

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50143314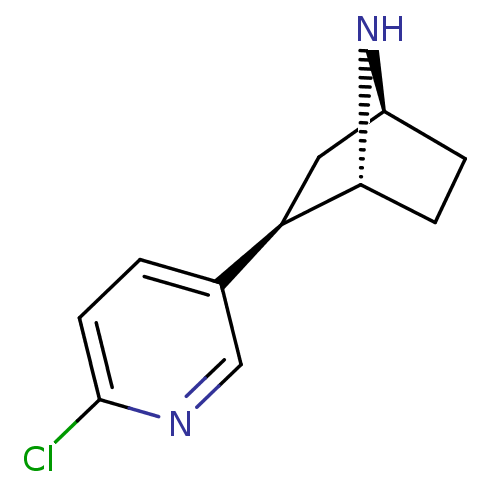 ((+)-Epibatidine | (-)-epibatidine | (1R,2R,4S)-2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-iodo-MLA binding to Nicotinic acetylcholine receptor alpha-7 of rat cerebral cortex | J Med Chem 47: 4588-94 (2004) Article DOI: 10.1021/jm040078g BindingDB Entry DOI: 10.7270/Q2DZ092M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312552 (3'-(3-nitrophenyl)epibatidine | CHEMBL1096352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085677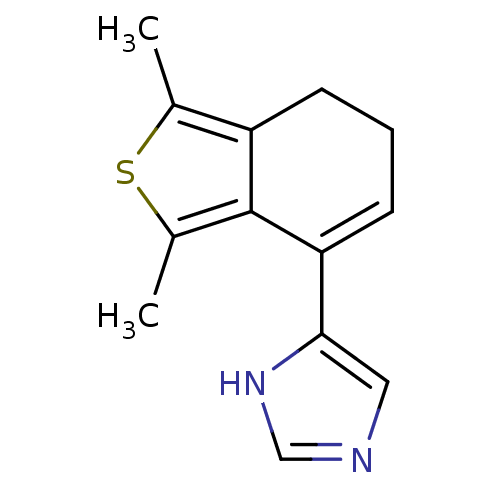 (4-(1,3-Dimethyl-6,7-dihydro-benzo[c]thiophen-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085677 (4-(1,3-Dimethyl-6,7-dihydro-benzo[c]thiophen-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM86812 (CAS_45263784 | NSC_45263784 | rac-2-(6-fluoro-5-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312557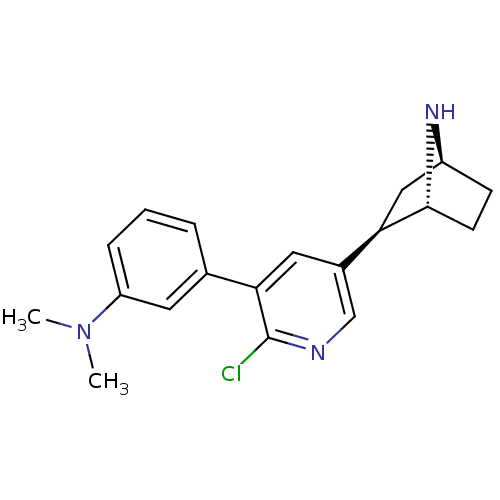 (3'-(3-Dimethylaminophenyl)epibatidine | CHEMBL1084...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM403653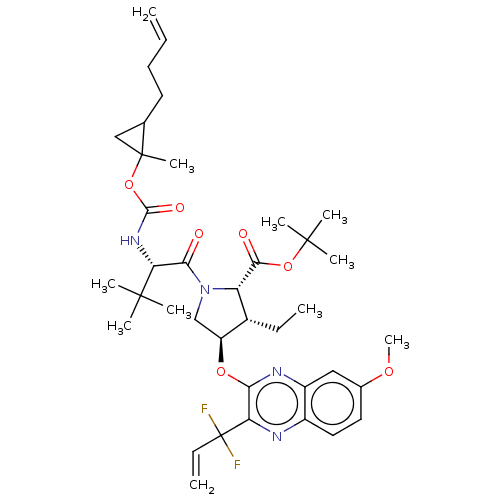 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403592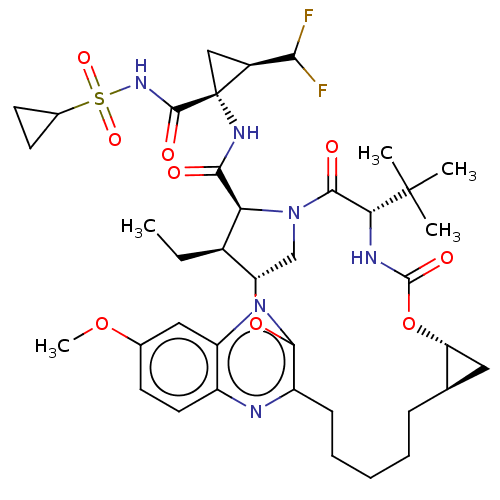 (Preparation of (1aR,5S,8S,9S,10R,22aR)-5-tert-buty...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144258 (4-[(8-Benzo[1,3]dioxol-5-ylmethyl-8-aza-bicyclo[3....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312547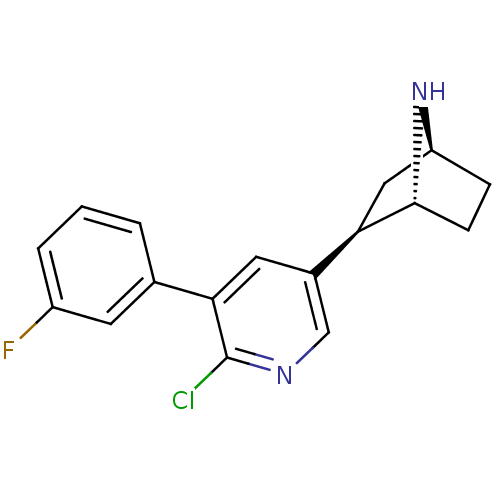 (3'-(3-Fluorophenyl)epibatidine | CHEMBL1097692) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312549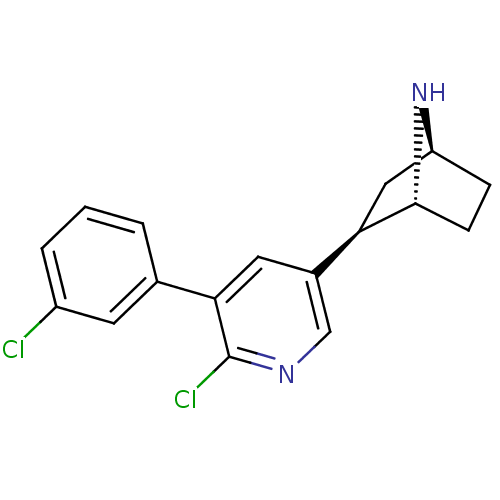 (3'-(3-Chlorophenyl)epibatidine | CHEMBL1098031) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50085683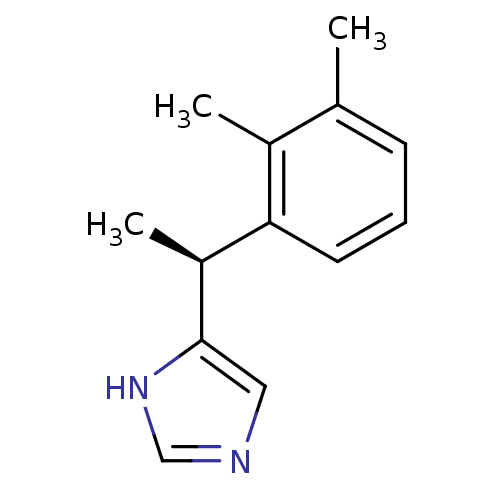 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312551 (3'-(4-Nitrophenyl)epibatidine | CHEMBL1098033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085683 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085683 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312550 (3'-(3-Bromophenyl)epibatidine | CHEMBL1098032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312554 (3'-(3-Aminophenyl)epibatidine | CHEMBL1076992) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312546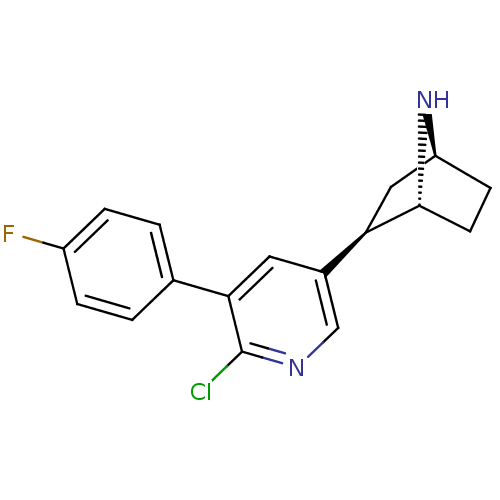 ((1R,2R,4S)-2-(6-chloro-5-(4-fluorophenyl)pyridin-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143314 ((+)-Epibatidine | (-)-epibatidine | (1R,2R,4S)-2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312556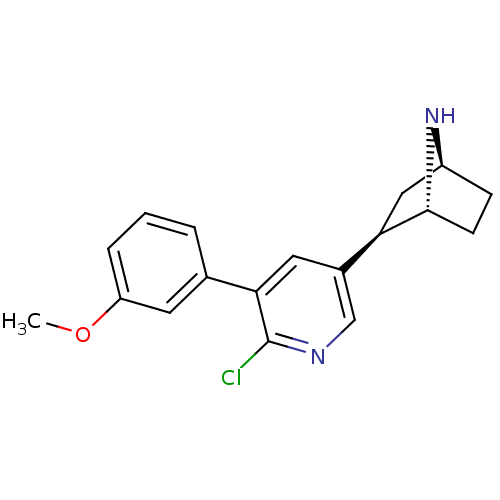 ((1R,2R,4S)-2-(6-chloro-5-(3-methoxyphenyl)pyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403677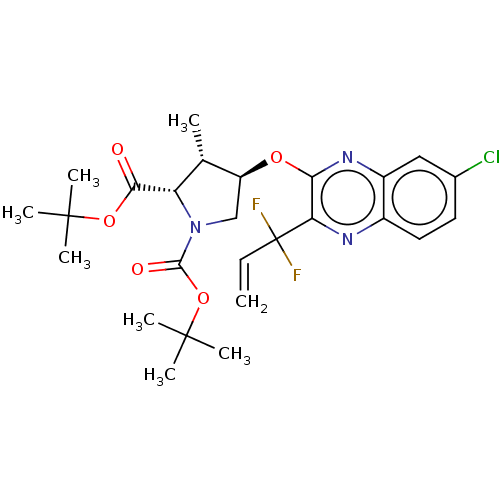 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-14-chloro-N-[...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100717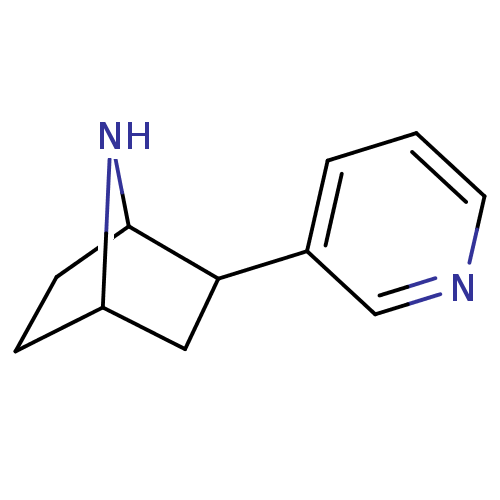 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50100717 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50162061 (2-(5-Ethynyl-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hep...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex | J Med Chem 48: 1221-8 (2005) Article DOI: 10.1021/jm040160b BindingDB Entry DOI: 10.7270/Q26W9BV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403609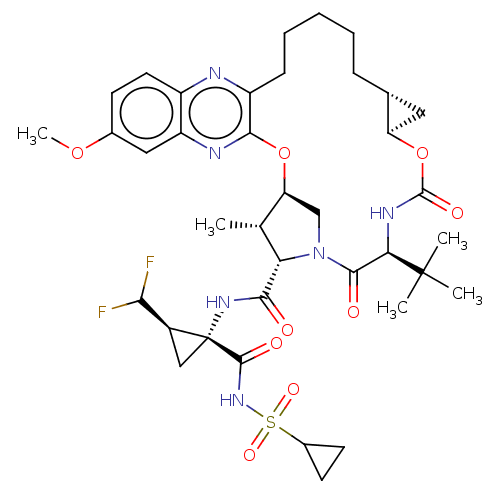 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-1-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403676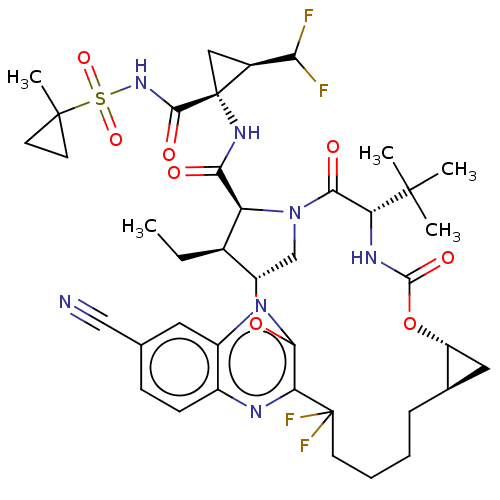 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-14-cyano-N-[(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312545 ((1R,2R,4S)-2-(6-chloro-5-phenylpyridin-3-yl)-7-aza...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312555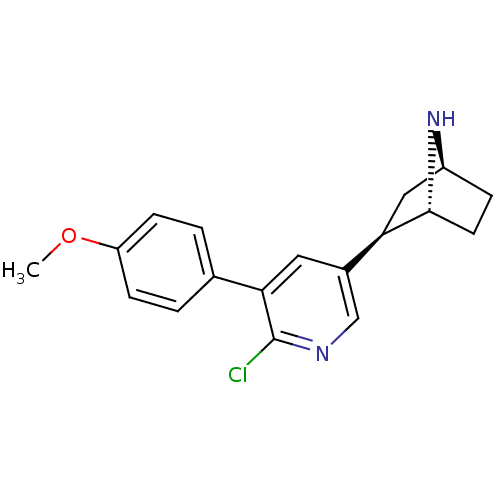 (3'-(4-Methoxylphenyl)epibatidine | CHEMBL1085916) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100715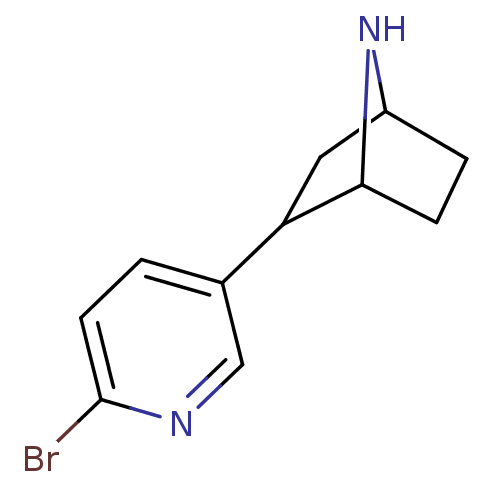 (2-(6-Bromo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hepta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144236 (4-[(R)-(S)-8-Aza-bicyclo[3.2.1]oct-(3Z)-ylidene-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144229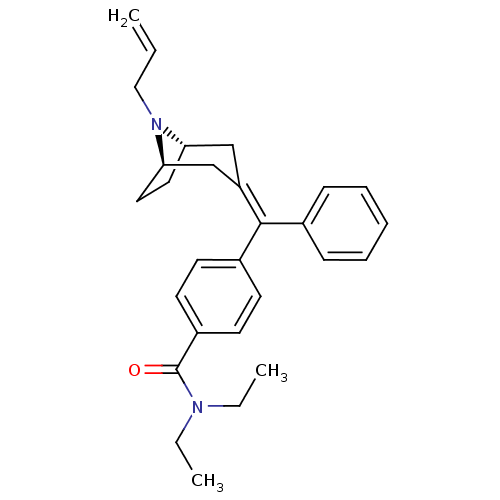 (4-{[(1S,5R)-8-Allyl-8-aza-bicyclo[3.2.1]oct-(3Z)-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Effective concentration against stimulation of [35S]-GTP-gammaS, binding in CHO cells transfected with the human opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50143320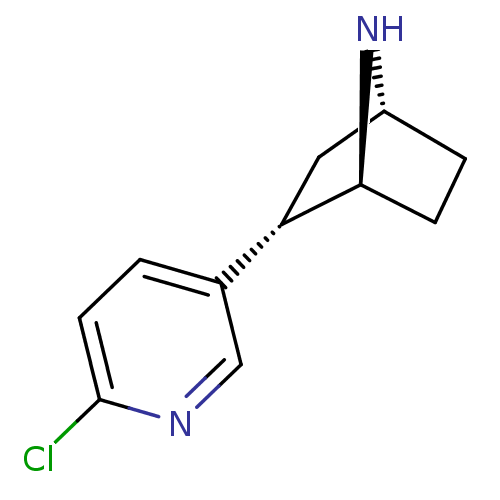 ((+)-epibatidine | (-)-1-epidatidine | (1S,2S,4R)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha-4-beta-2 nAChR | J Med Chem 50: 6383-91 (2007) Article DOI: 10.1021/jm0704696 BindingDB Entry DOI: 10.7270/Q25H7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143320 ((+)-epibatidine | (-)-1-epidatidine | (1S,2S,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143320 ((+)-epibatidine | (-)-1-epidatidine | (1S,2S,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex | J Med Chem 48: 1221-8 (2005) Article DOI: 10.1021/jm040160b BindingDB Entry DOI: 10.7270/Q26W9BV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100707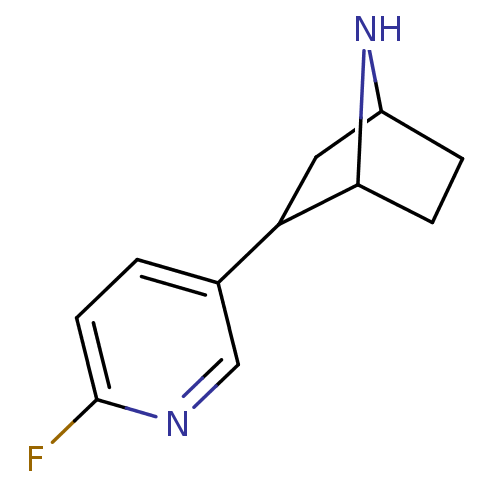 ((R)-2-(6-Fluoro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM86815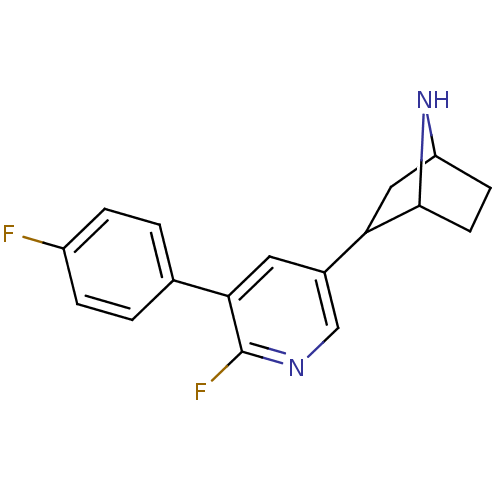 (CAS_45266019 | NSC_45266019 | rac-2-(6-fluoro-5-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50162062 (2-(5-Iodo-pyridin-3-yl)-7-methyl-7-aza-bicyclo[2.2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex | J Med Chem 48: 1221-8 (2005) Article DOI: 10.1021/jm040160b BindingDB Entry DOI: 10.7270/Q26W9BV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403666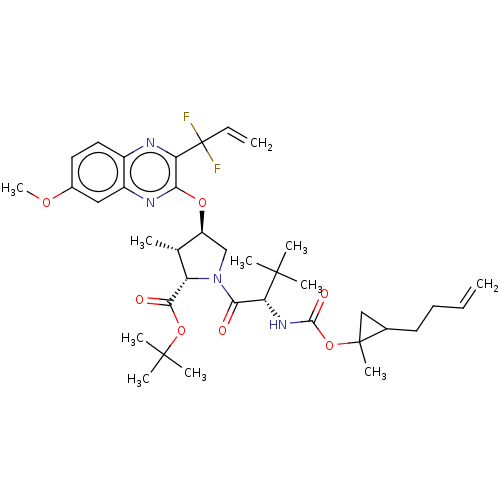 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403665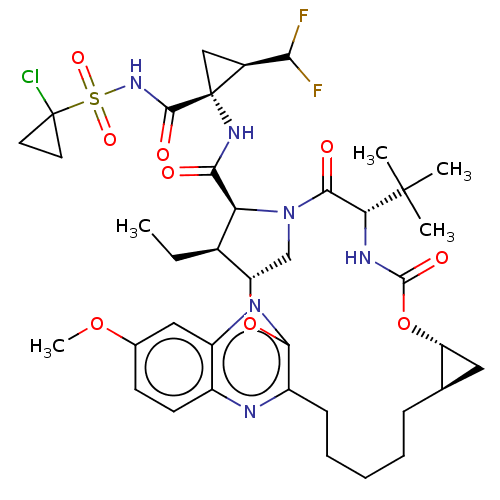 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-1-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403664 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403661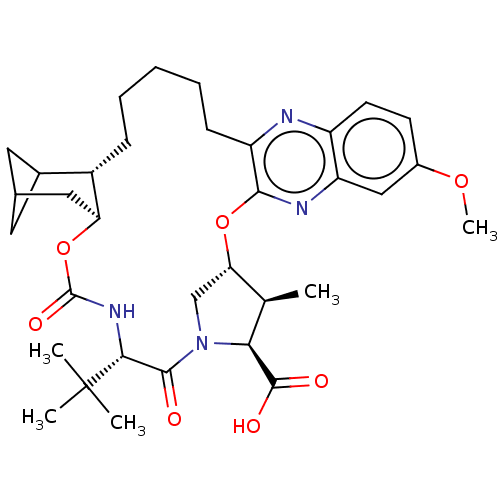 ((4aR,8S,11S,12S,13R,25aR)-8-tert-butyl-N-[(1R,2R)-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403653 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403671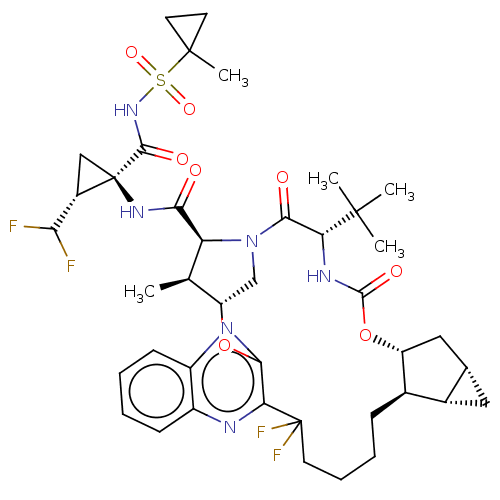 ((1aS,2aR,6S,9S,10S,11R,23aR,23bS)-6-tert-butyl-N-[...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403591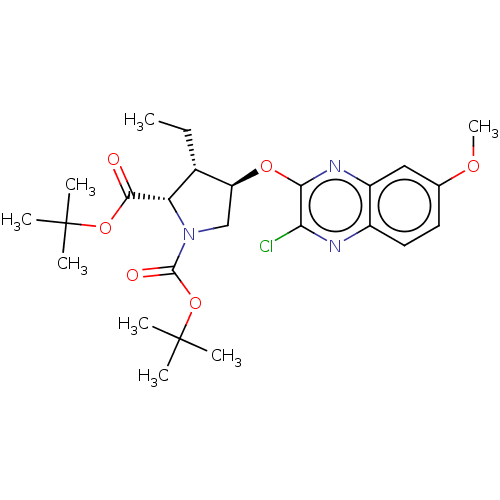 (Preparation of (1aR,5S,8S,9S,10R,22aR)-5-tert-buty...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403608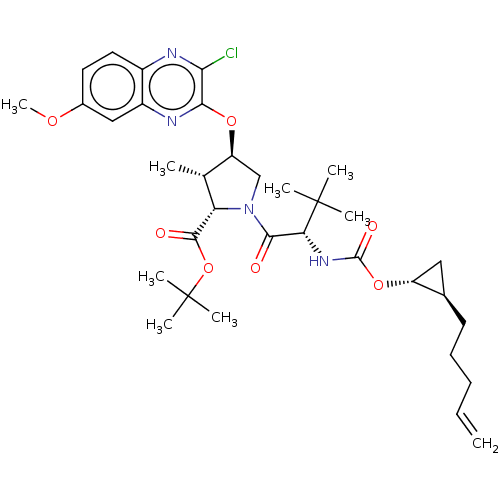 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM403674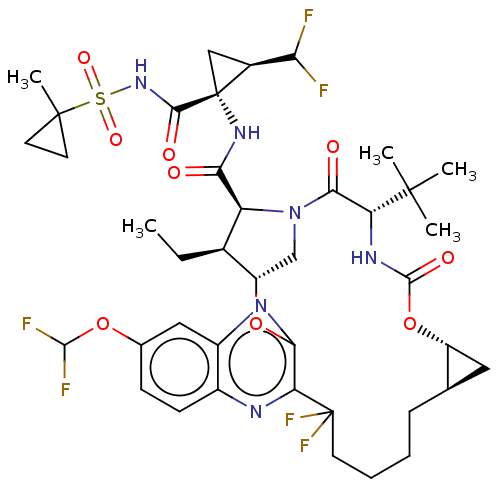 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-14-(difluorom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403678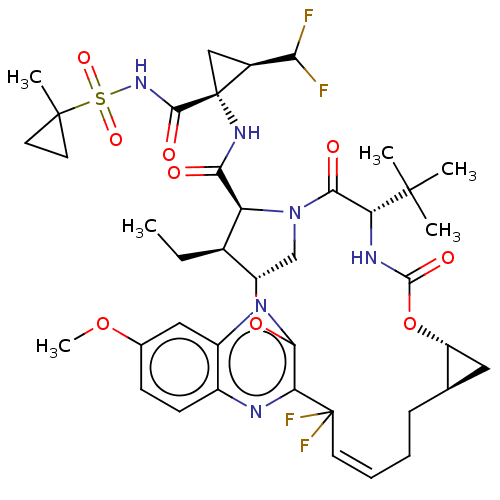 ((1aR,5S,8S,9S,10R,19E,22aR)-5-tert-butyl-N-[(1R,2R...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403617 ((3aR,7S10S,1S,12R,24aR)-7-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7642 total ) | Next | Last >> |