 Found 59 hits with Last Name = 'mason' and Initial = 'de'
Found 59 hits with Last Name = 'mason' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HER4 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HER4 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196094
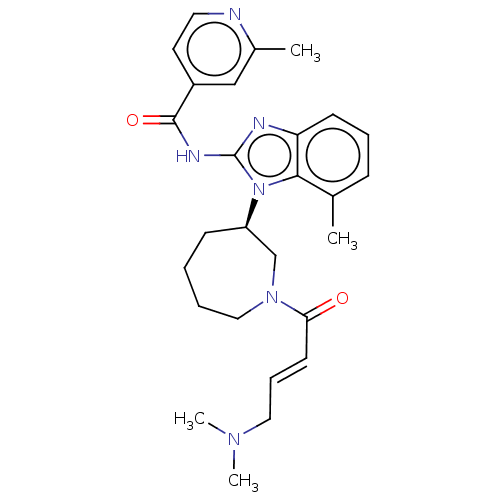
(CHEMBL3960167)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(C)c12 |r| Show InChI InChI=1S/C27H34N6O2/c1-19-9-7-11-23-25(19)33(27(29-23)30-26(35)21-13-14-28-20(2)17-21)22-10-5-6-16-32(18-22)24(34)12-8-15-31(3)4/h7-9,11-14,17,22H,5-6,10,15-16,18H2,1-4H3,(H,29,30,35)/b12-8+/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196093
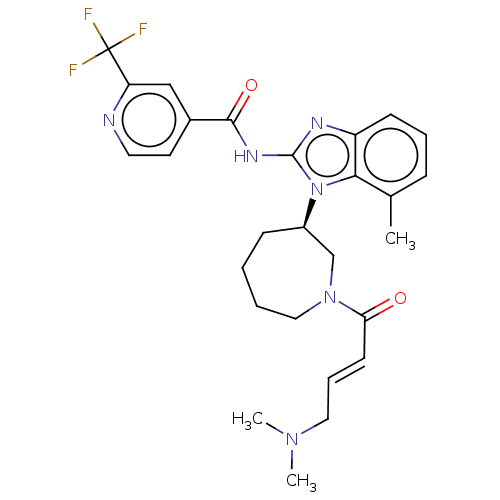
(CHEMBL3939913)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C27H31F3N6O2/c1-18-8-6-10-21-24(18)36(20-9-4-5-15-35(17-20)23(37)11-7-14-34(2)3)26(32-21)33-25(38)19-12-13-31-22(16-19)27(28,29)30/h6-8,10-13,16,20H,4-5,9,14-15,17H2,1-3H3,(H,32,33,38)/b11-7+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50160870
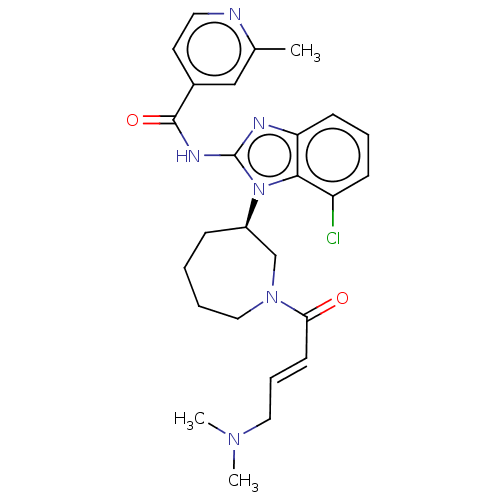
(CHEMBL3787344 | US11896597, Compound EGF816 | WO20...)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(Cl)c12 |r| Show InChI InChI=1S/C26H31ClN6O2/c1-18-16-19(12-13-28-18)25(35)30-26-29-22-10-6-9-21(27)24(22)33(26)20-8-4-5-15-32(17-20)23(34)11-7-14-31(2)3/h6-7,9-13,16,20H,4-5,8,14-15,17H2,1-3H3,(H,29,30,35)/b11-7+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Btk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196095
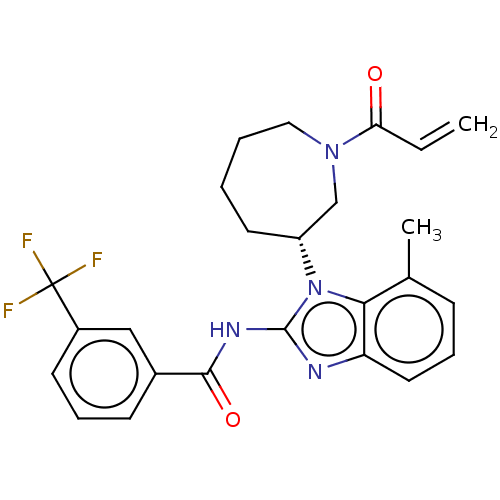
(CHEMBL3972316)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@@H]3CCCCN(C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-21(33)31-13-5-4-11-19(15-31)32-22-16(2)8-6-12-20(22)29-24(32)30-23(34)17-9-7-10-18(14-17)25(26,27)28/h3,6-10,12,14,19H,1,4-5,11,13,15H2,2H3,(H,29,30,34)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM149404
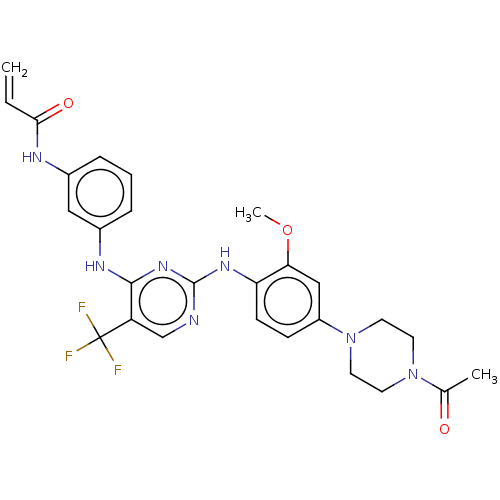
(AVL-301 | CHEMBL3545308 | CNX-419 | CO-1686 | Roci...)Show SMILES COc1cc(ccc1Nc1ncc(c(Nc2cccc(NC(=O)C=C)c2)n1)C(F)(F)F)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C27H28F3N7O3/c1-4-24(39)32-18-6-5-7-19(14-18)33-25-21(27(28,29)30)16-31-26(35-25)34-22-9-8-20(15-23(22)40-3)37-12-10-36(11-13-37)17(2)38/h4-9,14-16H,1,10-13H2,2-3H3,(H,32,39)(H2,31,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of wild type Bmx (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196096
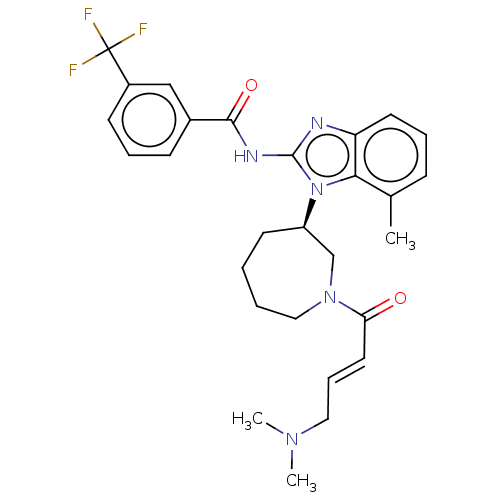
(CHEMBL3951434)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C28H32F3N5O2/c1-19-9-6-13-23-25(19)36(22-12-4-5-16-35(18-22)24(37)14-8-15-34(2)3)27(32-23)33-26(38)20-10-7-11-21(17-20)28(29,30)31/h6-11,13-14,17,22H,4-5,12,15-16,18H2,1-3H3,(H,32,33,38)/b14-8+/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 586 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Bmx (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50196095

(CHEMBL3972316)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@@H]3CCCCN(C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-21(33)31-13-5-4-11-19(15-31)32-22-16(2)8-6-12-20(22)29-24(32)30-23(34)17-9-7-10-18(14-17)25(26,27)28/h3,6-10,12,14,19H,1,4-5,11,13,15H2,2H3,(H,29,30,34)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50196095

(CHEMBL3972316)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@@H]3CCCCN(C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-21(33)31-13-5-4-11-19(15-31)32-22-16(2)8-6-12-20(22)29-24(32)30-23(34)17-9-7-10-18(14-17)25(26,27)28/h3,6-10,12,14,19H,1,4-5,11,13,15H2,2H3,(H,29,30,34)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196101
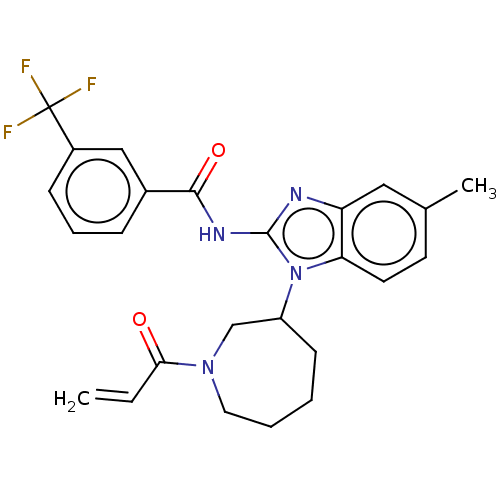
(CHEMBL3901943)Show SMILES Cc1ccc2n(C3CCCCN(C3)C(=O)C=C)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H25F3N4O2/c1-3-22(33)31-12-5-4-9-19(15-31)32-21-11-10-16(2)13-20(21)29-24(32)30-23(34)17-7-6-8-18(14-17)25(26,27)28/h3,6-8,10-11,13-14,19H,1,4-5,9,12,15H2,2H3,(H,29,30,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196237
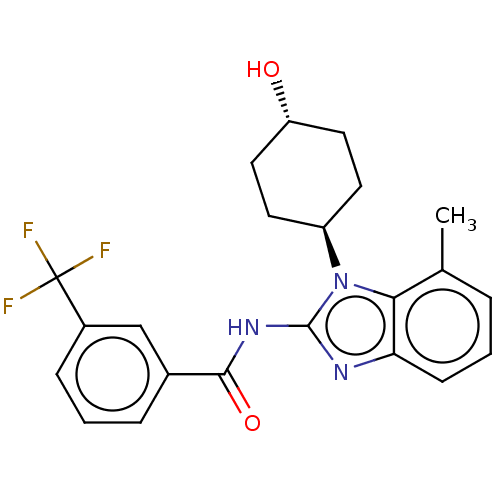
(CHEMBL3937373)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@H]3CC[C@H](O)CC3)c12 |r,wU:22.22,wD:25.26,(26.54,-21.82,;26.54,-20.28,;25.2,-19.51,;25.2,-17.97,;26.53,-17.2,;27.87,-17.96,;29.34,-17.48,;30.26,-18.73,;31.8,-18.73,;32.57,-17.4,;31.79,-16.07,;34.1,-17.4,;34.87,-16.07,;36.4,-16.06,;37.18,-17.4,;36.41,-18.74,;34.87,-18.74,;37.18,-20.07,;36.41,-21.4,;38.72,-20.07,;37.94,-21.4,;29.34,-19.99,;29.83,-21.46,;31.33,-21.77,;31.8,-23.24,;30.77,-24.39,;31.24,-25.85,;29.26,-24.06,;28.79,-22.6,;27.87,-19.51,)| Show InChI InChI=1S/C22H22F3N3O2/c1-13-4-2-7-18-19(13)28(16-8-10-17(29)11-9-16)21(26-18)27-20(30)14-5-3-6-15(12-14)22(23,24)25/h2-7,12,16-17,29H,8-11H2,1H3,(H,26,27,30)/t16-,17- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Btk (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50196093

(CHEMBL3939913)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C27H31F3N6O2/c1-18-8-6-10-21-24(18)36(20-9-4-5-15-35(17-20)23(37)11-7-14-34(2)3)26(32-21)33-25(38)19-12-13-31-22(16-19)27(28,29)30/h6-8,10-13,16,20H,4-5,9,14-15,17H2,1-3H3,(H,32,33,38)/b11-7+/t20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50160870

(CHEMBL3787344 | US11896597, Compound EGF816 | WO20...)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(Cl)c12 |r| Show InChI InChI=1S/C26H31ClN6O2/c1-18-16-19(12-13-28-18)25(35)30-26-29-22-10-6-9-21(27)24(22)33(26)20-8-4-5-15-32(17-20)23(34)11-7-14-31(2)3/h6-7,9-13,16,20H,4-5,8,14-15,17H2,1-3H3,(H,29,30,35)/b11-7+/t20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase STK11
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Lkb1 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196100
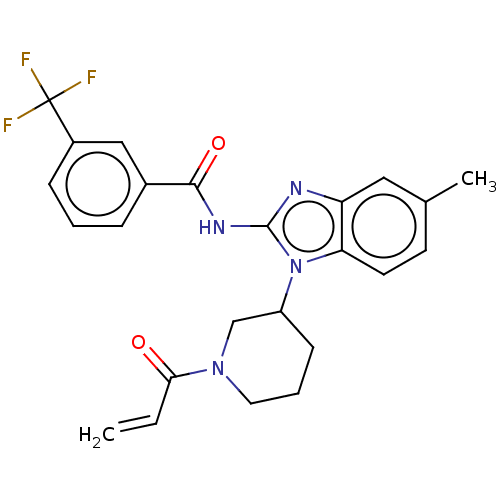
(CHEMBL3953921)Show SMILES Cc1ccc2n(C3CCCN(C3)C(=O)C=C)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C24H23F3N4O2/c1-3-21(32)30-11-5-8-18(14-30)31-20-10-9-15(2)12-19(20)28-23(31)29-22(33)16-6-4-7-17(13-16)24(25,26)27/h3-4,6-7,9-10,12-13,18H,1,5,8,11,14H2,2H3,(H,28,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
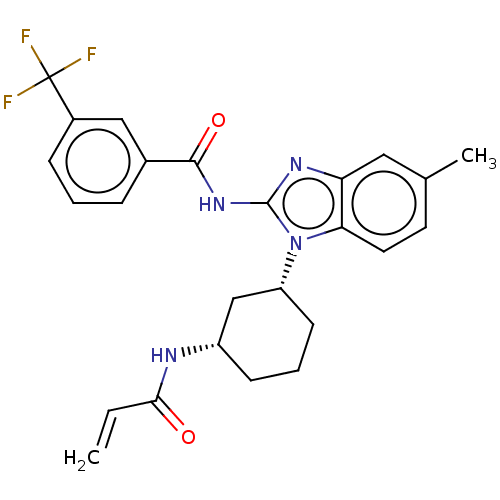
(Homo sapiens (Human)) | BDBM50196102
(CHEMBL3953221)Show SMILES Cc1ccc2n([C@@H]3CCC[C@@H](C3)NC(=O)C=C)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-22(33)29-18-8-5-9-19(14-18)32-21-11-10-15(2)12-20(21)30-24(32)31-23(34)16-6-4-7-17(13-16)25(26,27)28/h3-4,6-7,10-13,18-19H,1,5,8-9,14H2,2H3,(H,29,33)(H,30,31,34)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type human EGFR phosphorylation expressed in mouse NIH/3T3 cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
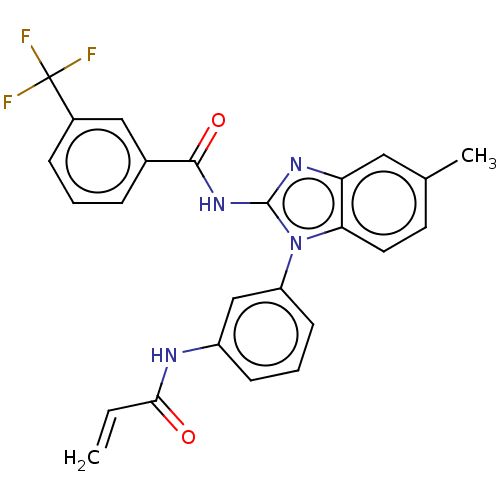
(Homo sapiens (Human)) | BDBM50196103
(CHEMBL3959632)Show SMILES Cc1ccc2n(c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1)-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C25H19F3N4O2/c1-3-22(33)29-18-8-5-9-19(14-18)32-21-11-10-15(2)12-20(21)30-24(32)31-23(34)16-6-4-7-17(13-16)25(26,27)28/h3-14H,1H2,2H3,(H,29,33)(H,30,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
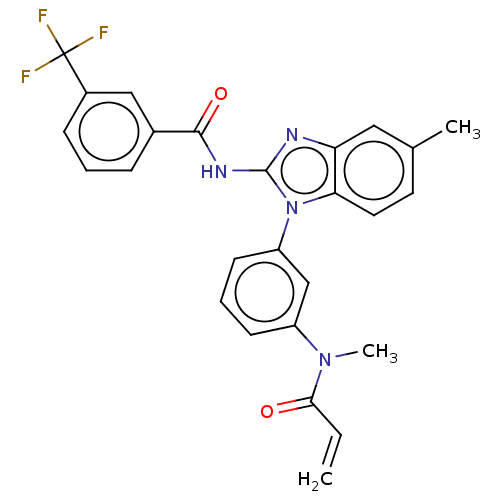
(Homo sapiens (Human)) | BDBM50196099
(CHEMBL3961202)Show SMILES CN(C(=O)C=C)c1cccc(c1)-n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(C)ccc12 Show InChI InChI=1S/C26H21F3N4O2/c1-4-23(34)32(3)19-9-6-10-20(15-19)33-22-12-11-16(2)13-21(22)30-25(33)31-24(35)17-7-5-8-18(14-17)26(27,28)29/h4-15H,1H2,2-3H3,(H,30,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196098
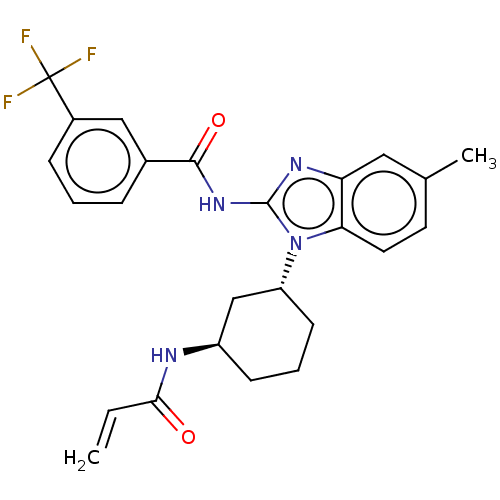
(CHEMBL3973973)Show SMILES Cc1ccc2n([C@@H]3CCC[C@H](C3)NC(=O)C=C)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-22(33)29-18-8-5-9-19(14-18)32-21-11-10-15(2)12-20(21)30-24(32)31-23(34)16-6-4-7-17(13-16)25(26,27)28/h3-4,6-7,10-13,18-19H,1,5,8-9,14H2,2H3,(H,29,33)(H,30,31,34)/t18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type human EGFR phosphorylation expressed in mouse NIH/3T3 cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196097
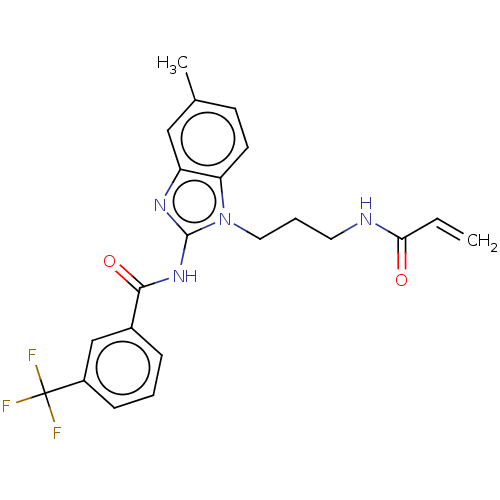
(CHEMBL3966111)Show SMILES Cc1ccc2n(CCCNC(=O)C=C)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C22H21F3N4O2/c1-3-19(30)26-10-5-11-29-18-9-8-14(2)12-17(18)27-21(29)28-20(31)15-6-4-7-16(13-15)22(23,24)25/h3-4,6-9,12-13H,1,5,10-11H2,2H3,(H,26,30)(H,27,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase STK11
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Lkb1 (unknown origin) |
Bioorg Med Chem Lett 18: 5916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.062
BindingDB Entry DOI: 10.7270/Q2M0457X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50196144
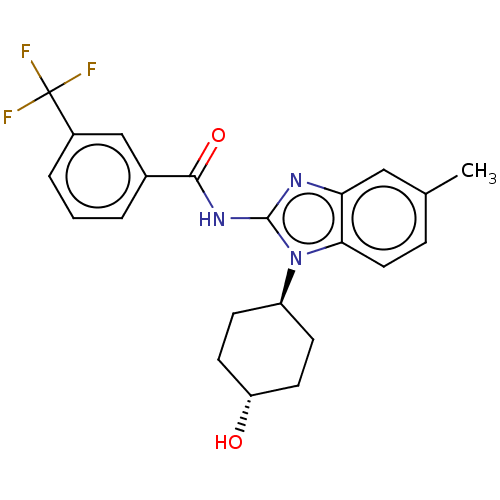
(CHEMBL3946259)Show SMILES Cc1ccc2n([C@H]3CC[C@H](O)CC3)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r,wU:6.5,wD:9.9,(4.39,-32.64,;5.72,-33.41,;5.72,-34.95,;7.05,-35.72,;8.39,-34.95,;9.86,-35.43,;10.35,-36.89,;11.85,-37.21,;12.32,-38.68,;11.29,-39.83,;11.76,-41.29,;9.78,-39.5,;9.31,-38.04,;10.77,-34.17,;12.31,-34.17,;13.08,-32.84,;12.31,-31.51,;14.62,-32.84,;15.38,-31.51,;16.92,-31.5,;17.7,-32.84,;16.93,-34.18,;15.39,-34.18,;17.7,-35.51,;16.93,-36.84,;19.24,-35.51,;18.46,-36.84,;9.86,-32.92,;8.39,-33.4,;7.05,-32.63,)| Show InChI InChI=1S/C22H22F3N3O2/c1-13-5-10-19-18(11-13)26-21(28(19)16-6-8-17(29)9-7-16)27-20(30)14-3-2-4-15(12-14)22(23,24)25/h2-5,10-12,16-17,29H,6-9H2,1H3,(H,26,27,30)/t16-,17- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50196096

(CHEMBL3951434)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C28H32F3N5O2/c1-19-9-6-13-23-25(19)36(22-12-4-5-16-35(18-22)24(37)14-8-15-34(2)3)27(32-23)33-26(38)20-10-7-11-21(17-20)28(29,30)31/h6-11,13-14,17,22H,4-5,12,15-16,18H2,1-3H3,(H,32,33,38)/b14-8+/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50196096

(CHEMBL3951434)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C28H32F3N5O2/c1-19-9-6-13-23-25(19)36(22-12-4-5-16-35(18-22)24(37)14-8-15-34(2)3)27(32-23)33-26(38)20-10-7-11-21(17-20)28(29,30)31/h6-11,13-14,17,22H,4-5,12,15-16,18H2,1-3H3,(H,32,33,38)/b14-8+/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50196096

(CHEMBL3951434)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cccc(C)c12 |r| Show InChI InChI=1S/C28H32F3N5O2/c1-19-9-6-13-23-25(19)36(22-12-4-5-16-35(18-22)24(37)14-8-15-34(2)3)27(32-23)33-26(38)20-10-7-11-21(17-20)28(29,30)31/h6-11,13-14,17,22H,4-5,12,15-16,18H2,1-3H3,(H,32,33,38)/b14-8+/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50160870

(CHEMBL3787344 | US11896597, Compound EGF816 | WO20...)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(Cl)c12 |r| Show InChI InChI=1S/C26H31ClN6O2/c1-18-16-19(12-13-28-18)25(35)30-26-29-22-10-6-9-21(27)24(22)33(26)20-8-4-5-15-32(17-20)23(34)11-7-14-31(2)3/h6-7,9-13,16,20H,4-5,8,14-15,17H2,1-3H3,(H,29,30,35)/b11-7+/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50160870

(CHEMBL3787344 | US11896597, Compound EGF816 | WO20...)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(Cl)c12 |r| Show InChI InChI=1S/C26H31ClN6O2/c1-18-16-19(12-13-28-18)25(35)30-26-29-22-10-6-9-21(27)24(22)33(26)20-8-4-5-15-32(17-20)23(34)11-7-14-31(2)3/h6-7,9-13,16,20H,4-5,8,14-15,17H2,1-3H3,(H,29,30,35)/b11-7+/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50196094

(CHEMBL3960167)Show SMILES CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2ccnc(C)c2)nc2cccc(C)c12 |r| Show InChI InChI=1S/C27H34N6O2/c1-19-9-7-11-23-25(19)33(27(29-23)30-26(35)21-13-14-28-20(2)17-21)22-10-5-6-16-32(18-22)24(34)12-8-15-31(3)4/h7-9,11-14,17,22H,5-6,10,15-16,18H2,1-4H3,(H,29,30,35)/b12-8+/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50196095

(CHEMBL3972316)Show SMILES Cc1cccc2nc(NC(=O)c3cccc(c3)C(F)(F)F)n([C@@H]3CCCCN(C3)C(=O)C=C)c12 |r| Show InChI InChI=1S/C25H25F3N4O2/c1-3-21(33)31-13-5-4-11-19(15-31)32-22-16(2)8-6-12-20(22)29-24(32)30-23(34)17-9-7-10-18(14-17)25(26,27)28/h3,6-10,12,14,19H,1,4-5,11,13,15H2,2H3,(H,29,30,34)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 59: 6671-89 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01985
BindingDB Entry DOI: 10.7270/Q2RF5X00 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data