,25-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human cloned kappa opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50232709

(CHEMBL4098442 | US11046670, Table 6.3)Show SMILES [H][C@]12C[C@@]([H])(N(CC1)C(=O)OCc1ccccc1)c1cc(ccc21)N1CCN(CC1)C1CCCCC1 |r,TLB:19:18:2:5.6.7,8:5:2:23.18,THB:22:23:2:5.6.7| Show InChI InChI=1S/C29H37N3O2/c33-29(34-21-22-7-3-1-4-8-22)32-14-13-23-19-28(32)27-20-25(11-12-26(23)27)31-17-15-30(16-18-31)24-9-5-2-6-10-24/h1,3-4,7-8,11-12,20,23-24,28H,2,5-6,9-10,13-19,21H2/t23-,28-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2GF0XN4 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50232709

(CHEMBL4098442 | US11046670, Table 6.3)Show SMILES [H][C@]12C[C@@]([H])(N(CC1)C(=O)OCc1ccccc1)c1cc(ccc21)N1CCN(CC1)C1CCCCC1 |r,TLB:19:18:2:5.6.7,8:5:2:23.18,THB:22:23:2:5.6.7| Show InChI InChI=1S/C29H37N3O2/c33-29(34-21-22-7-3-1-4-8-22)32-14-13-23-19-28(32)27-20-25(11-12-26(23)27)31-17-15-30(16-18-31)24-9-5-2-6-10-24/h1,3-4,7-8,11-12,20,23-24,28H,2,5-6,9-10,13-19,21H2/t23-,28-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2GF0XN4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human cloned mu opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50451625

(CHEMBL4217414)Show SMILES CC(C)C[C@H](NC(=O)c1cc(nn1C)C(C)(C)C)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C18H30N6O2/c1-12(2)9-13(17(26)24(8)22(6)11-19)20-16(25)14-10-15(18(3,4)5)21-23(14)7/h10,12-13H,9H2,1-8H3,(H,20,25)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Trypanosoma cruzi cruzain using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substra... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50451631

(CHEMBL4212454)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(nn1C)C(C)(C)C |r| Show InChI InChI=1S/C21H28N6O2/c1-21(2,3)18-13-17(26(5)24-18)19(28)23-16(12-15-10-8-7-9-11-15)20(29)27(6)25(4)14-22/h7-11,13,16H,12H2,1-6H3,(H,23,28)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Trypanosoma cruzi cruzain using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substra... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027236

(CHEMBL2113305)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccccc2Cl)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C30H33ClN2O4/c1-33-14-13-29-25-19-7-9-22(34)26(25)37-27(29)30(36-2)12-11-28(29,23(33)15-19)16-20(30)17-32-24(35)10-8-18-5-3-4-6-21(18)31/h3-10,20,23,27,34H,11-17H2,1-2H3,(H,32,35)/b10-8+/t20-,23-,27-,28-,29+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human cloned kappa opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027242

(CHEMBL2113308)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@]2(OC)[C@@H](CNC(=O)\C=C\c2ccc(cc2)[N+]([O-])=O)C1)ccc3O |THB:10:9:5.4.6:14| Show InChI InChI=1S/C30H33N3O6/c1-32-14-13-29-25-19-6-9-22(34)26(25)39-27(29)30(38-2)12-11-28(29,23(32)15-19)16-20(30)17-31-24(35)10-5-18-3-7-21(8-4-18)33(36)37/h3-10,20,23,27,34H,11-17H2,1-2H3,(H,31,35)/b10-5+/t20-,23-,27-,28-,29+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human cloned kappa opioid receptor |
J Med Chem 50: 5176-82 (2007)
Article DOI: 10.1021/jm070255o
BindingDB Entry DOI: 10.7270/Q2RR202P |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50370624
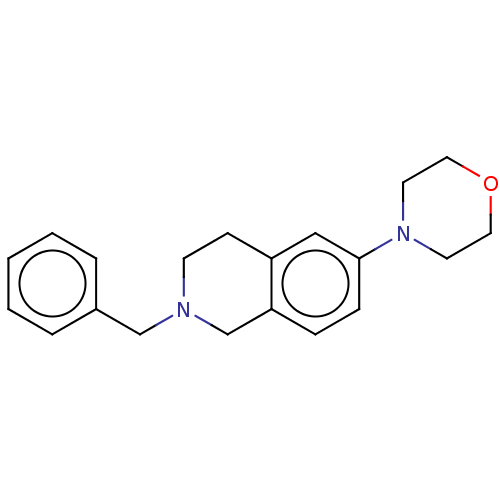
(CHEMBL4169752 | US10954217, Example 00234)Show InChI InChI=1S/C20H24N2O/c1-2-4-17(5-3-1)15-21-9-8-18-14-20(7-6-19(18)16-21)22-10-12-23-13-11-22/h1-7,14H,8-13,15-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
TBD |
US Patent US10954217 (2021)
BindingDB Entry DOI: 10.7270/Q2JH3Q9M |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50370624

(CHEMBL4169752 | US10954217, Example 00234)Show InChI InChI=1S/C20H24N2O/c1-2-4-17(5-3-1)15-21-9-8-18-14-20(7-6-19(18)16-21)22-10-12-23-13-11-22/h1-7,14H,8-13,15-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Pentazocine from sigma1 receptor in guinea pig brain membrane by microbeta scintillation counting method |
Eur J Med Chem 151: 557-567 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.024
BindingDB Entry DOI: 10.7270/Q28918FQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data