 Found 87 hits with Last Name = 'masjedizadeh' and Initial = 'm'
Found 87 hits with Last Name = 'masjedizadeh' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589205
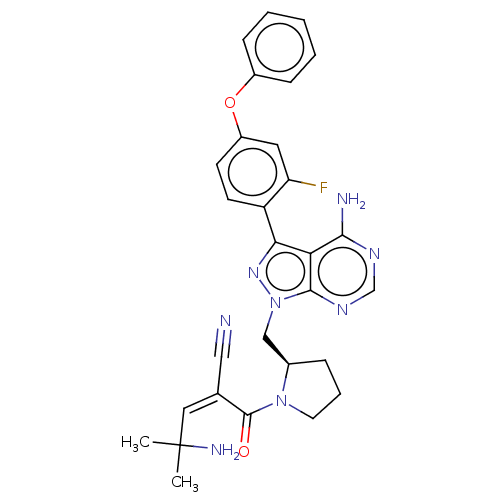
(CHEMBL5174021)Show SMILES CC(C)(N)\C=C(\C#N)C(=O)N1CCC[C@@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589186
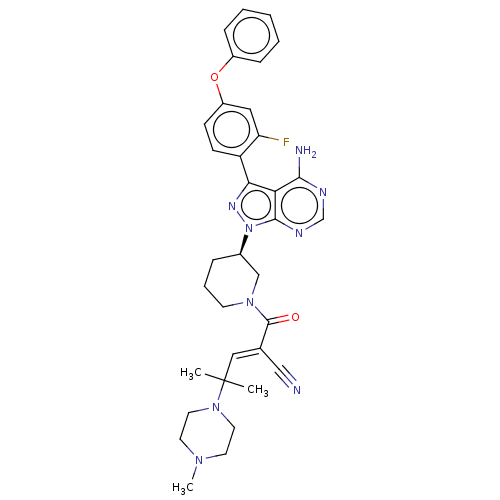
(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589207
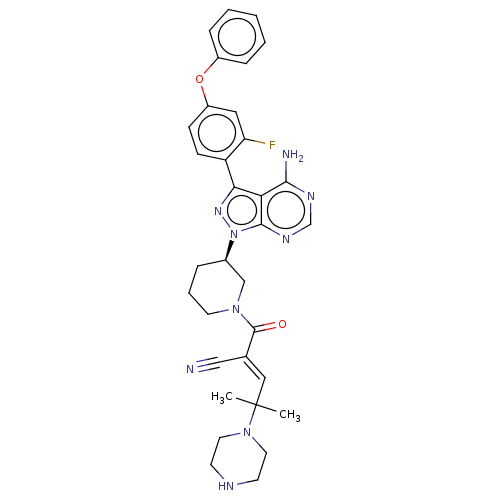
(CHEMBL5169419)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589202
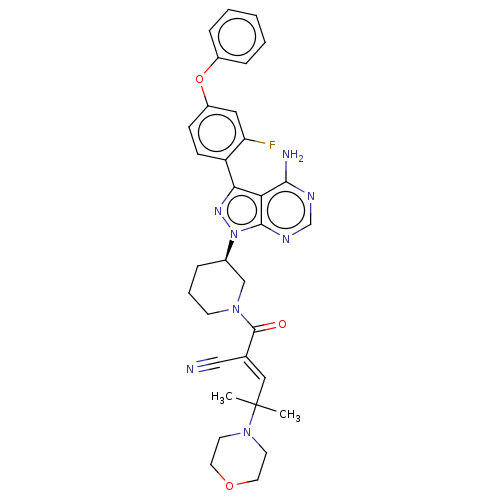
(CHEMBL5191633)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50557485
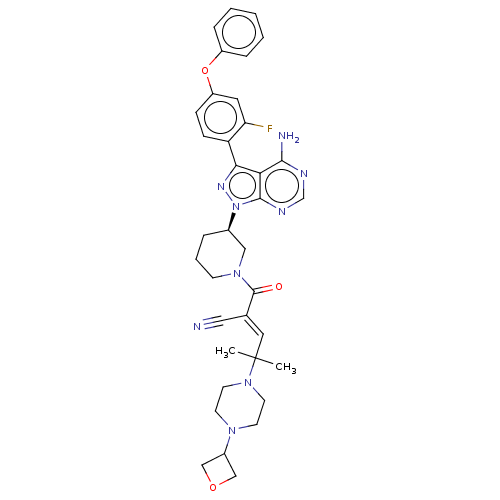
(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589212
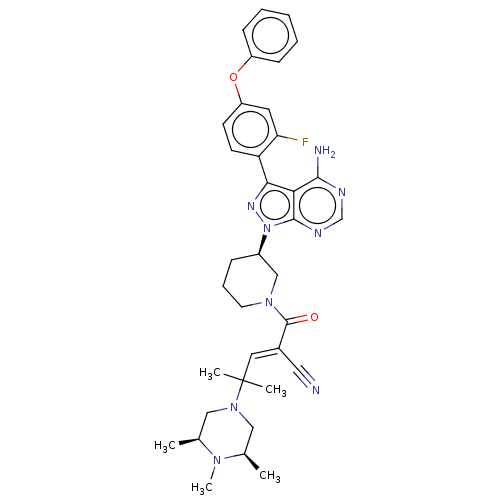
(CHEMBL5195816)Show SMILES C[C@H]1CN(C[C@@H](C)N1C)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589187
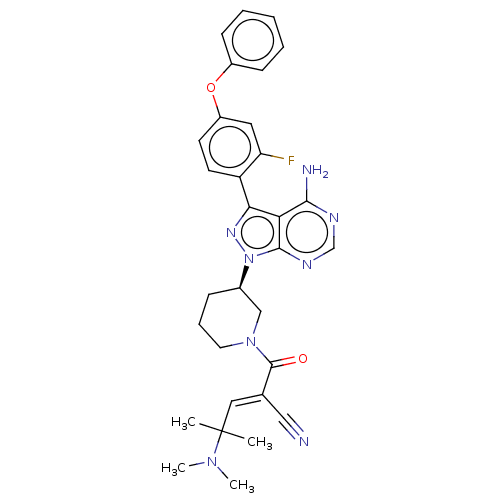
(CHEMBL5178314)Show SMILES CN(C)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589213
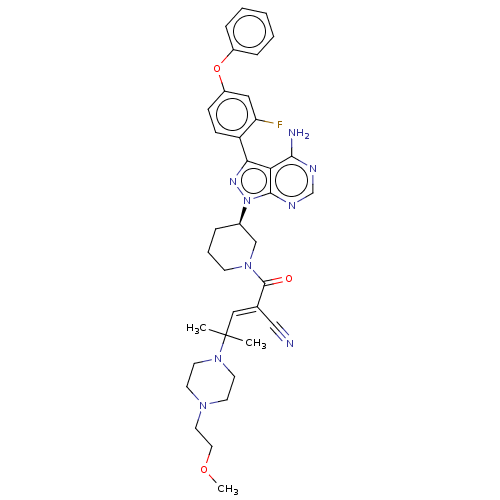
(CHEMBL3702850)Show SMILES COCCN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589200
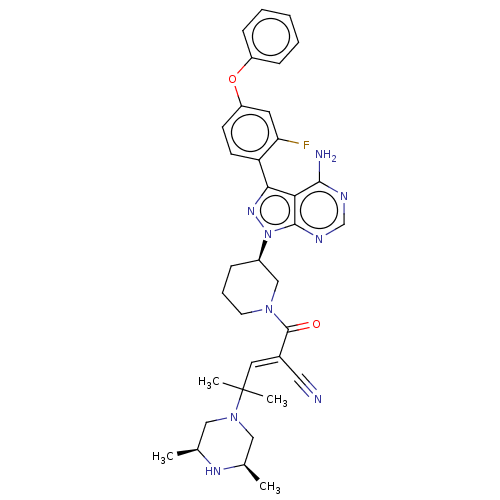
(CHEMBL5207901)Show SMILES C[C@H]1CN(C[C@@H](C)N1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589208

(CHEMBL3702851)Show SMILES CN1CCN(CC1=O)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589209
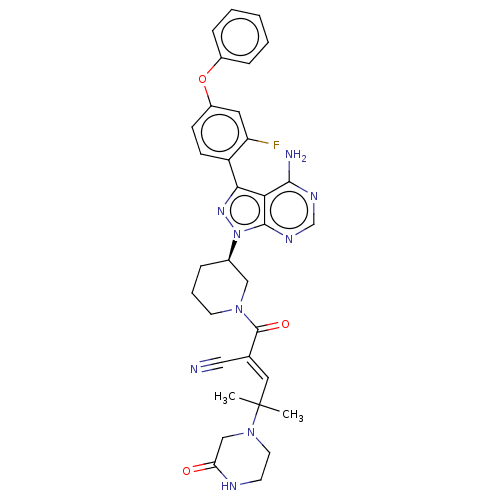
(CHEMBL3702858)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCNC(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589201
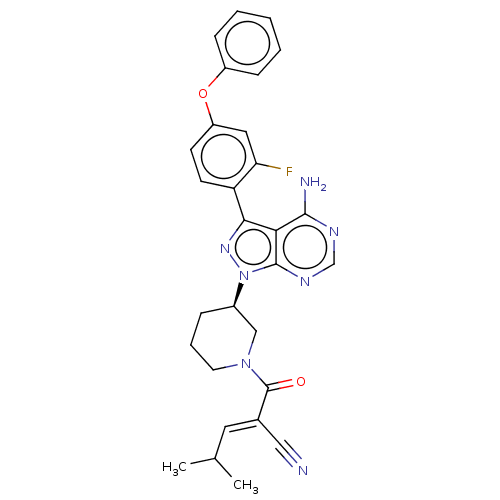
(CHEMBL5170583)Show SMILES CC(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589211
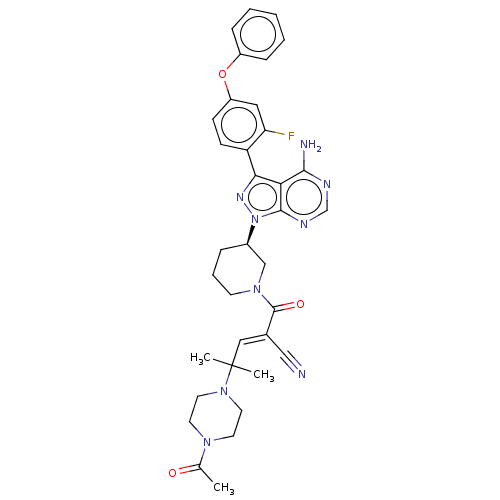
(CHEMBL3702855)Show SMILES CC(=O)N1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589192

(CHEMBL5204545)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3F)c12)[C@@H]1CCCN(C1)C(=O)C(=C\C1CC1)\C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301911

(CHEMBL583269 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1Cl |r,t:21| Show InChI InChI=1S/C24H25ClFN3O6S2/c1-24(2,3)21-20(30)18(23(31)29(21)12-13-5-7-14(26)8-6-13)19-22(25)37(34,35)17-11-15(28-36(4,32)33)9-10-16(17)27-19/h5-11,18,21-22,28H,12H2,1-4H3/t18?,21-,22?/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589210

(CHEMBL3702856)Show SMILES COC(=O)N1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589194
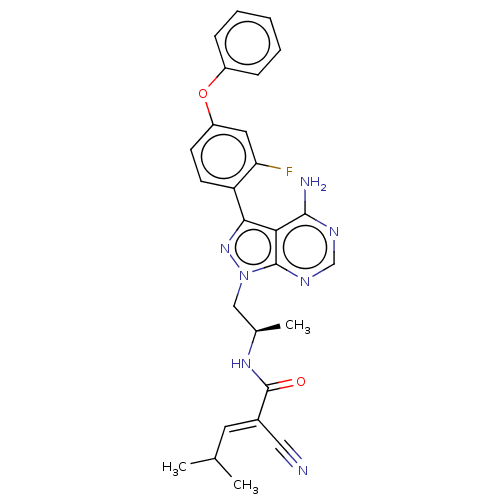
(CHEMBL4114703)Show SMILES CC(C)\C=C(/C#N)C(=O)N[C@H](C)Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589188
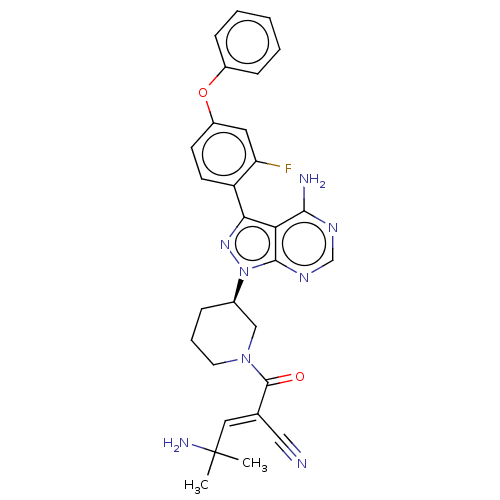
(CHEMBL5188669)Show SMILES CC(C)(N)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301902

(CHEMBL571825 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES Cc1cc(CN2[C@H](C(=O)C(C2=O)C2=Nc3ccc(NS(C)(=O)=O)cc3S(=O)(=O)C2)C(C)(C)C)ccc1F |r,t:13| Show InChI InChI=1S/C25H28FN3O6S2/c1-14-10-15(6-8-17(14)26)12-29-23(25(2,3)4)22(30)21(24(29)31)19-13-37(34,35)20-11-16(28-36(5,32)33)7-9-18(20)27-19/h6-11,21,23,28H,12-13H2,1-5H3/t21?,23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589189
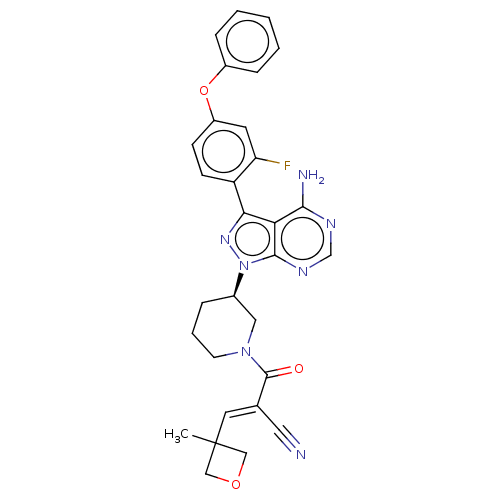
(CHEMBL3702827)Show SMILES CC1(COC1)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589193
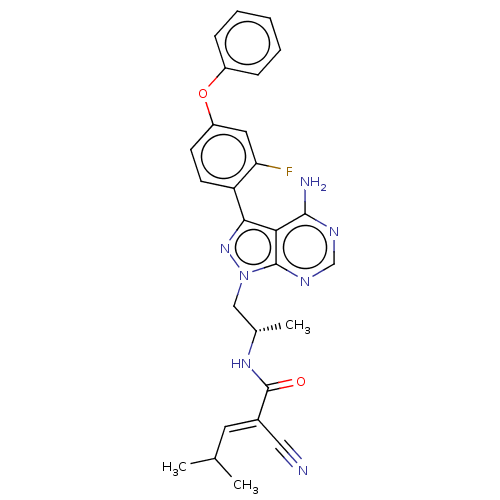
(CHEMBL3947620)Show SMILES [H][C@](C)(Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)NC(=O)C(=C\C(C)C)\C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301903

(CHEMBL569120 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES COc1cc(CN2[C@H](C(=O)C(C2=O)C2=Nc3ccc(NS(C)(=O)=O)cc3S(=O)(=O)C2)C(C)(C)C)ccc1F |r,t:14| Show InChI InChI=1S/C25H28FN3O7S2/c1-25(2,3)23-22(30)21(24(31)29(23)12-14-6-8-16(26)19(10-14)36-4)18-13-38(34,35)20-11-15(28-37(5,32)33)7-9-17(20)27-18/h6-11,21,23,28H,12-13H2,1-5H3/t21?,23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301914
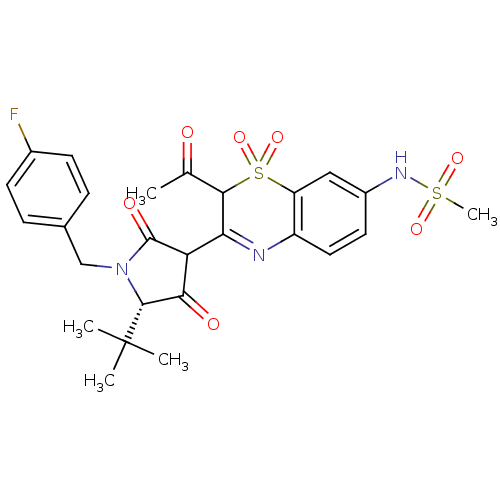
(CHEMBL570249 | N-{2-Acetyl-3-[(S)-5-tert-butyl-1-(...)Show SMILES CC(=O)C1C(=Nc2ccc(NS(C)(=O)=O)cc2S1(=O)=O)C1C(=O)[C@@H](N(Cc2ccc(F)cc2)C1=O)C(C)(C)C |r,c:4| Show InChI InChI=1S/C26H28FN3O7S2/c1-14(31)23-21(28-18-11-10-17(29-38(5,34)35)12-19(18)39(23,36)37)20-22(32)24(26(2,3)4)30(25(20)33)13-15-6-8-16(27)9-7-15/h6-12,20,23-24,29H,13H2,1-5H3/t20?,23?,24-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589195
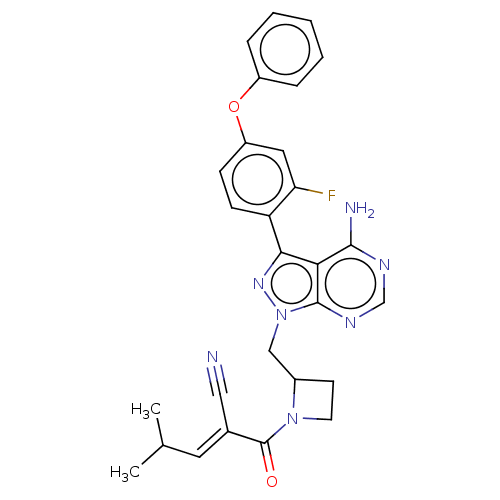
(CHEMBL3702838)Show SMILES CC(C)\C=C(/C#N)C(=O)N1CCC1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50369720
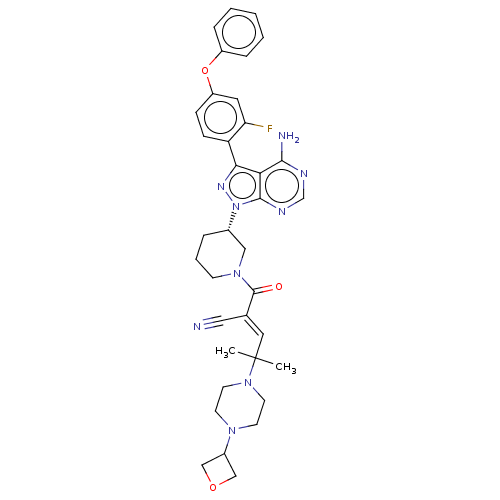
(CHEMBL3979355)Show SMILES [H][C@@]1(CCCN(C1)C(=O)C(=C\C(C)(C)N1CCN(CC1)C1COC1)\C#N)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| Show InChI InChI=1S/C36H40FN9O3/c1-36(2,45-15-13-43(14-16-45)26-21-48-22-26)18-24(19-38)35(47)44-12-6-7-25(20-44)46-34-31(33(39)40-23-41-34)32(42-46)29-11-10-28(17-30(29)37)49-27-8-4-3-5-9-27/h3-5,8-11,17-18,23,25-26H,6-7,12-16,20-22H2,1-2H3,(H2,39,40,41)/b24-18+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589198
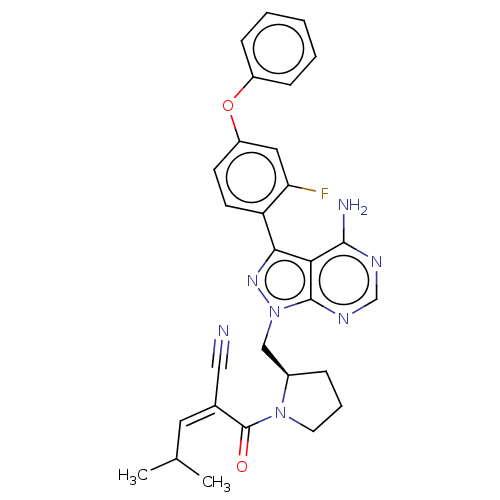
(CHEMBL5203733)Show SMILES CC(C)\C=C(\C#N)C(=O)N1CCC[C@@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50589206
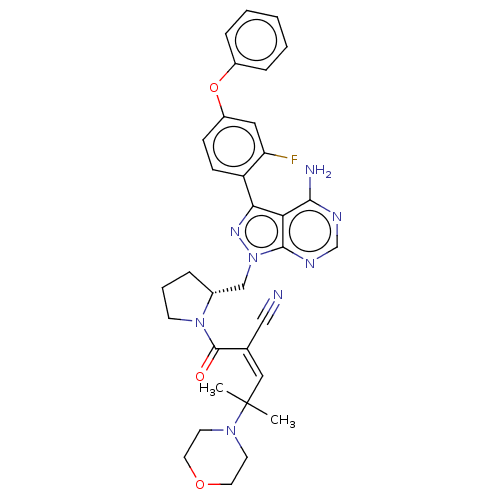
(CHEMBL5184084)Show SMILES CC(C)(\C=C(\C#N)C(=O)N1CCC[C@@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCOCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301901
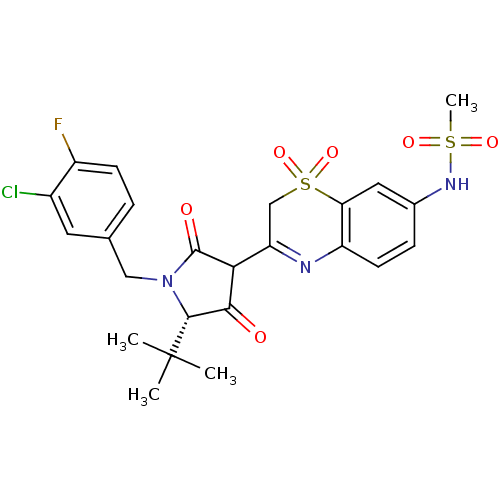
(CHEMBL570934 | N-{3-[(S)-5-tert-Butyl-1-(3-chloro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)c(Cl)c2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1 |r,t:22| Show InChI InChI=1S/C24H25ClFN3O6S2/c1-24(2,3)22-21(30)20(23(31)29(22)11-13-5-7-16(26)15(25)9-13)18-12-37(34,35)19-10-14(28-36(4,32)33)6-8-17(19)27-18/h5-10,20,22,28H,11-12H2,1-4H3/t20?,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301906
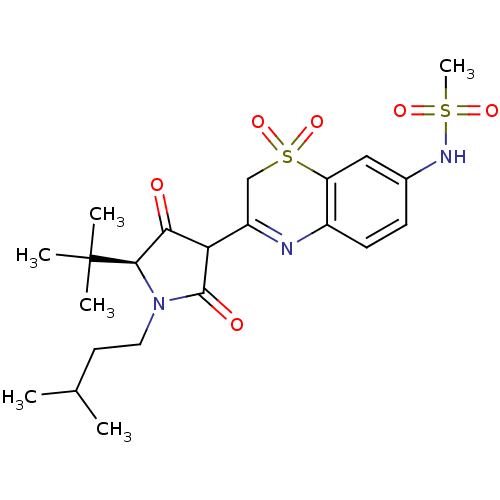
(CHEMBL571606 | N-{3-[(S)-5-tert-Butyl-4-hydroxy-1-...)Show SMILES CC(C)CCN1[C@H](C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1)C(C)(C)C |r,t:13| Show InChI InChI=1S/C22H31N3O6S2/c1-13(2)9-10-25-20(22(3,4)5)19(26)18(21(25)27)16-12-33(30,31)17-11-14(24-32(6,28)29)7-8-15(17)23-16/h7-8,11,13,18,20,24H,9-10,12H2,1-6H3/t18?,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589199
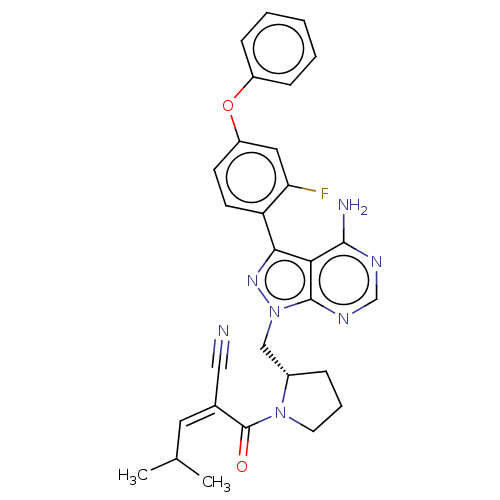
(CHEMBL5202266)Show SMILES CC(C)\C=C(\C#N)C(=O)N1CCC[C@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301913

(CHEMBL571564 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1C#N |r,t:21| Show InChI InChI=1S/C25H25FN4O6S2/c1-25(2,3)23-22(31)20(24(32)30(23)13-14-5-7-15(26)8-6-14)21-19(12-27)38(35,36)18-11-16(29-37(4,33)34)9-10-17(18)28-21/h5-11,19-20,23,29H,13H2,1-4H3/t19?,20?,23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301900
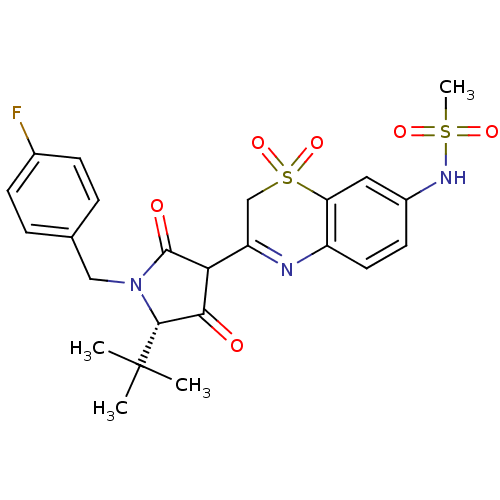
(CHEMBL568894 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1 |r,t:21| Show InChI InChI=1S/C24H26FN3O6S2/c1-24(2,3)22-21(29)20(23(30)28(22)12-14-5-7-15(25)8-6-14)18-13-36(33,34)19-11-16(27-35(4,31)32)9-10-17(19)26-18/h5-11,20,22,27H,12-13H2,1-4H3/t20?,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589189

(CHEMBL3702827)Show SMILES CC1(COC1)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301907
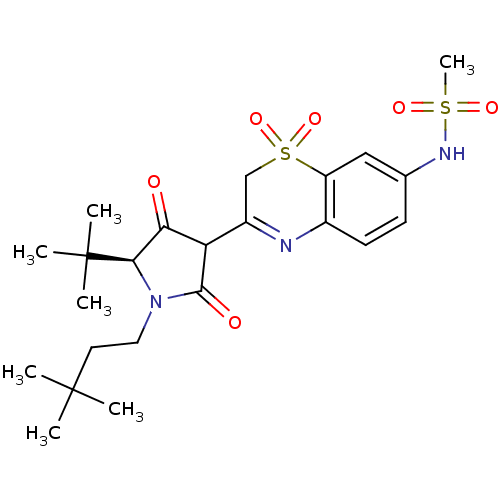
(CHEMBL572246 | N-{3-[(S)-5-tert-Butyl-1-(3,3-dimet...)Show SMILES CC(C)(C)CCN1[C@H](C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1)C(C)(C)C |r,t:14| Show InChI InChI=1S/C23H33N3O6S2/c1-22(2,3)10-11-26-20(23(4,5)6)19(27)18(21(26)28)16-13-34(31,32)17-12-14(25-33(7,29)30)8-9-15(17)24-16/h8-9,12,18,20,25H,10-11,13H2,1-7H3/t18?,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301899
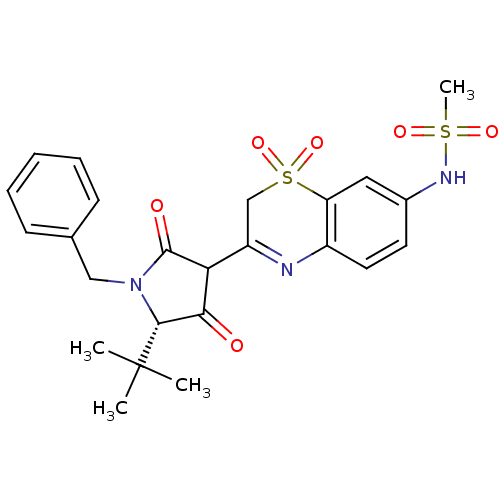
(CHEMBL585384 | N-[3-((S)-1-Benzyl-5-tert-butyl-4-h...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccccc2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1 |r,t:20| Show InChI InChI=1S/C24H27N3O6S2/c1-24(2,3)22-21(28)20(23(29)27(22)13-15-8-6-5-7-9-15)18-14-35(32,33)19-12-16(26-34(4,30)31)10-11-17(19)25-18/h5-12,20,22,26H,13-14H2,1-4H3/t20?,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data